Melatonin: Buffering the Immune System
Abstract
:1. Introduction
2. Pineal-Immune System Cross-Talk: From the Pineal Gland to the Immune System and Return
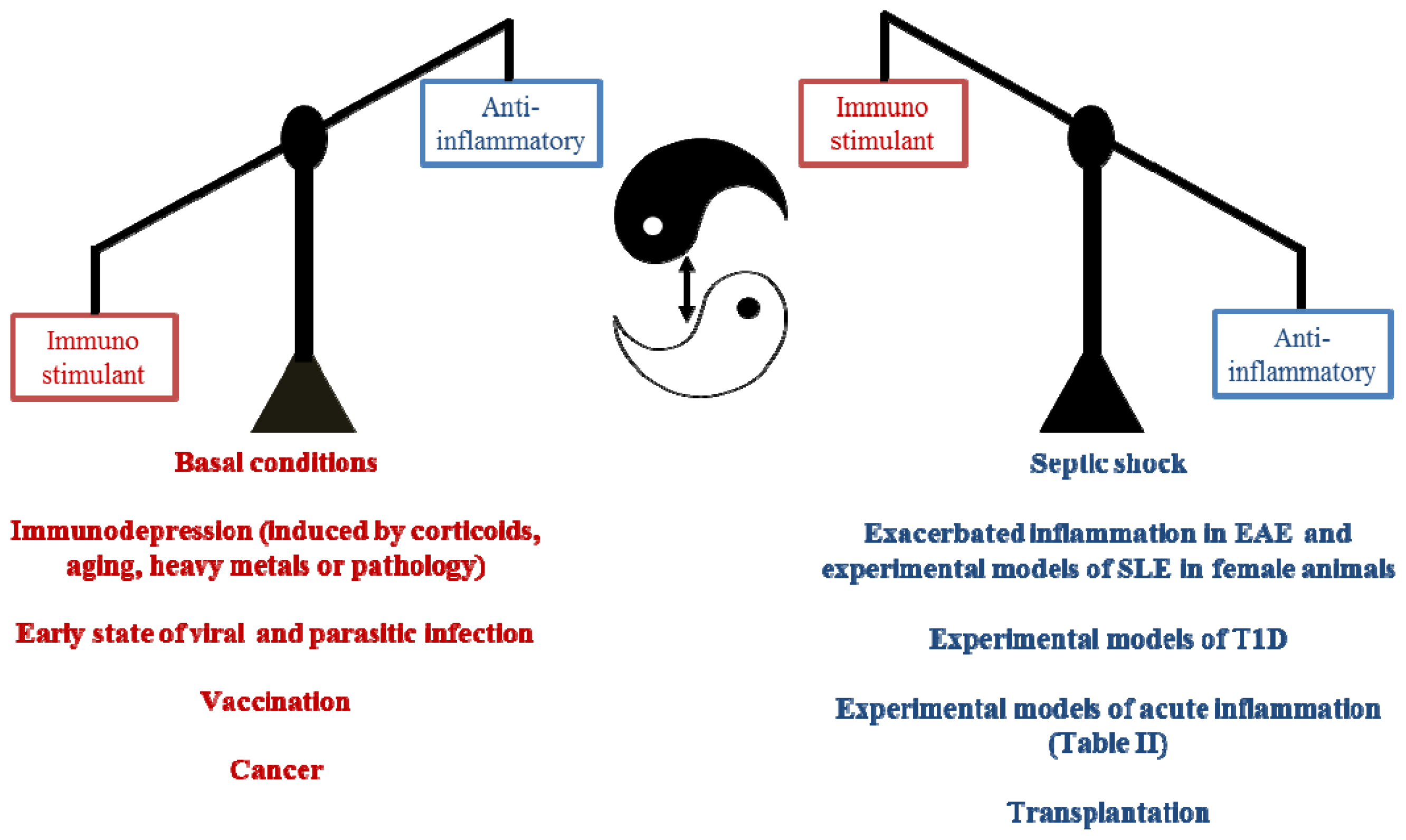
3. Pleiotropic Actions of Melatonin Administration on the Immune Response
3.1. Immunomodulatory Actions of Melatonin in the Innate Immune Response
3.2. Immunomodulatory Actions of Melatonin in the Specific Immune Response
4. Clinical Relevance of Melatonin
4.1. Melatonin and Infection
4.1.1. The Role of Melatonin in Viral Infections
4.1.2. The Role of Melatonin in Bacterial Infections
4.1.3. The Role of Melatonin in Parasite Infections
4.2. Melatonin and Autoimmunity: A Winding Road
4.2.1. Melatonin and Rheumatoid Arthritis
4.2.2. Melatonin and Multiple Sclerosis
4.2.3. Melatonin and Systemic Lupus Erythematosus
4.2.4. Melatonin and Type 1 Diabetes
4.2.5. Melatonin and Irritable Bowel Syndrome/Inflammatory Bowel Disease
4.3. Melatonin and Vaccination: A Worthwhile Area to Explore
4.4. Immunological Aspects of Melatonin in Transplantation
4.5. Melatonin and Immunosenescence
5. Conclusions
Acknowledgments
Conflicts of Interest
References
- Lerner, A.B.; Case, J.D.; Takahashi, Y.; Lee, T.H.; Mori, W. Isolation of melatonin, the pineal factor that lightens melanocytes. J. Am. Chem. Soc 1958, 80, 2587. [Google Scholar]
- Hardeland, R.; Poeggeler, B. Non-vertebrate melatonin. J. Pineal Res 2003, 34, 233–241. [Google Scholar]
- Axelrod, J.; Weissbach, H. Enzymatic o-methylation of n-acetylserotonin to melatonin. Science 1960, 131, 1312. [Google Scholar]
- Zawilska, J.B.; Skene, D.J.; Arendt, J. Physiology and pharmacology of melatonin in relation to biological rhythms. Pharmacol. Rep 2009, 61, 383–410. [Google Scholar]
- Rajaratnam, S.M.; Arendt, J. Health in a 24-h society. Lancet 2001, 358, 999–1005. [Google Scholar]
- Hardeland, R.; Cardinali, D.P.; Srinivasan, V.; Spence, D.W.; Brown, G.M.; Pandi-Perumal, S.R. Melatonin—A pleiotropic, orchestrating regulator molecule. Prog. Neurobiol 2011, 93, 350–384. [Google Scholar]
- Cutando, A.; Lopez-Valverde, A.; Arias-Santiago, S.; de Vicente, J.; de Diego, R.G. Role of melatonin in cancer treatment. Anticancer Res 2012, 32, 2747–2753. [Google Scholar]
- Radogna, F.; Diederich, M.; Ghibelli, L. Melatonin: A pleiotropic molecule regulating inflammation. Biochem. Pharmacol 2010, 80, 1844–1852. [Google Scholar]
- Hardeland, R.; Madrid, J.A.; Tan, D.X.; Reiter, R.J. Melatonin, the circadian multioscillator system and health: The need for detailed analyses of peripheral melatonin signaling. J. Pineal Res 2012, 52, 139–166. [Google Scholar]
- Grota, L.J.; Brown, G.M. Antibodies to indolealkylamines: Serotonin and melatonin. Can. J. Biochem 1974, 52, 196–202. [Google Scholar]
- Bubenik, G.A. Gastrointestinal melatonin: Localization, function, and clinical relevance. Dig. Dis. Sci 2002, 47, 2336–2348. [Google Scholar]
- Slominski, A.; Wortsman, J.; Tobin, D.J. The cutaneous serotoninergic/melatoninergic system: Securing a place under the sun. FASEB J 2005, 19, 176–194. [Google Scholar]
- Iuvone, P.M.; Tosini, G.; Pozdeyev, N.; Haque, R.; Klein, D.C.; Chaurasia, S.S. Circadian clocks, clock networks, arylalkylamine n-acetyltransferase, and melatonin in the retina. Prog. Retin. Eye Res 2005, 24, 433–456. [Google Scholar]
- Carrillo-Vico, A.; Guerrero, J.M.; Lardone, P.J.; Reiter, R.J. A review of the multiple actions of melatonin on the immune system. Endocrine 2005, 27, 189–200. [Google Scholar]
- Miossec, P.; Korn, T.; Kuchroo, V.K. Interleukin-17 and type 17 helper t cells. N. Engl. J. Med 2009, 361, 888–898. [Google Scholar]
- Xie, G.; Bonner, C.A.; Jensen, R.A. Dynamic diversity of the tryptophan pathway in chlamydiae: Reductive evolution and a novel operon for tryptophan recapture. Genome Biol 2002, 3, 51–56. [Google Scholar]
- Blalock, J.E.; Smith, E.M. Conceptual development of the immune system as a sixth sense. Brain Behav. Immun 2007, 21, 23–33. [Google Scholar]
- Carrillo-Vico, A.; Reiter, R.J.; Lardone, P.J.; Herrera, J.L.; Fernandez-Montesinos, R.; Guerrero, J.M.; Pozo, D. The modulatory role of melatonin on immune responsiveness. Curr. Opin. Investig. Drugs 2006, 7, 423–431. [Google Scholar]
- Withyachumnarnkul, B.; Nonaka, K.O.; Santana, C.; Attia, A.M.; Reiter, R.J. Interferon-gamma modulates melatonin production in rat pineal glands in organ culture. J. Interferon Res 1990, 10, 403–411. [Google Scholar]
- Mucha, S.; Zylinska, K.; Zerek-Melen, G.; Swietoslawski, J.; Stepien, H. Effect of interleukin-1 on in vivo melatonin secretion by the pineal gland in rats. Adv. Pineal Res 1994, 7, 177–181. [Google Scholar]
- Zylinska, K.; Komorowski, J.; Robak, T.; Mucha, S.; Stepien, H. Effect of granulocyte-macrophage colony stimulating factor and granulocyte colony stimulating factor on melatonin secretion in rats in vivo and in vitro studies. J. Neuroimmunol 1995, 56, 187–190. [Google Scholar]
- Youbicier-Simo, B.J.; Boudard, F.; Mekaouche, M.; Bayle, J.D.; Bastide, M. A role for bursa fabricii and bursin in the ontogeny of the pineal biosynthetic activity in the chicken. J. Pineal Res 1996, 21, 35–43. [Google Scholar]
- Markowska, M.; Bialecka, B.; Ciechanowska, M.; Koter, Z.; Laskowska, H.; Karkucinska-Wieckowska, A.; Skwarlo-Sonta, K. Effect of immunization on nocturnal nat activity in chicken pineal gland. Neuro Endocrinol. Lett 2000, 21, 367–373. [Google Scholar]
- Piesiewicz, A.; Kedzierska, U.; Adamska, I.; Usarek, M.; Zeman, M.; Skwarlo-Sonta, K.; Majewski, P.M. Pineal arylalkylamine n-acetyltransferase (aanat) gene expression as a target of inflammatory mediators in the chicken. Gen. Comp. Endocrinol 2012, 179, 143–151. [Google Scholar]
- Fernandes, P.A.; Cecon, E.; Markus, R.P.; Ferreira, Z.S. Effect of tnf-alpha on the melatonin synthetic pathway in the rat pineal gland: Basis for a “feedback” of the immune response on circadian timing. J. Pineal Res 2006, 41, 344–350. [Google Scholar]
- Pontes, G.N.; Cardoso, E.C.; Carneiro-Sampaio, M.M.; Markus, R.P. Injury switches melatonin production source from endocrine (pineal) to paracrine (phagocytes)—Melatonin in human colostrum and colostrum phagocytes. J. Pineal Res 2006, 41, 136–141. [Google Scholar]
- Pontes, G.N.; Cardoso, E.C.; Carneiro-Sampaio, M.M.; Markus, R.P. Pineal melatonin and the innate immune response: The tnf-alpha increase after cesarean section suppresses nocturnal melatonin production. J. Pineal Res 2007, 43, 365–371. [Google Scholar]
- Tamura, E.K.; Fernandes, P.A.; Marcola, M.; da Silveira Cruz-Machado, S.; Markus, R.P. Long-lasting priming of endothelial cells by plasma melatonin levels. PLoS One 2010, 5, 13958. [Google Scholar]
- Da Silveira Cruz-Machado, S.; Carvalho-Sousa, C.E.; Tamura, E.K.; Pinato, L.; Cecon, E.; Fernandes, P.A.; de Avellar, M.C.; Ferreira, Z.S.; Markus, R.P. Tlr4 and cd14 receptors expressed in rat pineal gland trigger nfkb pathway. J. Pineal Res 2010, 49, 183–192. [Google Scholar]
- Da Silveira Cruz-Machado, S.; Pinato, L.; Tamura, E.K.; Carvalho-Sousa, C.E.; Markus, R.P. Glia-pinealocyte network: The paracrine modulation of melatonin synthesis by tumor necrosis factor (tnf). PLoS One 2012, 7, 40142. [Google Scholar]
- Blalock, J.E. The immune system as the sixth sense. J. Intern. Med 2005, 257, 126–138. [Google Scholar]
- Tan, D.X.; Manchester, L.C.; Hardeland, R.; Lopez-Burillo, S.; Mayo, J.C.; Sainz, R.M.; Reiter, R.J. Melatonin: A hormone, a tissue factor, an autocoid, a paracoid, and an antioxidant vitamin. J. Pineal Res 2003, 34, 75–78. [Google Scholar]
- Raikhlin, N.T.; Kvetnoy, I.M.; Tolkachev, V.N. Melatonin may be synthesised in enterochromaffin cells. Nature 1975, 255, 344–345. [Google Scholar]
- Kvetnoy, I.M.; Ingel, I.E.; Kvetnaia, T.V.; Malinovskaya, N.K.; Rapoport, S.I.; Raikhlin, N.T.; Trofimov, A.V.; Yuzhakov, V.V. Gastrointestinal melatonin: Cellular identification and biological role. Neuro Endocrinol. Lett 2002, 23, 121–132. [Google Scholar]
- Stefulj, J.; Hortner, M.; Ghosh, M.; Schauenstein, K.; Rinner, I.; Wolfler, A.; Semmler, J.; Liebmann, P.M. Gene expression of the key enzymes of melatonin synthesis in extrapineal tissues of the rat. J. Pineal Res 2001, 30, 243–247. [Google Scholar]
- Finocchiaro, L.M.; Glikin, G.C. Intracellular melatonin distribution in cultured cell lines. J. Pineal Res 1998, 24, 22–34. [Google Scholar]
- Finocchiaro, L.M.; Nahmod, V.E.; Launay, J.M. Melatonin biosynthesis and metabolism in peripheral blood mononuclear leucocytes. Biochem. J 1991, 280, 727–731. [Google Scholar]
- Tan, D.X.; Manchester, L.C.; Reiter, R.J.; Qi, W.B.; Zhang, M.; Weintraub, S.T.; Cabrera, J.; Sainz, R.M.; Mayo, J.C. Identification of highly elevated levels of melatonin in bone marrow: Its origin and significance. Biochim. Biophys. Acta 1999, 1472, 206–214. [Google Scholar]
- Conti, A.; Conconi, S.; Hertens, E.; Skwarlo-Sonta, K.; Markowska, M.; Maestroni, J.M. Evidence for melatonin synthesis in mouse and human bone marrow cells. J. Pineal Res 2000, 28, 193–202. [Google Scholar]
- Martins, E., Jr; Ferreira, A.C.; Skorupa, A.L.; Afeche, S.C.; Cipolla-Neto, J.; Costa Rosa, LF. Tryptophan consumption and indoleamines production by peritoneal cavity macrophages. J. Leukoc. Biol 2004, 75, 1116–1121. [Google Scholar]
- Jimenez-Jorge, S.; Jimenez-Caliani, A.J.; Guerrero, J.M.; Naranjo, M.C.; Lardone, P.J.; Carrillo-Vico, A.; Osuna, C.; Molinero, P. Melatonin synthesis and melatonin-membrane receptor (mt1) expression during rat thymus development: Role of the pineal gland. J. Pineal Res 2005, 39, 77–83. [Google Scholar]
- Naranjo, M.C.; Guerrero, J.M.; Rubio, A.; Lardone, P.J.; Carrillo-Vico, A.; Carrascosa-Salmoral, M.P.; Jimenez-Jorge, S.; Arellano, M.V.; Leal-Noval, S.R.; Leal, M.; et al. Melatonin biosynthesis in the thymus of humans and rats. Cell Mol. Life Sci 2007, 64, 781–790. [Google Scholar]
- Gomez-Corvera, A.; Cerrillo, I.; Molinero, P.; Naranjo, M.C.; Lardone, P.J.; Sanchez-Hidalgo, M.; Carrascosa-Salmoral, M.P.; Medrano-Campillo, P.; Guerrero, J.M.; Rubio, A. Evidence of immune system melatonin production by two pineal melatonin deficient mice, c57bl/6 and swiss strains. J. Pineal Res 2009, 47, 15–22. [Google Scholar]
- Lardone, P.J.; Carrillo-Vico, A.; Naranjo, M.C.; de Felipe, B.; Vallejo, A.; Karasek, M.; Guerrero, J.M. Melatonin synthesized by jurkat human leukemic T cell line is implicated in il-2 production. J. Cell. Physiol 2006, 206, 273–279. [Google Scholar]
- Maldonado, M.D.; Mora-Santos, M.; Naji, L.; Carrascosa-Salmoral, M.P.; Naranjo, M.C.; Calvo, J.R. Evidence of melatonin synthesis and release by mast cells. Possible modulatory role on inflammation. Pharmacol. Res 2010, 62, 282–287. [Google Scholar]
- Pozo, D.; García-Mauriño, S.; Guerrero, J.M.; Calvo, J.R. Mrna expression of nuclear receptor rzr/roralpha, melatonin membrane receptor mt, and hydroxindole-o-methyltransferase in different populations of human immune cells. J. Pineal Res 2004, 37, 48–54. [Google Scholar]
- Carrillo-Vico, A.; García-Mauriño, S.; Calvo, J.R.; Guerrero, J.M. Melatonin counteracts the inhibitory effect of pge2 on il-2 production in human lymphocytes via its mt1 membrane receptor. FASEB J 2003, 17, 755–757. [Google Scholar]
- Lardone, P.J.; Carrillo-Vico, A.; Molinero, P.; Rubio, A.; Guerrero, J.M. A novel interplay between membrane and nuclear melatonin receptors in human lymphocytes: Significance in il-2 production. Cell. Mol. Life Sci 2009, 66, 516–525. [Google Scholar]
- Guerrero, J.M.; Pozo, D.; García-Mauriño, S.; Osuna, C.; Molinero, P.; Calvo, J.R. Involvement of nuclear receptors in the enhanced il-2 production by melatonin in jurkat cells. Ann. N. Y. Acad. Sci 2000, 917, 397–403. [Google Scholar]
- García-Mauriño, S.; Pozo, D.; Calvo, J.R.; Guerrero, J.M. Correlation between nuclear melatonin receptor expression and enhanced cytokine production in human lymphocytic and monocytic cell lines. J. Pineal Res 2000, 29, 129–137. [Google Scholar]
- Carrillo-Vico, A.; Lardone, P.J.; Fernandez-Santos, J.M.; Martin-Lacave, I.; Calvo, J.R.; Karasek, M.; Guerrero, J.M. Human lymphocyte-synthesized melatonin is involved in the regulation of the interleukin-2/interleukin-2 receptor system. J. Clin. Endocrinol. Metab 2005, 90, 992–1000. [Google Scholar]
- Lardone, P.J.; Rubio, A.; Cerrillo, I.; Gomez-Corvera, A.; Carrillo-Vico, A.; Sanchez-Hidalgo, M.; Guerrero, J.M.; Fernandez-Riejos, P.; Sanchez-Margalet, V.; Molinero, P. Blocking of melatonin synthesis and mt1 receptor impairs the activation of jurkat t cells. Cell. Mol. Life Sci 2010, 67, 3163–3172. [Google Scholar]
- Carrillo-Vico, A.; Garcia-Perganeda, A.; Naji, L.; Calvo, J.R.; Romero, M.P.; Guerrero, J.M. Expression of membrane and nuclear melatonin receptor mrna and protein in the mouse immune system. Cell. Mol. Life Sci 2003, 60, 2272–2278. [Google Scholar]
- Pozo, D.; Delgado, M.; Fernandez-Santos, J.M.; Calvo, J.R.; Gomariz, R.P.; Martin-Lacave, I.; Ortiz, G.G.; Guerrero, J.M. Expression of the mel1a-melatonin receptor mrna in t and b subsets of lymphocytes from rat thymus and spleen. FASEB J 1997, 11, 466–473. [Google Scholar]
- Ahmad, R.; Haldar, C. Melatonin and androgen receptor expression interplay modulates cell-mediated immunity in tropical rodent funambulus pennanti: An in vivo and in vitro study. Scand. J. Immunol 2010, 71, 420–430. [Google Scholar]
- Ahmad, R.; Haldar, C.; Gupta, S. Melatonin membrane receptor type mt1 modulates cell-mediated immunity in the seasonally breeding tropical rodent funambulus pennanti. Neuroimmunomodulation 2012, 19, 50–59. [Google Scholar]
- Ahmad, R.; Haldar, C. Photoperiodic regulation of mt1 and mt2 melatonin receptor expression is spleen and thymus of a tropical rodent funambulus pennanti during reproductively active and inactive phases. Chronobiol. Int 2010, 27, 446–462. [Google Scholar]
- Lahiri, S.; Haldar, C. Response of melatonin receptor mt1 in spleen of a tropical indian rodent, funambulus pennanti, to natural solar insolation and different photoperiodic conditions. Chronobiol. Int 2009, 26, 1559–1574. [Google Scholar]
- Gupta, S.; Haldar, C. Physiological crosstalk between melatonin and glucocorticoid receptor modulates t-cell mediated immune responses in a wild tropical rodent, funambulus pennanti. J. Steroid Biochem. Mol. Biol 2013, 134, 23–36. [Google Scholar]
- Drazen, D.L.; Nelson, R.J. Melatonin receptor subtype mt2 (mel 1b) and not mt1 (mel 1a) is associated with melatonin-induced enhancement of cell-mediated and humoral immunity. Neuroendocrinology 2001, 74, 178–184. [Google Scholar]
- Sánchez-Hidalgo, M.; Guerrero Montávez, J.M.; Carrascosa-Salmoral, M.D.P.; Naranjo Gutierrez, M.D.C.; Lardone, P.J.; de la Lastra Romero, C.A. Decreased mt1 and mt2 melatonin receptor expression in extrapineal tissues of the rat during physiological aging. J. Pineal Res 2009, 46, 29–35. [Google Scholar]
- Lotufo, C.M.; Lopes, C.; Dubocovich, M.L.; Farsky, S.H.; Markus, R.P. Melatonin and n-acetylserotonin inhibit leukocyte rolling and adhesion to rat microcirculation. Eur. J. Pharmacol 2001, 430, 351–357. [Google Scholar]
- Skwarlo-Sonta, K.; Majewski, P.; Markowska, M.; Oblap, R.; Olszanska, B. Bidirectional communication between the pineal gland and the immune system. Can. J. Physiol. Pharmacol 2003, 81, 342–349. [Google Scholar]
- Kumar Kharwar, R.; Haldar, C. Anatomical and histological profile of bronchus-associated lymphoid tissue and localization of melatonin receptor types (mel 1a and mel 1b) in the lung-associated immune system of a tropical bird, perdicula asiatica. Acta Histochem 2011, 113, 333–339. [Google Scholar]
- Yadav, S.K.; Haldar, C.; Singh, S.S. Variation in melatonin receptors (mel(1a) and mel(1b)) and androgen receptor (ar) expression in the spleen of a seasonally breeding bird, perdicula asiatica. J. Reprod. Immunol 2011, 92, 54–61. [Google Scholar]
- Espino, J.; Rodriguez, A.B.; Pariente, J.A. The inhibition of tnf-alpha-induced leucocyte apoptosis by melatonin involves membrane receptor mt1/mt2 interaction. J. Pineal Res. 2013. [Google Scholar] [CrossRef]
- Becker-André, M.; André, E.; DeLamarter, J.F. Identification of nuclear receptor mrnas by rt-pcr amplification of conserved zinc-finger motif sequences. Biochem. Biophys. Res. Commun 1993, 194, 1371–1379. [Google Scholar]
- Steinhilber, D.; Brungs, M.; Werz, O.; Wiesenberg, I.; Danielsson, C.; Kahlen, J.P.; Nayeri, S.; Schrader, M.; Carlberg, C. The nuclear receptor for melatonin represses 5-lipoxygenase gene expression in human b lymphocytes. J. Biol. Chem 1995, 270, 7037–7040. [Google Scholar]
- Lardone, P.J.; Guerrero, J.M.; Fernandez-Santos, J.M.; Rubio, A.; Martin-Lacave, I.; Carrillo-Vico, A. Melatonin synthesized by T lymphocytes as a ligand of the retinoic acid-related orphan receptor. J. Pineal Res 2011, 51, 454–462. [Google Scholar]
- Garcia-Mauriño, S.; Gonzalez-Haba, M.G.; Calvo, J.R.; Goberna, R.; Guerrero, J.M. Involvement of nuclear binding sites for melatonin in the regulation of il-2 and il-6 production by human blood mononuclear cells. J. Neuroimmunol 1998, 92, 76–84. [Google Scholar]
- Garcia-Maurino, S.; Gonzalez-Haba, M.G.; Calvo, J.R.; Rafii-El-Idrissi, M.; Sanchez-Margalet, V.; Goberna, R.; Guerrero, J.M. Melatonin enhances il-2, il-6, and ifn-gamma production by human circulating cd4+ cells: A possible nuclear receptor-mediated mechanism involving t helper type 1 lymphocytes and monocytes. J. Immunol 1997, 159, 574–581. [Google Scholar]
- Guerrero, J.M.; Pozo, D.; García-Mauriño, S.; Carrillo, A.; Osuna, C.; Molinero, P.; Calvo, J.R. Nuclear receptors are involved in the enhanced il-6 production by melatonin in u937 cells. Biol. Signals Recept 2000, 9, 197–202. [Google Scholar]
- Carrillo-Vico, A.; Calvo, J.R.; Abreu, P.; Lardone, P.J.; Garcia-Maurino, S.; Reiter, R.J.; Guerrero, J.M. Evidence of melatonin synthesis by human lymphocytes and its physiological significance: Possible role as intracrine, autocrine, and/or paracrine substance. FASEB J 2004, 18, 537–539. [Google Scholar]
- Fjaerli, O.; Lund, T.; Osterud, B. The effect of melatonin on cellular activation processes in human blood. J. Pineal Res 1999, 26, 50–55. [Google Scholar]
- Garcia-Maurino, S.; Pozo, D.; Carrillo-Vico, A.; Calvo, J.R.; Guerrero, J.M. Melatonin activates th1 lymphocytes by increasing il-12 production. Life Sci 1999, 65, 2143–2150. [Google Scholar]
- Muxel, S.M.; Pires-Lapa, M.A.; Monteiro, A.W.; Cecon, E.; Tamura, E.K.; Floeter-Winter, L.M.; Markus, R.P. Nf-kappab drives the synthesis of melatonin in raw 264.7 macrophages by inducing the transcription of the arylalkylamine-n-acetyltransferase (aa-nat) gene. PLoS One 2012, 7, e52010. [Google Scholar]
- Rai, S.; Haldar, C. Pineal control of immune status and hematological changes in blood and bone marrow of male squirrels (funambulus pennanti) during their reproductively active phase. Comp. Biochem. Physiol. C Toxicol. Pharmacol 2003, 136, 319–328. [Google Scholar]
- Vaughan, M.K.; Hubbard, G.B.; Champney, T.H.; Vaughan, G.M.; Little, J.C.; Reiter, R.J. Splenic hypertrophy and extramedullary hematopoiesis induced in male syrian hamsters by short photoperiod or melatonin injections and reversed by melatonin pellets or pinealectomy. Am. J. Anat 1987, 179, 131–136. [Google Scholar]
- Tian, Y.M.; Zhang, G.Y.; Dai, Y.R. Melatonin rejuvenates degenerated thymus and redresses peripheral immune functions in aged mice. Immunol. Lett 2003, 88, 101–104. [Google Scholar]
- Haldar, C.; Rai, S.; Singh, R. Melatonin blocks dexamethasone-induced immunosuppression in a seasonally breeding rodent indian palm squirrel, funambulus pennanti. Steroids 2004, 69, 367–377. [Google Scholar]
- Capelli, E.; Campo, I.; Panelli, S.; Damiani, G.; Barbone, M.G.; Lucchelli, A.; Cuccia, M. Evaluation of gene expression in human lymphocytes activated in the presence of melatonin. Int. Immunopharmacol 2002, 2, 885–892. [Google Scholar]
- Sze, S.F.; Liu, W.K.; Ng, T.B. Stimulation of murine splenocytes by melatonin and methoxytryptamine. J. Neural Transm. Gen. Sect 1993, 94, 115–126. [Google Scholar]
- Demas, G.E.; Nelson, R.J. Exogenous melatonin enhances cell-mediated, but not humoral, immune function in adult male deer mice (peromyscus maniculatus). J. Biol. Rhythms 1998, 13, 245–252. [Google Scholar]
- Kaur, C.; Ling, E.A. Effects of melatonin on macrophages/microglia in postnatal rat brain. J. Pineal Res 1999, 26, 158–168. [Google Scholar]
- Currier, N.L.; Sun, L.Z.; Miller, S.C. Exogenous melatonin: Quantitative enhancement in vivo of cells mediating non-specific immunity. J. Neuroimmunol 2000, 104, 101–108. [Google Scholar]
- Lissoni, P.; Marelli, O.; Mauri, R.; Resentini, M.; Franco, P.; Esposti, D.; Esposti, G.; Fraschini, F.; Halberg, F.; Sothern, R.B.; et al. Ultradian chronomodulation by melatonin of a placebo effect upon human killer cell activity. Chronobiologia 1986, 13, 339–343. [Google Scholar]
- Baeza, I.; Alvarado, C.; Alvarez, P.; Salazar, V.; Castillo, C.; Ariznavarreta, C.; Fdez-Tresguerres, J.A.; de la Fuente, M. Improvement of leucocyte functions in ovariectomised aged rats after treatment with growth hormone, melatonin, oestrogens or phyto-oestrogens. J. Reprod Immunol 2009, 80, 70–79. [Google Scholar]
- Pena, C.; Rincon, J.; Pedreanez, A.; Viera, N.; Mosquera, J. Chemotactic effect of melatonin on leukocytes. J. Pineal Res 2007, 43, 263–269. [Google Scholar]
- Pioli, C.; Caroleo, M.C.; Nistico, G.; Doria, G. Melatonin increases antigen presentation and amplifies specific and non specific signals for t-cell proliferation. Int. J. Immunopharmacol 1993, 15, 463–468. [Google Scholar]
- Rodriguez, A.B.; Terron, M.P.; Duran, J.; Ortega, E.; Barriga, C. Physiological concentrations of melatonin and corticosterone affect phagocytosis and oxidative metabolism of ring dove heterophils. J. Pineal Res 2001, 31, 31–38. [Google Scholar]
- Arias, J.; Melean, E.; Valero, N.; Pons, H.; Chacin-Bonilla, L.; Larreal, Y.; Bonilla, E. Effect of melatonin on lymphocyte proliferation and production of interleukin-2 (il-2) and interleukin-1 beta (il-1 beta) in mice splenocytes. Invest. Clin 2003, 44, 41–50. [Google Scholar]
- Wichmann, M.W.; Zellweger, R.; DeMaso, C.M.; Ayala, A.; Chaudry, I.H. Melatonin administration attenuates depressed immune functions trauma-hemorrhage. J. Surg. Res 1996, 63, 256–262. [Google Scholar]
- Morrey, K.M.; McLachlan, J.A.; Serkin, C.D.; Bakouche, O. Activation of human monocytes by the pineal hormone melatonin. J. Immunol 1994, 153, 2671–2680. [Google Scholar]
- Lin, X.J.; Mei, G.P.; Liu, J.; Li, Y.L.; Zuo, D.; Liu, S.J.; Zhao, T.B.; Lin, M.T. Therapeutic effects of melatonin on heatstroke-induced multiple organ dysfunction syndrome in rats. J. Pineal Res 2011, 50, 436–444. [Google Scholar]
- Chen, C.F.; Wang, D.; Reiter, R.J.; Yeh, D.Y. Oral melatonin attenuates lung inflammation and airway hyperreactivity induced by inhalation of aerosolized pancreatic fluid in rats. J. Pineal Res 2011, 50, 46–53. [Google Scholar]
- Jaworek, J.; Szklarczyk, J.; Jaworek, A.K.; Nawrot-Porabka, K.; Leja-Szpak, A.; Bonior, J.; Kot, M. Protective effect of melatonin on acute pancreatitis. Int. J. Inflam 2012, 2012, 173675, :1–173675:8.. [Google Scholar]
- Lee, M.Y.; Kuan, Y.H.; Chen, H.Y.; Chen, T.Y.; Chen, S.T.; Huang, C.C.; Yang, I.P.; Hsu, Y.S.; Wu, T.S.; Lee, E.J. Intravenous administration of melatonin reduces the intracerebral cellular inflammatory response following transient focal cerebral ischemia in rats. J. Pineal Res 2007, 42, 297–309. [Google Scholar]
- Lotufo, C.M.; Yamashita, C.E.; Farsky, S.H.; Markus, R.P. Melatonin effect on endothelial cells reduces vascular permeability increase induced by leukotriene b4. Eur. J. Pharmacol 2006, 534, 258–263. [Google Scholar]
- Yuan, X.; Li, B.; Li, H.; Xiu, R. Melatonin inhibits il-1beta-induced monolayer permeability of human umbilical vein endothelial cells via rac activation. J. Pineal Res 2011, 51, 220–225. [Google Scholar]
- Deng, W.G.; Tang, S.T.; Tseng, H.P.; Wu, K.K. Melatonin suppresses macrophage cyclooxygenase-2 and inducible nitric oxide synthase expression by inhibiting p52 acetylation and binding. Blood 2006, 108, 518–524. [Google Scholar]
- Ban, J.Y.; Kim, B.S.; Kim, S.C.; Kim, D.H.; Chung, J.H. Microarray analysis of gene expression profiles in response to treatment with melatonin in lipopolysaccharide activated raw 264.7 cells. Korean J. Physiol. Pharmacol 2011, 15, 23–29. [Google Scholar]
- Min, K.J.; Jang, J.H.; Kwon, T.K. Inhibitory effects of melatonin on the lipopolysaccharide-induced cc chemokine expression in bv2 murine microglial cells are mediated by suppression of akt-induced nf-kappab and stat/gas activity. J. Pineal Res 2012, 52, 296–304. [Google Scholar]
- Kim, G.D.; Lee, S.E.; Kim, T.H.; Jin, Y.H.; Park, Y.S.; Park, C.S. Melatonin suppresses acrolein-induced il-8 production in human pulmonary fibroblasts. J. Pineal Res 2012, 52, 356–364. [Google Scholar]
- Silva, S.O.; Rodrigues, M.R.; Ximenes, V.F.; Bueno-da-Silva, A.E.; Amarante-Mendes, G.P.; Campa, A. Neutrophils as a specific target for melatonin and kynuramines: Effects on cytokine release. J. Neuroimmunol 2004, 156, 146–152. [Google Scholar]
- Pieri, C.; Recchioni, R.; Moroni, F.; Marcheselli, F.; Marra, M.; Marinoni, S.; di Primio, R. Melatonin regulates the respiratory burst of human neutrophils and their depolarization. J. Pineal Res 1998, 24, 43–49. [Google Scholar]
- Akbulut, K.G.; Gonul, B.; Akbulut, H. The effects of melatonin on humoral immune responses of young and aged rats. Immunol. Invest 2001, 30, 17–20. [Google Scholar]
- Rai, S.; Haldar, C.; Singh, R. Modulation of immunity in young-adult and aged squirrel, funambulus pennanti by melatonin and p-chlorophenylalanine. Immun. Ageing 2009, 6, 5. [Google Scholar]
- Inserra, P.; Zhang, Z.; Ardestani, S.K.; Araghi-Niknam, M.; Liang, B.; Jiang, S.; Shaw, D.; Molitor, M.; Elliott, K.; Watson, R.R. Modulation of cytokine production by dehydroepiandrosterone (dhea) plus melatonin (mlt) supplementation of old mice. Proc. Soc. Exp. Biol. Med 1998, 218, 76–82. [Google Scholar]
- Maestroni, G.J.; Conti, A.; Pierpaoli, W. Role of the pineal gland in immunity. Circadian synthesis and release of melatonin modulates the antibody response and antagonizes the immunosuppressive effect of corticosterone. J. Neuroimmunol 1986, 13, 19–30. [Google Scholar]
- Kim, Y.O.; Pyo, M.Y.; Kim, J.H. Influence of melatonin on immunotoxicity of lead. Int. J. Immunopharmacol 2000, 22, 821–832. [Google Scholar]
- Veneroso, C.; Tunon, M.J.; Gonzalez-Gallego, J.; Collado, P.S. Melatonin reduces cardiac inflammatory injury induced by acute exercise. J. Pineal Res 2009, 47, 184–191. [Google Scholar]
- Mei, Q.; Yu, J.P.; Xu, J.M.; Wei, W.; Xiang, L.; Yue, L. Melatonin reduces colon immunological injury in rats by regulating activity of macrophages. Acta Pharmacol. Sin 2002, 23, 882–886. [Google Scholar]
- Agil, A.; Reiter, R.J.; Jimenez-Aranda, A.; Iban-Arias, R.; Navarro-Alarcon, M.; Marchal, J.A.; Adem, A.; Fernandez-Vazquez, G. Melatonin ameliorates low-grade inflammation and oxidative stress in young zucker diabetic fatty rats. J. Pineal Res 2012, 54, 381–388. [Google Scholar]
- Tsai, M.C.; Chen, W.J.; Tsai, M.S.; Ching, C.H.; Chuang, J.I. Melatonin attenuates brain contusion-induced oxidative insult, inactivation of signal transducers and activators of transcription 1, and upregulation of suppressor of cytokine signaling-3 in rats. J. Pineal Res 2011, 51, 233–245. [Google Scholar]
- Kang, J.W.; Koh, E.J.; Lee, S.M. Melatonin protects liver against ischemia and reperfusion injury through inhibition of toll-like receptor signaling pathway. J. Pineal Res 2011, 50, 403–411. [Google Scholar]
- Negi, G.; Kumar, A.; Sharma, S.S. Melatonin modulates neuroinflammation and oxidative stress in experimental diabetic neuropathy: Effects on nf-kappab and nrf2 cascades. J. Pineal Res 2011, 50, 124–131. [Google Scholar]
- Ara, C.; Dirican, A.; Unal, B.; Bay Karabulut, A.; Piskin, T. The effect of melatonin against fk506-induced renal oxidative stress in rats. Surg. Innov 2011, 18, 34–38. [Google Scholar]
- Tahan, G.; Gramignoli, R.; Marongiu, F.; Aktolga, S.; Cetinkaya, A.; Tahan, V.; Dorko, K. Melatonin expresses powerful anti-inflammatory and antioxidant activities resulting in complete improvement of acetic-acid-induced colitis in rats. Dig. Dis. Sci 2011, 56, 715–720. [Google Scholar]
- Jung, K.H.; Hong, S.W.; Zheng, H.M.; Lee, H.S.; Lee, H.; Lee, D.H.; Lee, S.Y.; Hong, S.S. Melatonin ameliorates cerulein-induced pancreatitis by the modulation of nuclear erythroid 2-related factor 2 and nuclear factor-kappab in rats. J. Pineal Res 2010, 48, 239–250. [Google Scholar]
- Yang, F.L.; Subeq, Y.M.; Lee, C.J.; Lee, R.P.; Peng, T.C.; Hsu, B.G. Melatonin ameliorates hemorrhagic shock-induced organ damage in rats. J. Surg. Res 2011, 167, 315–321. [Google Scholar]
- Jung, K.H.; Hong, S.W.; Zheng, H.M.; Lee, D.H.; Hong, S.S. Melatonin downregulates nuclear erythroid 2-related factor 2 and nuclear factor-kappab during prevention of oxidative liver injury in a dimethylnitrosamine model. J. Pineal Res 2009, 47, 173–183. [Google Scholar]
- Kireev, R.A.; Tresguerres, A.C.; Garcia, C.; Ariznavarreta, C.; Vara, E.; Tresguerres, J.A. Melatonin is able to prevent the liver of old castrated female rats from oxidative and pro-inflammatory damage. J. Pineal Res 2008, 45, 394–402. [Google Scholar]
- Ozen, I.O.; Ekingen, G.; Taslipinar, M.Y.; Bukan, N.; Demirogullari, B.; Karabulut, R.; Sonmez, K.; Basaklar, A.C.; Kale, N. Effect of melatonin on healing of colonic anastomosis in a rat model of peritonitis. Eur. Surg. Res 2007, 39, 122–127. [Google Scholar]
- Li, J.H.; Yu, J.P.; Yu, H.G.; Xu, X.M.; Yu, L.L.; Liu, J.; Luo, H.S. Melatonin reduces inflammatory injury through inhibiting nf-kappab activation in rats with colitis. Mediators Inflamm 2005, 2005, 185–193. [Google Scholar]
- Tyagi, E.; Agrawal, R.; Nath, C.; Shukla, R. Effect of melatonin on neuroinflammation and acetylcholinesterase activity induced by lps in rat brain. Eur. J. Pharmacol 2010, 640, 206–210. [Google Scholar]
- Kara, A.; Akman, S.; Ozkanlar, S.; Tozoglu, U.; Kalkan, Y.; Canakci, C.F.; Tozoglu, S. Immune modulatory and antioxidant effects of melatonin in experimental periodontitis in rats. Free Radic. Biol. Med 2013, 55, 21–26. [Google Scholar]
- Esposito, E.; Mazzon, E.; Riccardi, L.; Caminiti, R.; Meli, R.; Cuzzocrea, S. Matrix metalloproteinase-9 and metalloproteinase-2 activity and expression is reduced by melatonin during experimental colitis. J. Pineal Res 2008, 45, 166–173. [Google Scholar]
- Mazzon, E.; Esposito, E.; Crisafulli, C.; Riccardi, L.; Muia, C.; di Bella, P.; Meli, R.; Cuzzocrea, S. Melatonin modulates signal transduction pathways and apoptosis in experimental colitis. J. Pineal Res 2006, 41, 363–373. [Google Scholar]
- Gulben, K.; Ozdemir, H.; Berberoglu, U.; Mersin, H.; Yrkin, F.; Cakyr, E.; Aksaray, S. Melatonin modulates the severity of taurocholate-induced acute pancreatitis in the rat. Dig. Dis. Sci 2010, 55, 941–946. [Google Scholar]
- Sener, G.; Tugtepe, H.; Velioglu-Ogunc, A.; Cetinel, S.; Gedik, N.; Yegen, B.C. Melatonin prevents neutrophil-mediated oxidative injury in escherichia coli-induced pyelonephritis in rats. J. Pineal Res 2006, 41, 220–227. [Google Scholar]
- Yip, H.K.; Chang, Y.C.; Wallace, C.G.; Chang, L.T.; Tsai, T.H.; Chen, Y.L.; Chang, H.W.; Leu, S.; Zhen, Y.Y.; Tsai, C.Y.; et al. Melatonin treatment improves adipose-derived mesenchymal stem cell therapy for acute lung ischemia-reperfusion injury. J. Pineal Res 2013, 54, 207–221. [Google Scholar]
- Kaur, C.; Sivakumar, V.; Robinson, R.; Foulds, W.S.; Luu, C.D.; Ling, E.A. Neuroprotective effect of melatonin against hypoxia-induced retinal ganglion cell death in neonatal rats. J. Pineal Res 2013, 54, 190–206. [Google Scholar]
- Kunak, Z.I.; Macit, E.; Yaren, H.; Yaman, H.; Cakir, E.; Aydin, I.; Turker, T.; Kurt, Y.G.; Ozcan, A.; Uysal, B.; et al. Protective effects of melatonin and s-methylisothiourea on mechlorethamine induced nephrotoxicity. J. Surg. Res 2012, 175, 17–23. [Google Scholar]
- Wang, H.; Wei, W.; Zhang, S.Y.; Shen, Y.X.; Yue, L.; Wang, N.P.; Xu, S.Y. Melatonin-selenium nanoparticles inhibit oxidative stress and protect against hepatic injury induced by bacillus calmette-guerin/lipopolysaccharide in mice. J. Pineal Res 2005, 39, 156–163. [Google Scholar]
- Jang, S.S.; Kim, H.G.; Lee, J.S.; Han, J.M.; Park, H.J.; Huh, G.J.; Son, C.G. Melatonin reduces X-ray radiation-induced lung injury in mice by modulating oxidative stress and cytokine expression. Int. J.. Radiat. Biol 2013, 89, 97–105. [Google Scholar]
- Xu, D.X.; Wang, H.; Ning, H.; Zhao, L.; Chen, Y.H. Maternally administered melatonin differentially regulates lipopolysaccharide-induced proinflammatory and anti-inflammatory cytokines in maternal serum, amniotic fluid, fetal liver, and fetal brain. J. Pineal Res 2007, 43, 74–79. [Google Scholar]
- Ganguly, K.; Swarnakar, S. Chronic gastric ulceration causes matrix metalloproteinases-9 and -3 augmentation: Alleviation by melatonin. Biochimie 2012, 94, 2687–2698. [Google Scholar]
- Olcese, J.M.; Cao, C.; Mori, T.; Mamcarz, M.B.; Maxwell, A.; Runfeldt, M.J.; Wang, L.; Zhang, C.; Lin, X.; Zhang, G.; et al. Protection against cognitive deficits and markers of neurodegeneration by long-term oral administration of melatonin in a transgenic model of alzheimer disease. J. Pineal Res 2009, 47, 82–96. [Google Scholar]
- Gitto, E.; Aversa, S.; Salpietro, C.D.; Barberi, I.; Arrigo, T.; Trimarchi, G.; Reiter, R.J.; Pellegrino, S. Pain in neonatal intensive care: Role of melatonin as an analgesic antioxidant. J. Pineal Res 2012, 52, 291–295. [Google Scholar]
- Ochoa, J.J.; Diaz-Castro, J.; Kajarabille, N.; Garcia, C.; Guisado, I.M.; de Teresa, C.; Guisado, R. Melatonin supplementation ameliorates oxidative stress and inflammatory signaling induced by strenuous exercise in adult human males. J. Pineal Res 2011, 51, 373–380. [Google Scholar]
- Chahbouni, M.; Escames, G.; Venegas, C.; Sevilla, B.; Garcia, J.A.; Lopez, L.C.; Munoz-Hoyos, A.; Molina-Carballo, A.; Acuna-Castroviejo, D. Melatonin treatment normalizes plasma pro-inflammatory cytokines and nitrosative/oxidative stress in patients suffering from duchenne muscular dystrophy. J. Pineal Res 2010, 48, 282–289. [Google Scholar]
- Gitto, E.; Reiter, R.J.; Cordaro, S.P.; La Rosa, M.; Chiurazzi, P.; Trimarchi, G.; Gitto, P.; Calabro, M.P.; Barberi, I. Oxidative and inflammatory parameters in respiratory distress syndrome of preterm newborns: Beneficial effects of melatonin. Am. J. Perinatol 2004, 21, 209–216. [Google Scholar]
- Xia, M.Z.; Liang, Y.L.; Wang, H.; Chen, X.; Huang, Y.Y.; Zhang, Z.H.; Chen, Y.H.; Zhang, C.; Zhao, M.; Xu, D.X.; et al. Melatonin modulates tlr4-mediated inflammatory genes through myd88- and trif-dependent signaling pathways in lipopolysaccharide-stimulated raw264.7 cells. J. Pineal Res 2012, 53, 325–334. [Google Scholar]
- Choi, E.Y.; Jin, J.Y.; Lee, J.Y.; Choi, J.I.; Choi, I.S.; Kim, S.J. Melatonin inhibits prevotella intermedia lipopolysaccharide-induced production of nitric oxide and interleukin-6 in murine macrophages by suppressing nf-kappab and stat1 activity. J. Pineal Res 2011, 50, 197–206. [Google Scholar]
- Huang, S.H.; Cao, X.J.; Wei, W. Melatonin decreases tlr3-mediated inflammatory factor expression via inhibition of nf-kappa b activation in respiratory syncytial virus-infected raw264.7 macrophages. J. Pineal Res 2008, 45, 93–100. [Google Scholar]
- Tocharus, J.; Khonthun, C.; Chongthammakun, S.; Govitrapong, P. Melatonin attenuates methamphetamine-induced overexpression of pro-inflammatory cytokines in microglial cell lines. J. Pineal Res 2010, 48, 347–352. [Google Scholar]
- Hoppe, J.B.; Frozza, R.L.; Horn, A.P.; Comiran, R.A.; Bernardi, A.; Campos, M.M.; Battastini, A.M.; Salbego, C. Amyloid-beta neurotoxicity in organotypic culture is attenuated by melatonin: Involvement of gsk-3beta, tau and neuroinflammation. J. Pineal Res 2010, 48, 230–238. [Google Scholar]
- Park, J.H.; Chung, E.J.; Kwon, H.J.; Im, S.S.; Lim, J.G.; Song, D.K. Protective effect of melatonin on tnf-alpha-induced muscle atrophy in L6 myotubes. J. Pineal Res. 2012. [Google Scholar] [CrossRef]
- Petrovsky, N.; Harrison, L.C. Diurnal rhythmicity of human cytokine production: A dynamic disequilibrium in t helper cell type 1/t helper cell type 2 balance? J. Immunol 1997, 158, 5163–5168. [Google Scholar]
- Drazen, D.L.; Klein, S.L.; Yellon, S.M.; Nelson, R.J. In vitro melatonin treatment enhances splenocyte proliferation in prairie voles. J. Pineal Res 2000, 28, 34–40. [Google Scholar]
- Wu, C.C.; Lu, K.C.; Lin, G.J.; Hsieh, H.Y.; Chu, P.; Lin, S.H.; Sytwu, H.K. Melatonin enhances endogenous heme oxygenase-1 and represses immune responses to ameliorate experimental murine membranous nephropathy. J. Pineal Res 2012, 52, 460–469. [Google Scholar]
- Maestroni, G.J. T-helper-2 lymphocytes as a peripheral target of melatonin. J. Pineal Res 1995, 18, 84–89. [Google Scholar]
- Dimitrov, S.; Lange, T.; Tieken, S.; Fehm, H.L.; Born, J. Sleep associated regulation of t helper 1/t helper 2 cytokine balance in humans. Brain Behav. Immun 2004, 18, 341–348. [Google Scholar]
- Kelestimur, H.; Sahin, Z.; Sandal, S.; Bulmus, O.; Ozdemir, G.; Yilmaz, B. Melatonin-related alterations in th1/th2 polarisation in primary thymocyte cultures of pinealectomized rats. Front. Neuroendocrinol 2006, 27, 103–110. [Google Scholar]
- Raghavendra, V.; Singh, V.; Kulkarni, S.K.; Agrewala, J.N. Melatonin enhances th2 cell mediated immune responses: Lack of sensitivity to reversal by naltrexone or benzodiazepine receptor antagonists. Mol. Cell. Biochem 2001, 221, 57–62. [Google Scholar]
- Majewska, M.; Zajac, K.; Zemelka, M.; Szczepanik, M. Influence of melatonin and its precursor l-tryptophan on th1 dependent contact hypersensitivity. J. Physiol. Pharmacol 2007, 58, 125–132. [Google Scholar]
- Hemadi, M.; Shokri, S.; Pourmatroud, E.; Moramezi, F.; Khodadai, A. Follicular dynamic and immunoreactions of the vitrified ovarian graft after host treatment with variable regimens of melatonin. Am. J. Reprod. Immunol 2012, 67, 401–412. [Google Scholar]
- Carrillo-Vico, A.; Lardone, P.J.; Naji, L.; Fernandez-Santos, J.M.; Martin-Lacave, I.; Guerrero, J.M.; Calvo, J.R. Beneficial pleiotropic actions of melatonin in an experimental model of septic shock in mice: Regulation of pro-/anti-inflammatory cytokine network, protection against oxidative damage and anti-apoptotic effects. J. Pineal Res 2005, 39, 400–408. [Google Scholar]
- Kim, T.H.; Jung, J.A.; Kim, G.D.; Jang, A.H.; Ahn, H.J.; Park, Y.S.; Park, C.S. Melatonin inhibits the development of 2,4-dinitrofluorobenzene-induced atopic dermatitis-like skin lesions in nc/nga mice. J. Pineal Res 2009, 47, 324–329. [Google Scholar]
- Cernysiov, V.; Gerasimcik, N.; Mauricas, M.; Girkontaite, I. Regulation of t-cell-independent and t-cell-dependent antibody production by circadian rhythm and melatonin. Int. Immunol 2010, 22, 25–34. [Google Scholar]
- Yang, X.O.; Pappu, B.P.; Nurieva, R.; Akimzhanov, A.; Kang, H.S.; Chung, Y.; Ma, L.; Shah, B.; Panopoulos, A.D.; Schluns, K.S.; et al. T helper 17 lineage differentiation is programmed by orphan nuclear receptors ror alpha and ror gamma. Immunity 2008, 28, 29–39. [Google Scholar]
- Du, J.; Huang, C.; Zhou, B.; Ziegler, S.F. Isoform-specific inhibition of ror alpha-mediated transcriptional activation by human foxp3. J. Immunol 2008, 180, 4785–4792. [Google Scholar]
- Vigore, L.; Messina, G.; Brivio, F.; Fumagalli, L.; Rovelli, F.; di Gede, G.; Lissoni, P. Psychoneuroendocrine modulation of regulatory t lymphocyte system: In vivo and in vitro effects of the pineal immunomodulating hormone melatonin. In Vivo 2010, 24, 787–789. [Google Scholar]
- Liu, H.; Xu, L.; Wei, J.E.; Xie, M.R.; Wang, S.E.; Zhou, R.X. Role of cd4+ cd25+ regulatory T cells in melatonin-mediated inhibition of murine gastric cancer cell growth in vivo and in vitro. Anat Rec. (Hoboken) 2011, 294, 781–788. [Google Scholar]
- Srinivasan, V.; Mohamed, M.; Kato, H. Melatonin in bacterial and viral infections with focus on sepsis: A review. Recent Pat. Endocr. Metab. Immune Drug Discov 2012, 6, 30–39. [Google Scholar]
- Bagnaresi, P.; Nakabashi, M.; Thomas, A.P.; Reiter, R.J.; Garcia, C.R. The role of melatonin in parasite biology. Mol. Biochem. Parasitol 2012, 181, 1–6. [Google Scholar]
- Reiter, R.J.; Tan, D.X.; Rosales-Corral, S.; Manchester, L.C. The universal nature, unequal distribution and antioxidant functions of melatonin and its derivatives. Mini Rev. Med. Chem 2012, 13, 373–384. [Google Scholar]
- Bonilla, E.; Valero-Fuenmayor, N.; Pons, H.; Chacin-Bonilla, L. Melatonin protects mice infected with venezuelan equine encephalomyelitis virus. Cell. Mol. Life Sci 1997, 53, 430–434. [Google Scholar]
- Bonilla, E.; Rodon, C.; Valero, N.; Pons, H.; Chacin-Bonilla, L.; Garcia Tamayo, J.; Rodriguez, Z.; Medina-Leendertz, S.; Anez, F. Melatonin prolongs survival of immunodepressed mice infected with the venezuelan equine encephalomyelitis virus. Trans. R. Soc. Trop Med. Hyg 2001, 95, 207–210. [Google Scholar]
- Valero, N.; Bonilla, E.; Pons, H.; Chacin-Bonilla, L.; Anez, F.; Espina, L.M.; Medina-Leendertz, S.; Garcia Tamayo, J. Melatonin induces changes to serum cytokines in mice infected with the venezuelan equine encephalomyelitis virus. Trans. R. Soc. Trop Med. Hyg 2002, 96, 348–351. [Google Scholar]
- Bonilla, E.; Valero, N.; Chacin-Bonilla, L.; Pons, H.; Larreal, Y.; Medina-Leendertz, S.; Espina, L.M. Melatonin increases interleukin-1beta and decreases tumor necrosis factor alpha in the brain of mice infected with the venezuelan equine encephalomyelitis virus. Neurochem. Res 2003, 28, 681–686. [Google Scholar]
- Valero, N.; MarinaEspina, L.; Bonilla, E.; Mosquera, J. Melatonin decreases nitric oxide production and lipid peroxidation and increases interleukin-1 beta in the brain of mice infected by the venezuelan equine encephalomyelitis virus. J. Pineal Res 2007, 42, 107–112. [Google Scholar]
- Maestroni, G.J.; Conti, A.; Pierpaoli, W. Role of the pineal gland in immunity. III. Melatonin antagonizes the immunosuppressive effect of acute stress via an opiatergic mechanism. Immunology 1988, 63, 465–469. [Google Scholar]
- Ellis, L.C. Melatonin reduces mortality from aleutian disease in mink (mustela vison). J. Pineal. Res 1996, 21, 214–217. [Google Scholar]
- Ben-Nathan, D.; Maestroni, G.J.; Lustig, S.; Conti, A. Protective effects of melatonin in mice infected with encephalitis viruses. Arch. Virol 1995, 140, 223–230. [Google Scholar]
- Zhang, Z.; Araghi-Niknam, M.; Liang, B.; Inserra, P.; Ardestani, S.K.; Jiang, S.; Chow, S.; Watson, R.R. Prevention of immune dysfunction and vitamin e loss by dehydroepiandrosterone and melatonin supplementation during murine retrovirus infection. Immunology 1999, 96, 291–297. [Google Scholar]
- Nunnari, G.; Nigro, L.; Palermo, F.; Leto, D.; Pomerantz, R.J.; Cacopardo, B. Reduction of serum melatonin levels in hiv-1-infected individuals’ parallel disease progression: Correlation with serum interleukin-12 levels. Infection 2003, 31, 379–382. [Google Scholar]
- Wiid, I.; Hoal-van Helden, E.; Hon, D.; Lombard, C.; van Helden, P. Potentiation of isoniazid activity against mycobacterium tuberculosis by melatonin. Antimicrob. Agents Chemother 1999, 43, 975–977. [Google Scholar]
- Ozkan, E.; Yaman, H.; Cakir, E.; Deniz, O.; Oztosun, M.; Gumus, S.; Akgul, E.O.; Agilli, M.; Cayci, T.; Kurt, Y.G.; et al. Plasma melatonin and urinary 6-hydroxymelatonin levels in patients with pulmonary tuberculosis. Inflammation 2012, 35, 1429–1434. [Google Scholar]
- Thorpe, L.E.; Frieden, T.R.; Laserson, K.F.; Wells, C.; Khatri, G.R. Seasonality of tuberculosis in india: Is it real and what does it tell us? Lancet 2004, 364, 1613–1614. [Google Scholar]
- Nagayama, N.; Ohmori, M. Seasonality in various forms of tuberculosis. Int. J. Tuberc. Lung. Dis 2006, 10, 1117–1122. [Google Scholar]
- Singh, S.S.; Haldar, C. Peripheral melatonin modulates seasonal immunity and reproduction of indian tropical male bird perdicula asiatica. Comp. Biochem. Physiol. A Mol. Integr. Physiol 2007, 146, 446–450. [Google Scholar]
- Pantoja, L.G.; Miller, R.D.; Ramirez, J.A.; Molestina, R.E.; Summersgill, J.T. Inhibition of chlamydia pneumoniae replication in human aortic smooth muscle cells by gamma interferon-induced indoleamine 2,3-dioxygenase activity. Infect. Immun 2000, 68, 6478–6481. [Google Scholar]
- Rahman, M.A.; Azuma, Y.; Fukunaga, H.; Murakami, T.; Sugi, K.; Fukushi, H.; Miura, K.; Suzuki, H.; Shirai, M. Serotonin and melatonin, neurohormones for homeostasis, as novel inhibitors of infections by the intracellular parasite chlamydia. J. Antimicrob. Chemother 2005, 56, 861–868. [Google Scholar]
- Tekbas, O.F.; Ogur, R.; Korkmaz, A.; Kilic, A.; Reiter, R.J. Melatonin as an antibiotic: New insights into the actions of this ubiquitous molecule. J. Pineal Res 2008, 44, 222–226. [Google Scholar]
- Annane, D.; Bellissant, E.; Cavaillon, J.M. Septic shock. Lancet 2005, 365, 63–78. [Google Scholar]
- Escames, G.; Acuna-Castroviejo, D.; Lopez, L.C.; Tan, D.X.; Maldonado, M.D.; Sanchez-Hidalgo, M.; Leon, J.; Reiter, R.J. Pharmacological utility of melatonin in the treatment of septic shock: Experimental and clinical evidence. J. Pharm. Pharmacol 2006, 58, 1153–1165. [Google Scholar]
- Maestroni, G.J.M. Melatonin as a therapeutic agent in experimental endotoxic shock. J. Pineal Res 1996, 20, 84–89. [Google Scholar]
- Zhang, H.; Liu, D.; Wang, X.; Chen, X.; Long, Y.; Chai, W.; Zhou, X.; Rui, X.; Zhang, Q.; Wang, H. Melatonin improved rat cardiac mitochondria and survival rate in septic heart injury. J. Pineal Res. 2013. [Google Scholar] [CrossRef]
- Nava, F.; Calapai, G.; Facciola, G.; Cuzzocrea, S.; Giuliani, G.; DeSarro, A.; Caputi, A.P. Melatonin effects on inhibition of thirst and fever induced by lipopolysaccharide in rat. Eur. J. Pharmacol 1997, 331, 267–274. [Google Scholar]
- Reynolds, F.D.; Dauchy, R.; Blask, D.; Dietz, P.A.; Lynch, D.; Zuckerman, R. The pineal gland hormone melatonin improves survival in a rat model of sepsis/shock induced by zymosan A. Surgery 2003, 134, 474–479. [Google Scholar]
- Lowes, D.A.; Webster, N.R.; Murphy, M.P.; Galley, H.F. Antioxidants that protect mitochondria reduce interleukin-6 and oxidative stress, improve mitochondrial function, and reduce biochemical markers of organ dysfunction in a rat model of acute sepsis. Br. J. Anaesth 2013, 110, 472–480. [Google Scholar]
- Prendergast, B.J.; Hotchkiss, A.K.; Bilbo, S.D.; Kinsey, S.G.; Nelson, R.J. Photoperiodic adjustments in immune function protect siberian hamsters from lethal endotoxemia. J. Biol. Rhythm 2003, 18, 51–62. [Google Scholar]
- Erbaş, O.; Ergenoglu, A.M.; Akdemir, A.; Yeniel, A.Ö.; Taskiran, D. Comparison of melatonin and oxytocin in the prevention of critical illness polyneuropathy in rats with experimentally induced sepsis. J. Surg. Res. 2012. [Google Scholar] [CrossRef]
- Baykal, A.; Iskit, A.B.; Hamaloglu, E.; Guc, M.O.; Hascelik, G.; Sayek, I. Melatonin modulates mesenteric blood flow and tnf alpha concentrations after lipopolysaccharide challenge. Eur. J. Surg 2000, 166, 722–727. [Google Scholar]
- Shang, Y.; Xu, S.P.; Wu, Y.; Jiang, Y.X.; Wu, Z.Y.; Yuan, S.Y.; Yao, S.L. Melatonin reduces acute lung injury in endotoxemic rats. Chin. Med. J 2009, 122, 1388–1393. [Google Scholar]
- Sewerynek, E.; Melchiorri, D.; Reiter, R.J.; Ortiz, G.G.; Lewinski, A. Lipopolysaccharide-induced hepatotoxicity is inhibited by the antioxidant melatonin. Eur. J. Pharmacol. Environ. Toxicol. Pharmacol. Sect 1995, 293, 327–334. [Google Scholar]
- Wu, J.-Y.; Tsou, M.-Y.; Chen, T.-H.; Chen, S.-J.; Tsao, C.-M.; Wu, C.-C. Therapeutic effects of melatonin on peritonitis-induced septic shock with multiple organ dysfunction syndrome in rats. J. Pineal Res 2008, 45, 106–116. [Google Scholar]
- Crespo, E.; Macias, M.; Pozo, D.; Escames, G.; Martin, M.; Vives, F.; Guerrero, J.M.; Acuna-Castroviejo, D. Melatonin inhibits expression of the inducible no synthase II in liver and lung and prevents endotoxemia in lipopolysaccharide-induced multiple organ dysfunction syndrome in rats. FASEB J 1999, 13, 1537–1546. [Google Scholar]
- Wu, C.C.; Chiao, C.W.; Hsiao, G.; Chen, A.; Yen, M.H. Melatonin prevents endotoxin-induced circulatory failure in rats. J. Pineal Res 2001, 30, 147–156. [Google Scholar]
- Escames, G.; Lopez, L.C.; Ortiz, F.; Lopez, A.; Garcia, J.A.; Ros, E.; Acuna-Castroviejo, D. Attenuation of cardiac mitochondrial dysfunction by melatonin in septic mice. FEBS J 2007, 274, 2135–2147. [Google Scholar]
- De Filippis, D.; Iuvone, T.; Esposito, G.; Steardo, L.; Herman, A.G.; Pelckmans, P.A.; de Man, J.G.; de Winter, B.Y. Melatonin reverses lipopolysaccharide-induced gastro-intestinal motility disturbances through the inhibition of oxidative stress. J. Pineal Res 2008, 44, 45–51. [Google Scholar]
- D’Emmanuele di Villa Bianca, R.; Marzocco, S.; di Paola, R.; Autore, G.; Pinto, A.; Cuzzocrea, S.; Sorrentino, R. Melatonin prevents lipopolysaccharide-induced hyporeactivity in rat. J. Pineal Res 2004, 36, 146–154. [Google Scholar]
- Mundigler, G.; Delle-Karth, G.; Koreny, M.; Zehetgruber, M.; Steindl-Munda, P.; Marktl, W.; Ferti, L.; Siostrzonek, P. Impaired circadian rhythm of melatonin secretion in sedated critically ill patients with severe sepsis. Crit Care Med 2002, 30, 536–540. [Google Scholar]
- Verceles, A.C.; Silhan, L.; Terrin, M.; Netzer, G.; Shanholtz, C.; Scharf, S.M. Circadian rhythm disruption in severe sepsis: The effect of ambient light on urinary 6-sulfatoxymelatonin secretion. Intensive Care Med 2012, 38, 804–810. [Google Scholar]
- Perras, B.; Kurowski, V.; Dodt, C. Nocturnal melatonin concentration is correlated with illness severity in patients with septic disease. Intensive Care Med 2006, 32, 624–625. [Google Scholar]
- Bagci, S.; Horoz, Ö.; Yildizdas, D.; Reinsberg, J.; Bartmann, P.; Müller, A. Melatonin status in pediatric intensive care patients with sepsis. Pediatr. Crit Care Med 2012, 13, 120–123. [Google Scholar]
- Bagci, S.; Yildizdas, D.; Horoz, O.O.; Reinsberg, J.; Bartmann, P.; Mueller, A. Use of nocturnal melatonin concentration and urinary 6-sulfatoxymelatonin excretion to evaluate melatonin status in children with severe sepsis. J. Pediatr. Endocrinol. Metab 2011, 24, 1025–1030. [Google Scholar]
- Gitto, E.; Karbownik, M.; Reiter, R.J.; Tan, D.X.; Cuzzocrea, S.; Chiurazzi, P.; Cordaro, S.; Corona, G.; Trimarchi, G.; Barberi, I. Effects of melatonin treatment in septic newborns. Pediatr. Res 2001, 50, 756–760. [Google Scholar]
- Abrahamsohn, I.A. Cytokines in innate and acquired immunity to trypanosoma cruzi infection. Braz. J. Med. Biol. Res 1998, 31, 117–121. [Google Scholar]
- Oliveira, L.G.; Kuehn, C.C.; Santos, C.D.; Toldo, M.P.; do Prado, J.C., Jr. Enhanced protection by melatonin and meloxicam combination in experimental infection by trypanosoma cruzi. Parasite Immunol 2010, 32, 245–251. [Google Scholar]
- Santos, C.D.; Toldo, M.P.; Santello, F.H.; Filipin Mdel, V.; Brazao, V.; do Prado Junior, J.C. Dehydroepiandrosterone increases resistance to experimental infection by trypanosoma cruzi. Vet. Parasitol 2008, 153, 238–243. [Google Scholar]
- Santello, F.H.; Frare, E.O.; dos Santos, C.D.; Toldo, M.P.; Kawasse, L.M.; Zucoloto, S.; do Prado, J.C., Jr. Melatonin treatment reduces the severity of experimental trypanosoma cruzi infection. J. Pineal Res 2007, 42, 359–363. [Google Scholar]
- Brazao, V.; del Vecchio Filipin, M.; Santello, F.H.; Caetano, L.C.; Abrahao, A.A.; Toldo, M.P.; do Prado, J.C., Jr. Melatonin and zinc treatment: Distinctive modulation of cytokine production in chronic experimental trypanosoma cruzi infection. Cytokine 2011, 56, 627–632. [Google Scholar]
- Alves, E.; Bartlett, P.J.; Garcia, C.R.; Thomas, A.P. Melatonin and ip3-induced Ca2+ release from intracellular stores in the malaria parasite plasmodium falciparum within infected red blood cells. J. Biol. Chem 2011, 286, 5905–5912. [Google Scholar]
- Mozzanica, N.; Tadini, G.; Radaelli, A.; Negri, M.; Pigatto, P.; Morelli, M.; Frigerio, U.; Finzi, A.; Esposti, G.; Rossi, D.; et al. Plasma melatonin levels in psoriasis. Acta Dermato-venereol 1988, 68, 312–316. [Google Scholar]
- Lopez-Gonzalez, M.A.; Guerrero, J.M.; Sanchez, B.; Delgado, F. Melatonin induces hyporeactivity caused by type II collagen in peripheral blood lymphocytes from patients with autoimmune hearing losses. Neurosci. Lett 1997, 239, 1–4. [Google Scholar]
- McInnes, I.B.; Schett, G. The pathogenesis of rheumatoid arthritis. N. Engl. J. Med 2011, 365, 2205–2219. [Google Scholar]
- Hansson, I.; Holmdahl, R.; Mattsson, R. Constant darkness enhances autoimmunity to type II collagen and exaggerates development of collagen-induced arthritis in dba/1 mice. J. Neuroimmunol 1990, 27, 79–84. [Google Scholar]
- Hansson, I.; Holmdahl, R.; Mattsson, R. Pinealectomy ameliorates collagen II-induced arthritis in mice. Clin. Exp. Immunol 1993, 92, 432–436. [Google Scholar]
- Hansson, I.; Holmdahl, R.; Mattsson, R. The pineal hormone melatonin exaggerates development of collagen-induced arthritis in mice. J. Neuroimmunol 1992, 39, 23–30. [Google Scholar]
- Jimenez-Caliani, A.J.; Jimenez-Jorge, S.; Molinero, P.; Guerrero, J.M.; Fernandez-Santos, J.M.; Martin-Lacave, I.; Osuna, C. Dual effect of melatonin as proinflammatory and antioxidant in collagen-induced arthritis in rats. J. Pineal Res 2005, 38, 93–99. [Google Scholar]
- Chen, Q.; Wei, W. Effects and mechanisms of melatonin on inflammatory and immune responses of adjuvant arthritis rat. Int. Immunopharmacol 2002, 2, 1443–1449. [Google Scholar]
- Cutolo, M.; Masi, A.T. Circadian rhythms and arthritis. Rheum Dis. Clin. North. Am 2005, 31, 115–129. [Google Scholar]
- Arkema, E.V.; Hart, J.E.; Bertrand, K.A.; Laden, F.; Grodstein, F.; Rosner, B.A.; Karlson, E.W.; Costenbader, K.H. Exposure to ultraviolet-b and risk of developing rheumatoid arthritis among women in the nurses’ health study. Ann. Rheum Dis 2013, 72, 506–511. [Google Scholar]
- Brainard, G.C.; Podolin, P.L.; Leivy, S.W.; Rollag, M.D.; Cole, C.; Barker, F.M. Near-ultraviolet radiation suppresses pineal melatonin content. Endocrinology 1986, 119, 2201–2205. [Google Scholar]
- Petrovsky, N.; Harrison, L.C. The chronobiology of human cytokine production. Int. Rev. Immunol 1998, 16, 635–649. [Google Scholar]
- Cutolo, M.; Maestroni, G.J.; Otsa, K.; Aakre, O.; Villaggio, B.; Capellino, S.; Montagna, P.; Fazzuoli, L.; Veldi, T.; Peets, T.; et al. Circadian melatonin and cortisol levels in rheumatoid arthritis patients in winter time: A north and south europe comparison. Ann. Rheum Dis 2005, 64, 212–216. [Google Scholar]
- Sulli, A.; Maestroni, G.J.; Villaggio, B.; Hertens, E.; Craviotto, C.; Pizzorni, C.; Briata, M.; Seriolo, B.; Cutolo, M. Melatonin serum levels in rheumatoid arthritis. Ann. N. Y. Acad. Sci 2002, 966, 276–283. [Google Scholar]
- El-Awady, H.M.; El-Wakkad, A.S.; Saleh, M.T.; Muhammad, S.I.; Ghaniema, E.M. Serum melatonin in juvenile rheumatoid arthritis: Correlation with disease activity. Pak. J. Biol. Sci 2007, 10, 1471–1476. [Google Scholar]
- West, S.K.; Oosthuizen, J.M. Melatonin levels are decreased in rheumatoid arthritis. J. Basic Clin. Physiol. Pharmacol 1992, 3, 33–40. [Google Scholar]
- Cano, P.; Cardinali, D.P.; Chacon, F.; Reyes Toso, C.F.; Esquifino, A.I. Nighttime changes in norepinephrine and melatonin content and serotonin turnover in pineal glands of young and old rats injected with freund’s adjuvant. Neuro Endocrinol. Lett 2002, 23, 49–53. [Google Scholar]
- Maestroni, G.J.; Sulli, A.; Pizzorni, C.; Villaggio, B.; Cutolo, M. Melatonin in rheumatoid arthritis: Synovial macrophages show melatonin receptors. Ann. N. Y. Acad. Sci 2002, 966, 271–275. [Google Scholar]
- Cutolo, M.; Villaggio, B.; Candido, F.; Valenti, S.; Giusti, M.; Felli, L.; Sulli, A.; Accardo, S. Melatonin influences interleukin-12 and nitric oxide production by primary cultures of rheumatoid synovial macrophages and thp-1 cells. Ann. N. Y. Acad. Sci 1999, 876, 246–254. [Google Scholar]
- Kouri, V.P.; Olkkonen, J.; Kaivosoja, E.; Ainola, M.; Juhila, J.; Hovatta, I.; Konttinen, Y.T.; Mandelin, J. Circadian timekeeping is disturbed in rheumatoid arthritis at molecular level. PLoS One 2013, 8, e54049. [Google Scholar]
- Nah, S.S.; Won, H.J.; Park, H.J.; Ha, E.; Chung, J.H.; Cho, H.Y.; Baik, H.H. Melatonin inhibits human fibroblast-like synoviocyte proliferation via extracellular signal-regulated protein kinase/p21(cip1)/p27(kip1) pathways. J. Pineal Res 2009, 47, 70–74. [Google Scholar]
- Forrest, C.M.; Mackay, G.M.; Stoy, N.; Stone, T.W.; Darlington, L.G. Inflammatory status and kynurenine metabolism in rheumatoid arthritis treated with melatonin. Br. J. Clin. Pharmacol 2007, 64, 517–526. [Google Scholar]
- Lassmann, H.; van Horssen, J. The molecular basis of neurodegeneration in multiple sclerosis. FEBS Lett 2011, 585, 3715–3723. [Google Scholar]
- Pugliatti, M.; Sotgiu, S.; Rosati, G. The worldwide prevalence of multiple sclerosis. Clin. Neurol. Neurosurg 2002, 104, 182–191. [Google Scholar]
- Kurtzke, J.F. Geography in multiple sclerosis. J. Neurol 1977, 215, 1–26. [Google Scholar]
- Islam, T.; Gauderman, W.J.; Cozen, W.; Mack, T.M. Childhood sun exposure influences risk of multiple sclerosis in monozygotic twins. Neurology 2007, 69, 381–388. [Google Scholar]
- Van der Mei, I.A.; Ponsonby, A.L.; Dwyer, T.; Blizzard, L.; Simmons, R.; Taylor, B.V.; Butzkueven, H.; Kilpatrick, T. Past exposure to sun, skin phenotype, and risk of multiple sclerosis: Case-control study. BMJ 2003, 327, 316. [Google Scholar]
- Kurtzke, J.F. On the fine structure of the distribution of multiple sclerosis. Acta Neurol. Scand 1967, 43, 257–282. [Google Scholar]
- Hedstrom, A.K.; Akerstedt, T.; Hillert, J.; Olsson, T.; Alfredsson, L. Shift work at young age is associated with increased risk for multiple sclerosis. Ann. Neurol 2011, 70, 733–741. [Google Scholar]
- Sandyk, R.; Awerbuch, G.I. Nocturnal plasma melatonin and alpha-melanocyte stimulating hormone levels during exacerbation of multiple sclerosis. Int. J. Neurosci 1992, 67, 173–186. [Google Scholar]
- Melamud, L.; Golan, D.; Luboshitzky, R.; Lavi, I.; Miller, A. Melatonin dysregulation, sleep disturbances and fatigue in multiple sclerosis. J. Neurol. Sci 2012, 314, 37–40. [Google Scholar]
- Akpinar, Z.; Tokgoz, S.; Gokbel, H.; Okudan, N.; Uguz, F.; Yilmaz, G. The association of nocturnal serum melatonin levels with major depression in patients with acute multiple sclerosis. Psychiatry Res 2008, 161, 253–257. [Google Scholar]
- Sandyk, R. Diurnal variations in vision and relations to circadian melatonin secretion in multiple sclerosis. Int J. Neurosci 1995, 83, 1–6. [Google Scholar]
- Constantinescu, C.S.; Hilliard, B.; Ventura, E.; Rostami, A. Luzindole, a melatonin receptor antagonist, suppresses experimental autoimmune encephalomyelitis. Pathobiology 1997, 65, 190–194. [Google Scholar]
- Sandyk, R. Influence of the pineal gland on the expression of experimental allergic encephalomyelitis: Possible relationship to the aquisition of multiple sclerosis. Int. J. Neurosci 1997, 90, 129–133. [Google Scholar]
- Kang, J.C.; Ahn, M.; Kim, Y.S.; Moon, C.; Lee, Y.; Wie, M.B.; Lee, Y.J.; Shin, T. Melatonin ameliorates autoimmune encephalomyelitis through suppression of intercellular adhesion molecule-1. J. Vet. Sci 2001, 2, 85–89. [Google Scholar]
- Tsokos, G.C. Systemic lupus erythematosus. N. Engl. J. Med 2011, 365, 2110–2121. [Google Scholar]
- Lechner, O.; Dietrich, H.; Oliveira dos Santos, A.; Wiegers, G.J.; Schwarz, S.; Harbutz, M.; Herold, M.; Wick, G. Altered circadian rhythms of the stress hormone and melatonin response in lupus-prone mrl/mp-fas(ipr) mice. J. Autoimmun 2000, 14, 325–333. [Google Scholar]
- Haga, H.J.; Brun, J.G.; Rekvig, O.P.; Wetterberg, L. Seasonal variations in activity of systemic lupus erythematosus in a subarctic region. Lupus 1999, 8, 269–273. [Google Scholar]
- Lenz, S.P.; Izui, S.; Benediktsson, H.; Hart, D.A. Lithium chloride enhances survival of nzb/w lupus mice: Influence of melatonin and timing of treatment. Int. J. Immunopharmacol 1995, 17, 581–592. [Google Scholar]
- Jimenez-Caliani, A.J.; Jimenez-Jorge, S.; Molinero, P.; Fernandez-Santos, J.M.; Martin-Lacave, I.; Rubio, A.; Guerrero, J.M.; Osuna, C. Sex-dependent effect of melatonin on systemic erythematosus lupus developed in mrl/mpj-faslpr mice: It ameliorates the disease course in females, whereas it exacerbates it in males. Endocrinology 2006, 147, 1717–1724. [Google Scholar]
- Zhou, L.L.; Wei, W.; Si, J.F.; Yuan, D.P. Regulatory effect of melatonin on cytokine disturbances in the pristane-induced lupus mice. Mediators Inflamm 2010, 2010, 951210, :1–951210:7.. [Google Scholar]
- Daneman, D. Type 1 diabetes. Lancet 2006, 367, 847–858. [Google Scholar]
- Ponsonby, A.L.; Lucas, R.M.; van der Mei, I.A. Uvr, vitamin d and three autoimmune diseases—Multiple sclerosis, type 1 diabetes, rheumatoid arthritis. Photochem. Photobiol 2005, 81, 1267–1275. [Google Scholar]
- Peschke, E.; Hofmann, K.; Bahr, I.; Streck, S.; Albrecht, E.; Wedekind, D.; Muhlbauer, E. The insulin-melatonin antagonism: Studies in the lew.1ar1-iddm rat (an animal model of human type 1 diabetes mellitus). Diabetologia 2011, 54, 1831–1840. [Google Scholar]
- Conti, A.; Maestroni, G.J. Role of the pineal gland and melatonin in the development of autoimmune diabetes in non-obese diabetic mice. J. Pineal Res 1996, 20, 164–172. [Google Scholar]
- Lin, G.J.; Huang, S.H.; Chen, Y.W.; Hueng, D.Y.; Chien, M.W.; Chia, W.T.; Chang, D.M.; Sytwu, H.K. Melatonin prolongs islet graft survival in diabetic nod mice. J. Pineal Res 2009, 47, 284–292. [Google Scholar]
- Reyes-Toso, C.F.; Roson, M.I.; Albornoz, L.E.; Damiano, P.F.; Linares, L.M.; Cardinali, D.P. Vascular reactivity in diabetic rats: Effect of melatonin. J. Pineal Res 2002, 33, 81–86. [Google Scholar]
- Cavallo, A.; Daniels, S.R.; Dolan, L.M.; Bean, J.A.; Khoury, J.C. Blood pressure-lowering effect of melatonin in type 1 diabetes. J. Pineal Res 2004, 36, 262–266. [Google Scholar]
- Motilva, V.; Garcia-Maurino, S.; Talero, E.; Illanes, M. New paradigms in chronic intestinal inflammation and colon cancer: Role of melatonin. J. Pineal Res 2011, 51, 44–60. [Google Scholar]
- Chen, C.Q.; Fichna, J.; Bashashati, M.; Li, Y.Y.; Storr, M. Distribution, function and physiological role of melatonin in the lower gut. World J. Gastroenterol 2011, 17, 3888–3898. [Google Scholar]
- Thor, P.J.; Krolczyk, G.; Gil, K.; Zurowski, D.; Nowak, L. Melatonin and serotonin effects on gastrointestinal motility. J. Physiol. Pharmacol 2007, 58, 97–103. [Google Scholar]
- Song, G.H.; Leng, P.H.; Gwee, K.A.; Moochhala, S.M.; Ho, K.Y. Melatonin improves abdominal pain in irritable bowel syndrome patients who have sleep disturbances: A randomised, double blind, placebo controlled study. Gut 2005, 54, 1402–1407. [Google Scholar]
- Lu, W.Z.; Gwee, K.A.; Moochhalla, S.; Ho, K.Y. Melatonin improves bowel symptoms in female patients with irritable bowel syndrome: A double-blind placebo-controlled study. Aliment. Pharmacol. Ther 2005, 22, 927–934. [Google Scholar]
- Saha, L.; Malhotra, S.; Rana, S.; Bhasin, D.; Pandhi, P. A preliminary study of melatonin in irritable bowel syndrome. J. Clin. Gastroenterol 2007, 41, 29–32. [Google Scholar]
- Preuss, F.; Tang, Y.; Laposky, A.D.; Arble, D.; Keshavarzian, A.; Turek, F.W. Adverse effects of chronic circadian desynchronization in animals in a “challenging” environment. Am. J. Physiol. Regul. Integr. Comp. Physiol 2008, 295, R2034–R2040. [Google Scholar]
- Tang, Y.; Preuss, F.; Turek, F.W.; Jakate, S.; Keshavarzian, A. Sleep deprivation worsens inflammation and delays recovery in a mouse model of colitis. Sleep Med 2009, 10, 597–603. [Google Scholar]
- Mickle, A.; Sood, M.; Zhang, Z.; Shahmohammadi, G.; Sengupta, J.N.; Miranda, A. Antinociceptive effects of melatonin in a rat model of post-inflammatory visceral hyperalgesia: A centrally mediated process. Pain 2010, 149, 555–564. [Google Scholar]
- Pentney, P.T.; Bubenik, G.A. Melatonin reduces the severity of dextran-induced colitis in mice. J. Pineal Res 1995, 19, 31–39. [Google Scholar]
- Cuzzocrea, S.; Mazzon, E.; Serraino, I.; Lepore, V.; Terranova, M.L.; Ciccolo, A.; Caputi, A.P. Melatonin reduces dinitrobenzene sulfonic acid-induced colitis. J. Pineal Res 2001, 30, 1–12. [Google Scholar]
- Dong, W.G.; Mei, Q.; Yu, J.P.; Xu, J.M.; Xiang, L.; Xu, Y. Effects of melatonin on the expression of inos and cox-2 in rat models of colitis. World J. Gastroenterol 2003, 9, 1307–1311. [Google Scholar]
- Akcan, A.; Kucuk, C.; Sozuer, E.; Esel, D.; Akyildiz, H.; Akgun, H.; Muhtaroglu, S.; Aritas, Y. Melatonin reduces bacterial translocation and apoptosis in trinitrobenzene sulphonic acid-induced colitis of rats. World J. Gastroenterol 2008, 14, 918–924. [Google Scholar]
- Mann, S. Melatonin for ulcerative colitis? Am. J. Gastroenterol 2003, 98, 232–233. [Google Scholar]
- Maldonado, M.D.; Calvo, J.R. Melatonin usage in ulcerative colitis: A case report. J. Pineal Res 2008, 45, 339–340. [Google Scholar]
- Calvo, J.R.; Guerrero, J.M.; Osuna, C.; Molinero, P.; Carrillo-Vico, A. Melatonin triggers crohn’s disease symptoms. J. Pineal Res 2002, 32, 277–278. [Google Scholar]
- Regodon, S.; Martin-Palomino, P.; Fernandez-Montesinos, R.; Herrera, J.L.; Carrascosa-Salmoral, M.P.; Piriz, S.; Vadillo, S.; Guerrero, J.M.; Pozo, D. The use of melatonin as a vaccine agent. Vaccine 2005, 23, 5321–5327. [Google Scholar]
- Katz, M.E.; Howarth, P.M.; Yong, W.K.; Riffkin, G.G.; Depiazzi, L.J.; Rood, J.I. Identification of three gene regions associated with virulence in dichelobacter nodosus, the causative agent of ovine footrot. J. Gen. Microbiol 1991, 137, 2117–2124. [Google Scholar]
- Regodon, S.; Ramos, A.; Morgado, S.; Tarazona, R.; Martin-Palomino, P.; Rosado, J.A.; Miguez, M.D.P. Melatonin enhances the immune response to vaccination against a1 and c strains of dichelobacter nodosus. Vaccine 2009, 27, 1566–1570. [Google Scholar]
- Regodon, S.; del Prado Miguez, M.; Jardin, I.; Lopez, J.J.; Ramos, A.; Paredes, S.D.; Rosado, J.A. Melatonin, as an adjuvant-like agent, enhances platelet responsiveness. J. Pineal Res 2009, 46, 275–285. [Google Scholar]
- Regodon, S.; Ramos, A.; Miguez, M.P.; Carrillo-Vico, A.; Rosado, J.A.; Jardin, I. Vaccination prepartum enhances the beneficial effects of melatonin on the immune response and reduces platelet responsiveness in sheep. BMC Vet. Res 2012, 8, 84–91. [Google Scholar]
- Connor, T.P. Melatonin as an adjuvant to therapeutic prostate cancer vaccines. J. Pineal Res 2008, 45, 224. [Google Scholar]
- Soliman, M.F.; El Shenawy, N.S.; El Arabi, S.E. Schistosoma mansoni: Melatonin enhances efficacy of cercarial and soluble worm antigens in the induction of protective immunity against infection in the hamster. Exp. Parasitol 2008, 119, 291–295. [Google Scholar]
- Tan, D.X.; Manchester, L.C.; Terron, M.P.; Flores, L.J.; Reiter, R.J. One molecule, many derivatives: A never-ending interaction of melatonin with reactive oxygen and nitrogen species? J. Pineal Res 2007, 42, 28–42. [Google Scholar]
- Jesudason, E.P.; Baben, B.; Ashok, B.S.; Masilamoni, J.G.; Kirubagaran, R.; Jebaraj, W.C.; Jayakumar, R. Anti-inflammatory effect of melatonin on a beta vaccination in mice. Mol. Cell. Biochem 2007, 298, 69–81. [Google Scholar]
- Ramos, A.; Laguna, I.; de Lucia, M.L.; Martin-Palomino, P.; Regodon, S.; Miguez, M.P. Evolution of oxidative/nitrosative stress biomarkers during an open-field vaccination procedure in sheep: Effect of melatonin. Vet. Immunol. Immunopathol 2010, 133, 16–24. [Google Scholar]
- Jung, F.J.; Yang, L.; Harter, L.; Inci, I.; Schneiter, D.; Lardinois, D.; Keel, M.; Weder, W.; Korom, S. Melatonin in vivo prolongs cardiac allograft survival in rats. J. Pineal Res 2004, 37, 36–41. [Google Scholar]
- Inci, I.; Inci, D.; Dutly, A.; Boehler, A.; Weder, W. Melatonin attenuates posttransplant lung ischemia-reperfusion injury. Ann. Thorac Surg 2002, 73, 220–225. [Google Scholar]
- Sapmaz, E.; Ayar, A.; Celik, H.; Sapmaz, T.; Kilic, N.; Yasar, M.A. Effects of melatonin and oxytetracycline in autologous intraperitoneal ovary transplantation in rats. Neuro Endocrinol. Lett 2003, 24, 350–354. [Google Scholar]
- Friedman, O.; Orvieto, R.; Fisch, B.; Felz, C.; Freud, E.; Ben-Haroush, A.; Abir, R. Possible improvements in human ovarian grafting by various host and graft treatments. Hum. Reprod 2012, 27, 474–482. [Google Scholar]
- Fildes, J.E.; Yonan, N.; Keevil, B.G. Melatonin—A pleiotropic molecule involved in pathophysiological processes following organ transplantation. Immunology 2009, 127, 443–449. [Google Scholar]
- Baykara, B.; Tekmen, I.; Pekcetin, C.; Ulukus, C.; Tuncel, P.; Sagol, O.; Ormen, M.; Ozogul, C. The protective effects of carnosine and melatonin in ischemia-reperfusion injury in the rat liver. Acta Histochem 2009, 111, 42–51. [Google Scholar]
- Zaouali, M.A.; Reiter, R.J.; Padrissa-Altes, S.; Boncompagni, E.; Garcia, J.J.; Ben Abnennebi, H.; Freitas, I.; Garcia-Gil, F.A.; Rosello-Catafau, J. Melatonin protects steatotic and nonsteatotic liver grafts against cold ischemia and reperfusion injury. J. Pineal Res 2011, 50, 213–221. [Google Scholar]
- Moussavian, M.R.; Scheuer, C.; Schmidt, M.; Kollmar, O.; Wagner, M.; von Heesen, M.; Schilling, M.K.; Menger, M.D. Multidrug donor preconditioning prevents cold liver preservation and reperfusion injury. Langenbecks Arch. Surg 2011, 396, 231–241. [Google Scholar]
- Von Heesen, M.; Seibert, K.; Hulser, M.; Scheuer, C.; Wagner, M.; Menger, M.D.; Schilling, M.K.; Moussavian, M.R. Multidrug donor preconditioning protects steatotic liver grafts against ischemia-reperfusion injury. Am. J. Surg 2012, 203, 168–176. [Google Scholar]
- Cardell, M.; Jung, F.J.; Zhai, W.; Hillinger, S.; Welp, A.; Manz, B.; Weder, W.; Korom, S. Acute allograft rejection and immunosuppression: Influence on endogenous melatonin secretion. J. Pineal Res 2008, 44, 261–266. [Google Scholar]
- Castle, S.C. Clinical relevance of age-related immune dysfunction. Clin. Infect. Dis 2000, 31, 578–585. [Google Scholar]
- Arlt, W.; Hewison, M. Hormones and immune function: Implications of aging. Aging Cell 2004, 3, 209–216. [Google Scholar]
- Karasek, M. Melatonin, human aging, and age-related diseases. Exp. Gerontol 2004, 39, 1723–1729. [Google Scholar]
- Zeitzer, J.M.; Daniels, J.E.; Duffy, J.F.; Klerman, E.B.; Shanahan, T.L.; Dijk, D.J.; Czeisler, C.A. Do plasma melatonin concentrations decline with age? Am. J. Med 1999, 107, 432–436. [Google Scholar]
- Fourtillan, J.B.; Brisson, A.M.; Fourtillan, M.; Ingrand, I.; Decourt, J.P.; Girault, J. Melatonin secretion occurs at a constant rate in both young and older men and women. Am. J. Physiol. Endocrinol. Metab 2001, 280, E11–E22. [Google Scholar]
- Iguchi, H. Age dependent changes in the serum melatonin concentrations in healthy human subjects and in patients with endocrine and hepatic disorders and renal failure (author’s transl). Fukuoka Igaku Zasshi 1981, 72, 423–430. [Google Scholar]
- Girotti, L.; Lago, M.; Ianovsky, O.; Carbajales, J.; Elizari, M.V.; Brusco, L.I.; Cardinali, D.P. Low urinary 6-sulphatoxymelatonin levels in patients with coronary artery disease. J. Pineal Res 2000, 29, 138–142. [Google Scholar]
- Siegrist, C.; Benedetti, C.; Orlando, A.; Beltran, J.M.; Tuchscherr, L.; Noseda, C.M.; Brusco, L.I.; Cardinali, D.P. Lack of changes in serum prolactin, fsh, tsh, and estradiol after melatonin treatment in doses that improve sleep and reduce benzodiazepine consumption in sleep-disturbed, middle-aged, and elderly patients. J. Pineal Res 2001, 30, 34–42. [Google Scholar]
- Mishima, K.; Okawa, M.; Hozumi, S.; Hishikawa, Y. Supplementary administration of artificial bright light and melatonin as potent treatment for disorganized circadian rest-activity and dysfunctional autonomic and neuroendocrine systems in institutionalized demented elderly persons. Chronobiol. Int 2000, 17, 419–432. [Google Scholar]
- Mishima, K.; Okawa, M.; Shimizu, T.; Hishikawa, Y. Diminished melatonin secretion in the elderly caused by insufficient environmental illumination. J. Clin. Endocrinol. Metab 2001, 86, 129–134. [Google Scholar]
- Luboshitzky, R.; Shen-Orr, Z.; Tzischichinsky, O.; Maldonado, M.; Herer, P.; Lavie, P. Actigraphic sleep-wake patterns and urinary 6-sulfatoxymelatonin excretion in patients with alzheimer’s disease. Chronobiol. Int 2001, 18, 513–524. [Google Scholar]
- Maestroni, G.J. The immunotherapeutic potential of melatonin. Expert Opin. Investig Drugs 2001, 10, 467–476. [Google Scholar]
- Tian, Y.M.; Li, P.P.; Jiang, X.F.; Zhang, G.Y.; Dai, Y.R. Rejuvenation of degenerative thymus by oral melatonin administration and the antagonistic action of melatonin against hydroxyl radical-induced apoptosis of cultured thymocytes in mice. J. Pineal Res 2001, 31, 214–221. [Google Scholar]
- Sainz, R.M.; Mayo, J.C.; Uria, H.; Kotler, M.; Antolin, I.; Rodriguez, C.; Menendez-Pelaez, A. The pineal neurohormone melatonin prevents in vivo and in vitro apoptosis in thymocytes. J. Pineal Res 1995, 19, 178–188. [Google Scholar]
- Espino, J.; Pariente, J.A.; Rodriguez, A.B. Oxidative stress and immunosenescence: Therapeutic effects of melatonin. Oxid. Med. Cell. Longev 2012, 2012, 670294, :1–670294:9.. [Google Scholar]
- Espino, J.; Bejarano, I.; Paredes, S.D.; Barriga, C.; Reiter, R.J.; Pariente, J.A.; Rodriguez, A.B. Melatonin is able to delay endoplasmic reticulum stress-induced apoptosis in leukocytes from elderly humans. Age (Dordr) 2011, 33, 497–507. [Google Scholar]
- Vishwas, D.K.; Mukherjee, A.; Haldar, C.; Dash, D.; Nayak, M.K. Improvement of oxidative stress and immunity by melatonin: An age dependent study in golden hamster. Exp. Gerontol 2013, 48, 168–182. [Google Scholar]
- Caroleo, M.C.; Doria, G.; Nistico, G. Melatonin restores immunodepression in aged and cyclophosphamide-treated mice. Ann. N. Y. Acad. Sci 1994, 719, 343–352. [Google Scholar]
- Cuesta, S.; Kireev, R.; Forman, K.; Garcia, C.; Escames, G.; Ariznavarreta, C.; Vara, E.; Tresguerres, J.A. Melatonin improves inflammation processes in liver of senescence-accelerated prone male mice (samp8). Exp. Gerontol 2010, 45, 950–956. [Google Scholar]
- Sharman, K.G.; Sharman, E.H.; Yang, E.; Bondy, S.C. Dietary melatonin selectively reverses age-related changes in cortical cytokine mrna levels, and their responses to an inflammatory stimulus. Neurobiol. Aging 2002, 23, 633–638. [Google Scholar]
- Malow, B.; Adkins, K.W.; McGrew, S.G.; Wang, L.; Goldman, S.E.; Fawkes, D.; Burnette, C. Melatonin for sleep in children with autism: A controlled trial examining dose, tolerability, and outcomes. J. Autism. Dev. Disord 2012, 42, 1729–1737. [Google Scholar]

| Receptor | Distribution | Effector mechanism | References | |
|---|---|---|---|---|
| Membrane receptors | MT1 | Human PBMCs | Regulation of IL-2 production Regulation of cAMP levels | [46–48] |
| Different subsets of human T lymphocytes, B cells and monocytes | [46] | |||
| Jurkat (human T cells) | Regulation of IL-2 production and IL-2R (CD25) levels Regulation of cAMP levels | [44,48–52] | ||
| U937 (human monocyte cells) | [50] | |||
| Mouse thymus | [53] | |||
| Mouse spleen | [53] | |||
| Mouse peritoneal macrophages | Regulation of cAMP levels | [51] | ||
| Rat thymus | [54] | |||
| Rat spleen | [54] | |||
| Rat B cells | [54] | |||
| Rat CD4+, CD8+ and CD4+ CD8+ thymocytes | [54] | |||
| RBL-2H3 (rat mast cells) | [45] | |||
| Spleen of palm squirrel | Regulation of organ weight Regulation of splenocyte proliferation | [55–59] | ||
| Thymus of palm squirrel | Regulation of thymocyte proliferation Regulation of IL-2 production | [55,57,59] | ||
| PBMCs of palm squirrel | [59] | |||
| BALT of quail | ||||
| MT2 | Jurkat | [44] | ||
| Mouse thymus | [53] | |||
| Mouse splenocytes | Increased proliferation | [60] | ||
| Rat thymus | [61] | |||
| Rat spleen | [61] | |||
| Rat leukocytes | Inhibition of leukocyte rolling | [62] | ||
| RBL-2H3 | [45] | |||
| Chicken spleen | Regulation of splenocyte proliferation Regulation of cAMP and IP3 levels | [41] | ||
| Chicken thymus | [63] | |||
| Chicken lymphocytes | [63] | |||
| BALT of quail | [64] | |||
| Spleen of quail | [65] | |||
| MT1/MT2 | Human PBMCs | Inhibition of TNFα-induced apoptosis Activation of ERK signaling pathway | [66] | |
| Nuclear receptors | RZRα | Human PBMCs, including different subsets of T lymphocytes, B cells and monocytes | [46,67] | |
| Jurkat | [49] | |||
| RPMI 1788 and P16 (human B cells) | Repression of 5-LOX expression | [68] | ||
| HL-60 (promyelocytes) | [68] | |||
| Mono Mac 6 (monocytes) | [68] | |||
| RORα | Human PBMCs | [48] | ||
| Human cytotoxic T lymphocytes [RORα1] | [46] | |||
| Human PBMCs, including different subsets of T lymphocytes, B cells and monocytes [RORα2] | [46] | |||
| Jurkat [RORα1, RORα2, RORα3] | [49,50,69] | |||
| U937 [RORα1, RORα2] | Regulation of IL-6 production | [50] | ||
| RPMI 1788 and P16 [RORα2, RORα3] | [68] | |||
| HL-60 [RORα2, RORα3] | [68] | |||
| Mono Mac 6 [RORα2, RORα3] | [68] | |||
| Mouse thymus and spleen | [53] | |||
| RZR/ROR | Human PBMCs | Regulation of IL-2 and IL-6 production and of IL-2R (CD25) | [51,70,71] | |
| Jurkat | Regulation of IL-2 production | [44,49] | ||
| U937 | Regulation of IL-6 production | [72] |
| Immune condition | Melatonin effects | Melatonin administration | References |
|---|---|---|---|
| Basal | Increases lymphocyte counts and the blastogenic stimulation ratio of spleen and thymus Increases cellularity in thymus and spleen | 25 mg/kg to adult male squirrels for 60 consecutive days during May–June | [77] |
| Basal | Splenic hypertrophy and extramedullary hematopoiesis | 25 mg/kg to adult male Syrian hamsters kept under a long photoperiod | [78] |
| Basal | Increase in cell numbers of macrophages/microglia and upregulation of MHC and CD4 antigens | Multiple daily injections of melatonin in the pineal gland and different regions of 1-day-old rat brains for 7–11 days | [84] |
| Basal | Increases bone marrow NK cells and monocytes | Daily administration through diet (7–14 days) to young adult male mice | [85] |
| Basal | Increases neutrophil chemotactic response to a physiological chemoattractant and the expression of intracellular chemokines | 20 mg daily to human volunteers | [88] |
| Basal | Increases in splenocyte proliferation and IL-2 and IL-1β levels | 500 mg/kg to young mice | [91] |
| Aging-induced immunosuppression | Reverses thymic and splenic involution, total numbers of thymocytes and splenocytes, mitogen responsiveness and NK cell activity | 15 mg/kg in drinking water to 22-month-old female C57BL mice for 60 consecutive days | [79] |
| Aging-induced immunosuppression | Increases humoral response (IgG1 and IgM levels) | Subcutaneous injection of 10 mg/kg for 7 days to 28-month-old male Wistar rats | [106] |
| Aging-induced immunosuppression | Increases total leukocyte and lymphocyte counts, mitogenic response of splenocytes and delayed type hypersensitivity response | Daily subcutaneous administration of 0.25 mg/kg to squirrels | [107] |
| Aging-induced immunosuppression | Increases B cell proliferation and Th1 cytokines and decreases Th2 response | Injection of old (16.5 months) female C57BL/6 mice | [108] |
| Immunosenescence induced by aging plus ovariectomy | Restores impaired chemotaxis, mitogenic response, IL-2 release and NK cell activity | 1 mg/kg in drinking water to Wistar albino female rats | [87] |
| Dexamethasone-induced immunosuppression | Restores decreased thymus and spleen activities, lymphoid tissues mass, total leukocyte counts and bone marrow and T cell-mediated immune function | 25 mg/kg to adult squirrels along with dexamethasone for 60 consecutive days | [80] |
| Dexamethasone-induced immunosuppression | Enhances IL-2 production and thymic and splenic lymphocyte proliferation by membrane receptor-mediated mechanism | Daily subcutaneous administration of 0.25 mg/kg to squirrels during evening hours | [59] |
| Corticosterone-induced immunosuppression | Antagonizes the depression of antibody production | Evening injections to mice | [109] |
| Immunosuppression due to propranolol- and PCPA-induced pineal inactivation | Reverses the suppression of the humoral response and autologous mixed lymphocyte reaction | Evening injections to mice | [109] |
| Immunosuppression by trauma-hemorrhage | Improves reduced IL-1, IL-2 and IL-6 release and splenocyte proliferative capacity | Subcutaneous injection of 10 mg/kg in the evening of the day of surgery (soft-tissue trauma and hemorrhagic shock) and on the following evening in C3H/HeN mice | [92] |
| Lead-induced immunotoxicity | Increases thymus weight and splenic T and B cell and NK, T and B cell functions | 10 or 50 mg/kg orally administered to ICR mice daily for 28 days, 2 h before Pb treatment | [110] |
| Inflammatory condition | Melatonin effects | Molecular mechanism | References | |
|---|---|---|---|---|
| Rat | Exercise-induced cardiac inflammatory injury | Reduces TNF-α, IL-1β and IL-6 production | [111] | |
| Experimental colitis | Counteracts the high production of TNF-α, IL-1 and NO | Downregulation of NF-κB | [112] | |
| Diabetes-associated low-grade inflammation | Lowers TNF-α, IL-6 and CRP | [113] | ||
| Experimental model of traumatic brain injury | Reduces upregulation of IL-6, iNOS, SOCS-3 and oxidative stress | Overcomes STAT-1 inactivation | [114] | |
| Heatstroke-induced multiple organ dysfunction syndrome | Attenuates high production of TNF-α, IL-1β and IL-6 and promotes IL-10 production | [94] | ||
| Ischemia reperfusion-induced liver damage | Attenuates enhanced levels of TNF-α, IL-6 and NO | Suppresses the increase in MyD88, ERK, phosphorylated JNK and c-Jun and nuclear translocation of NF-κB | [115] | |
| Neuroinflammation in experimental diabetic neuropathy | Attenuates elevated levels of TNF-α, IL-6, iNOS, COX-2 and oxidative stress | Decreases NF-κB cascade activation | [116] | |
| FK506-induced nephropathy | Lowers TNF-α, IL-6 and NO levels | [117] | ||
| Acetic acid-induced colitis | Reverses increased levels of TNF-α, IL-1β, IL-6, MPO and oxidative stress | [118] | ||
| Cerulein-induced pancreatitis | Reduces expression of TNF-α, IL-1β, IL-6, IL-8 and iNOS | Inhibition of elevated nuclear binding of NF-κB | [119] | |
| Hemorrhagic shock | Suppresses the release of TNF-α and IL-6 | [120] | ||
| DMN-induced liver injury | Decreases expression of TNF-α, IL-1β, IL-6 and iNOS | Inhibition of increased nuclear binding of NF-κB | [121] | |
| Aging | Attenuates increased levels of TNF-α, IL-1β, IL-6, iNOS and LPO | [122] | ||
| Cecal dissection-induced bacterial peritonitis | Lowers levels of TNF-α, IL-6 and MDA | [123] | ||
| Pancreatic fluid-induced lung inflammation and airway hyperreactivity | Reduces TNF-α and iNOS concentrations | [95] | ||
| TNBS-induced colitis | Decreases high levels of TNF-α, IL-1 and NO | NF-κB inhibition and blockade of IκBα degradation | [112,124] | |
| Neuro-inflammation induced by intra-cerebroventricular administration of LPS | Decreases TNF-α, IL-1β and oxidative stress | [125] | ||
| Experimental periodontitis | Reduces TNF-α, IL-1β and MDA | [126] | ||
| DNBS-induced colon injury | Reduces expression of TNF-α and MMP-9 and MMP-2 activities | Reduction in NF-κB activation and phosphorylation of c-Jun | [127,128] | |
| Taurocholate-induced acute pancreatitis | Reduces TNF-α and amylase levels | [129] | ||
| Escherichia coli-induced pyelonephritis | Reverses increased levels of TNF-α and MDA | [130] | ||
| Spinal cord injury | Decreases expression of TNF-α and MMP-9 and MMP-2 | [127] | ||
| Lung ischemia-reperfusion injury | Diminishes levels of TNF-α | Inhibition of NF-κB protein levels | [131] | |
| Hypoxia-induced retinal ganglion cell death | Reverses the upregulation of TNF-α, IL-1β and LPO | [132] | ||
| Mechlorethamine-induced nephrotoxicity | Ameliorates the increased production of TNF-α and IL-1β | [133] | ||
| Mouse | Immunological liver injury | Attenuates the increases in TNF-α and IL-1β | [134] | |
| Radiation-induced lung injury | Reduces TNF-α, TGF-1 and oxidative stress | [135] | ||
| Maternal LPS-induced inflammation | Attenuates elevation of TNF-α in maternal serum and fetal brain | [136] | ||
| Indomethacin-induced chronic gastric ulcer | Blocks expression of TNF-α, IL-1β and IL-8 | Inhibition of ERK and JNK phosphorylation and NF-κB, c-Fos and c-Jun expression | [137] | |
| Alzheimer’s transgenic mice | Decreases TNF-α levels in hippocampus | [138] | ||
| Human | Infant endotracheal intubation | Lowers IL-6, IL-8, IL-10 and IL-12 | [139] | |
| Strenuous exercise | Prevents overexpression of TNF-α, IL-6, IL-1ra and oxidative stress | [140] | ||
| Duchenne muscular dystrophy | Reduces levels of TNF-α, IL-1β, IL-6, IL-2, IFN-γ and oxidative stress | [141] | ||
| Respiratory distress syndrome | Limits serum rise in TNF-α, IL-6 and IL-8 | [142] |
© 2013 by the authors; licensee MDPI, Basel, Switzerland This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Carrillo-Vico, A.; Lardone, P.J.; Álvarez-Sánchez, N.; Rodríguez-Rodríguez, A.; Guerrero, J.M. Melatonin: Buffering the Immune System. Int. J. Mol. Sci. 2013, 14, 8638-8683. https://doi.org/10.3390/ijms14048638
Carrillo-Vico A, Lardone PJ, Álvarez-Sánchez N, Rodríguez-Rodríguez A, Guerrero JM. Melatonin: Buffering the Immune System. International Journal of Molecular Sciences. 2013; 14(4):8638-8683. https://doi.org/10.3390/ijms14048638
Chicago/Turabian StyleCarrillo-Vico, Antonio, Patricia J. Lardone, Nuria Álvarez-Sánchez, Ana Rodríguez-Rodríguez, and Juan M. Guerrero. 2013. "Melatonin: Buffering the Immune System" International Journal of Molecular Sciences 14, no. 4: 8638-8683. https://doi.org/10.3390/ijms14048638
APA StyleCarrillo-Vico, A., Lardone, P. J., Álvarez-Sánchez, N., Rodríguez-Rodríguez, A., & Guerrero, J. M. (2013). Melatonin: Buffering the Immune System. International Journal of Molecular Sciences, 14(4), 8638-8683. https://doi.org/10.3390/ijms14048638







