Antioxidant Systems from Pepper (Capsicum annuum L.): Involvement in the Response to Temperature Changes in Ripe Fruits
Abstract
:1. Introduction
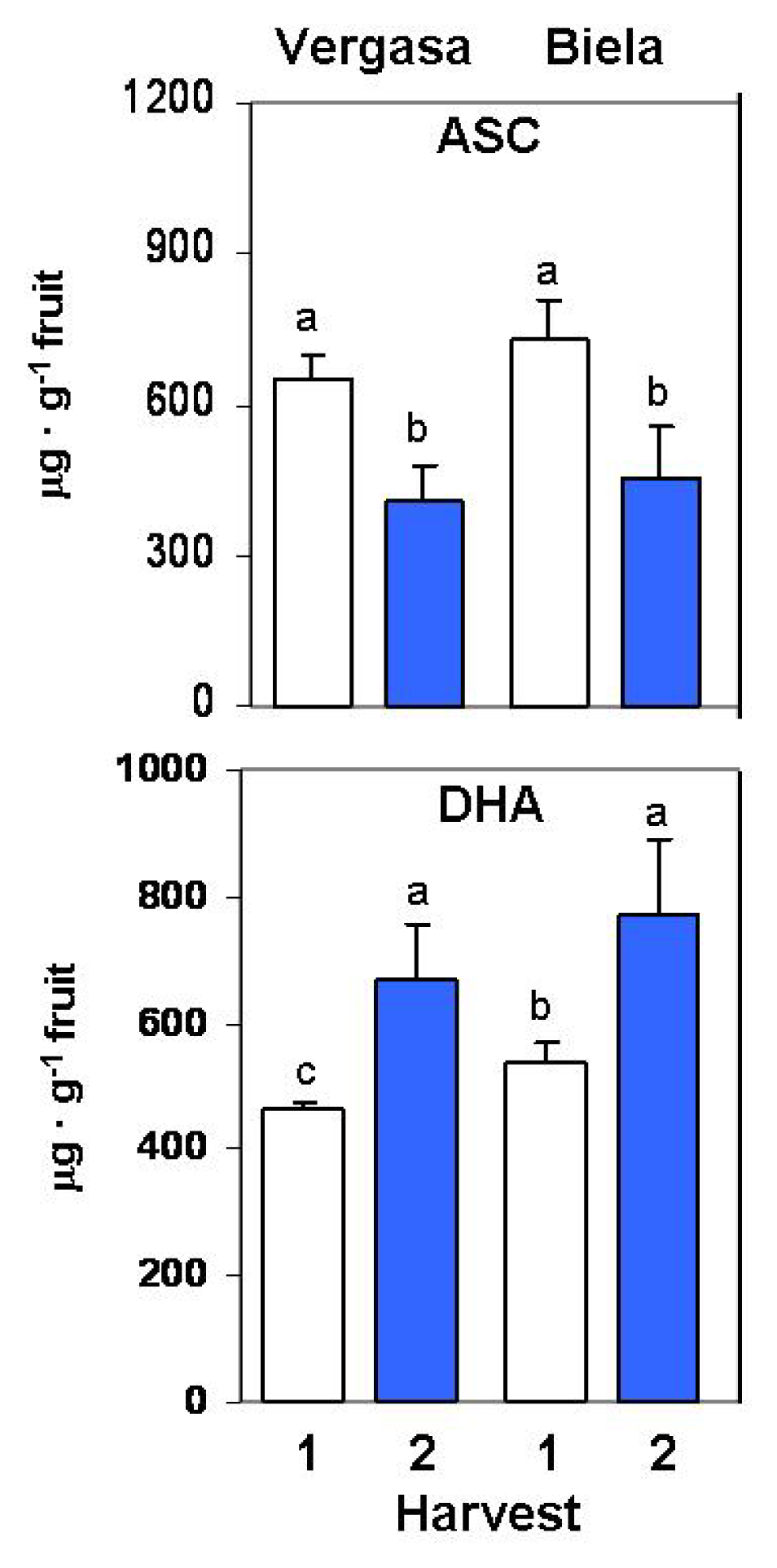
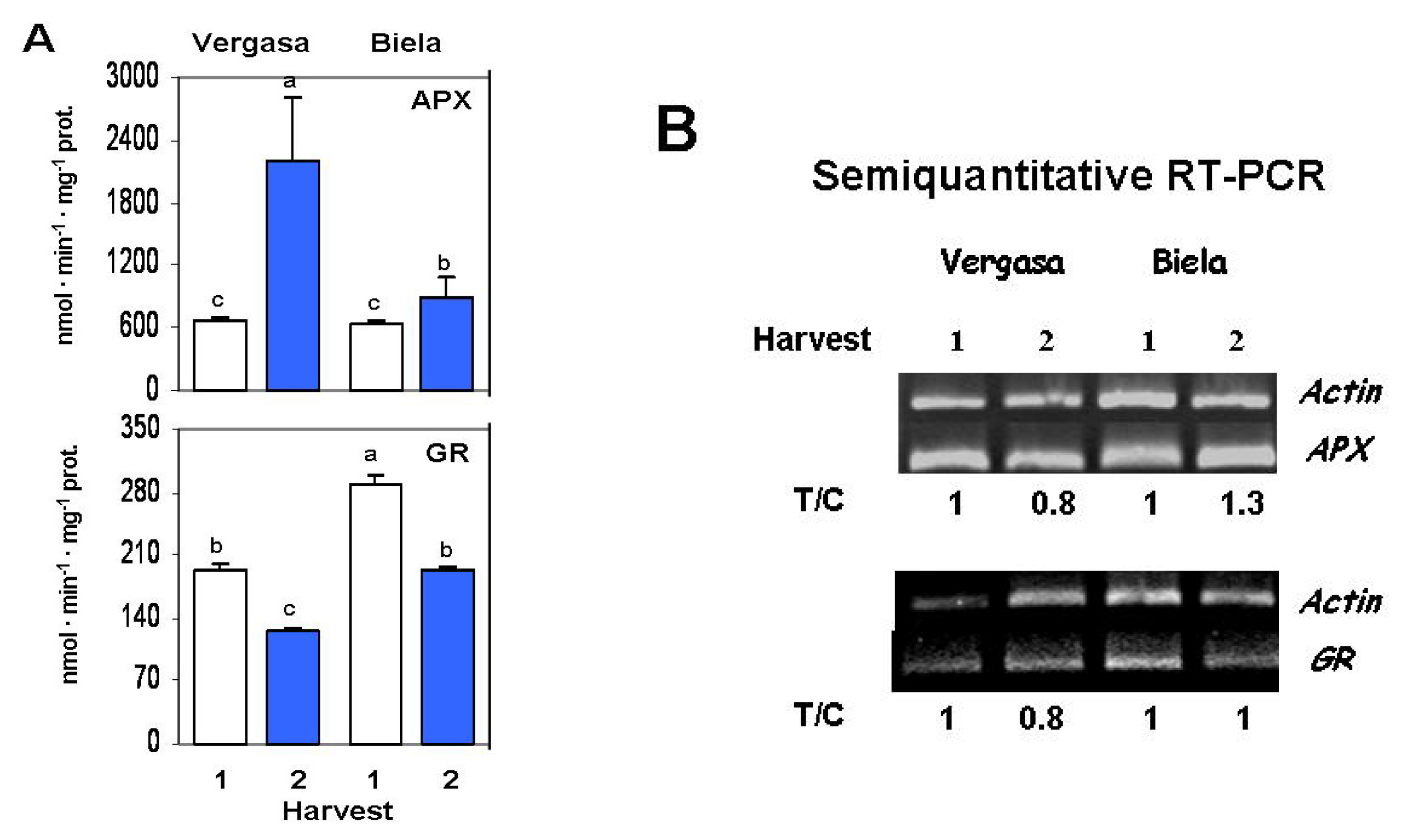
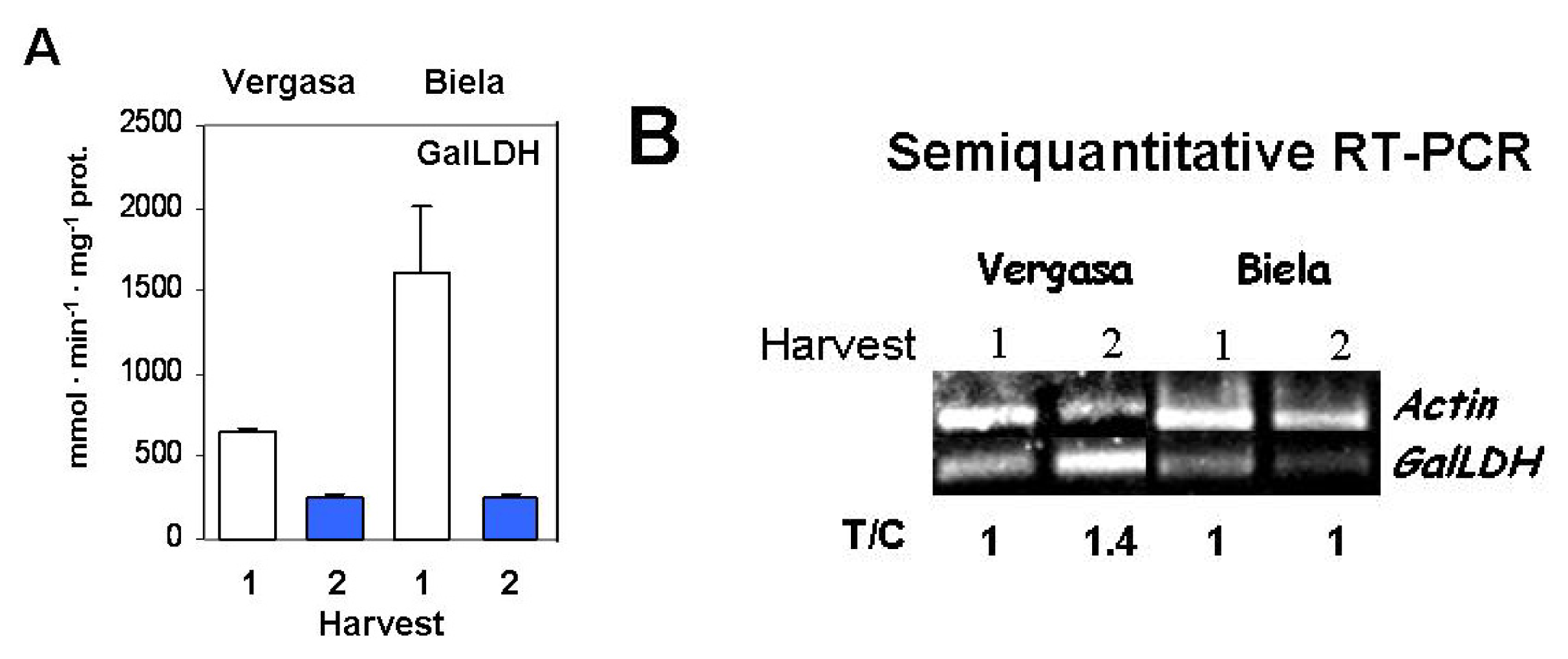
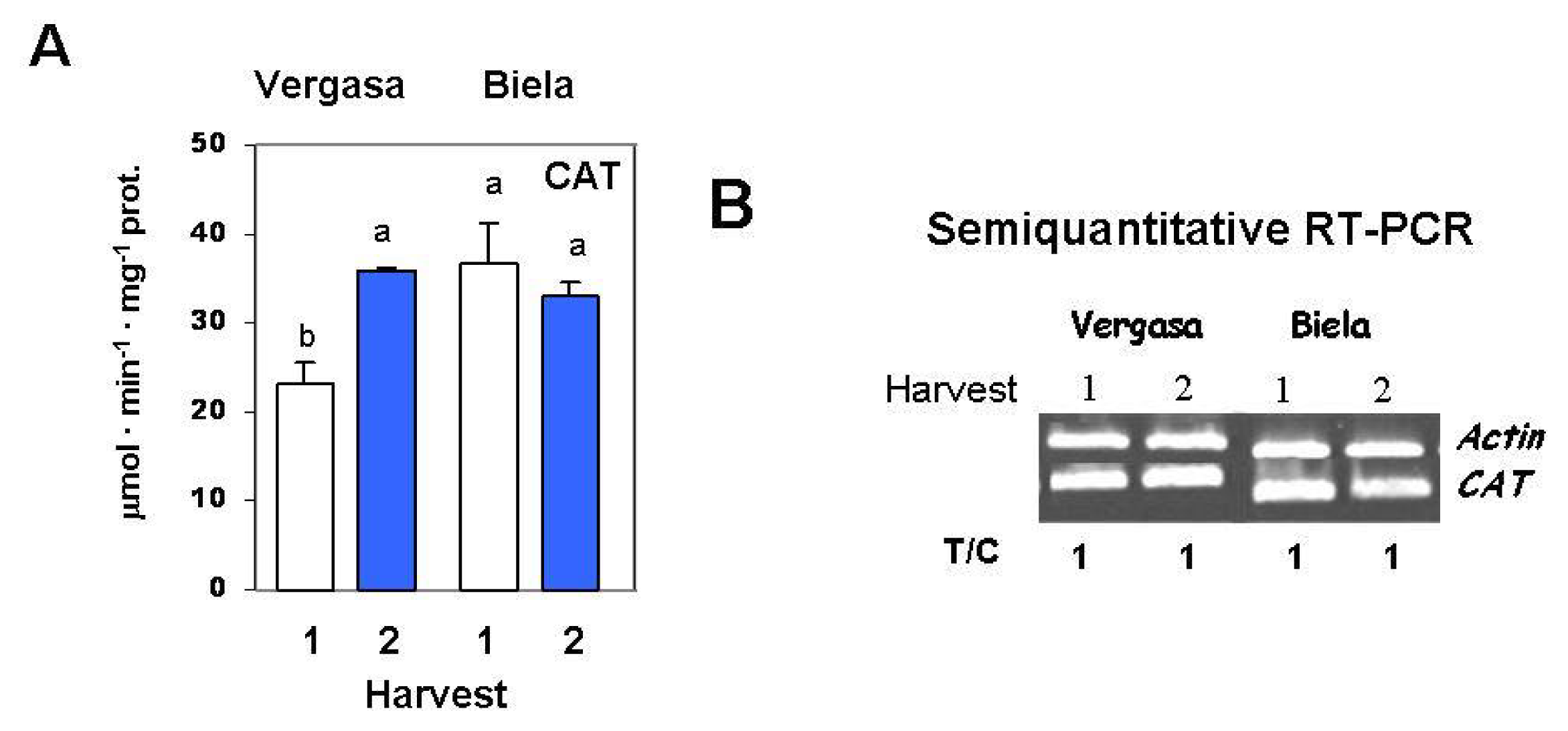
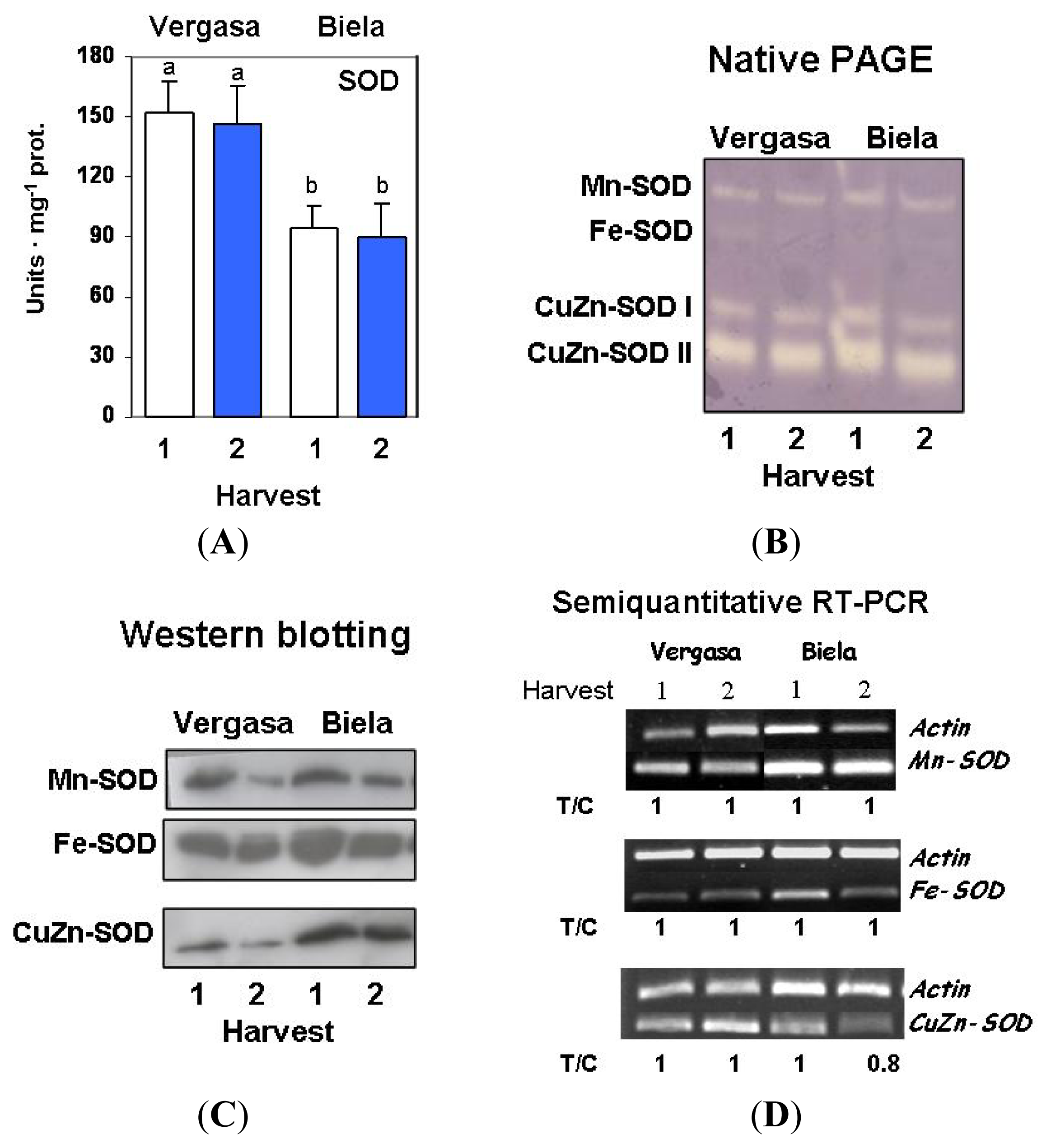
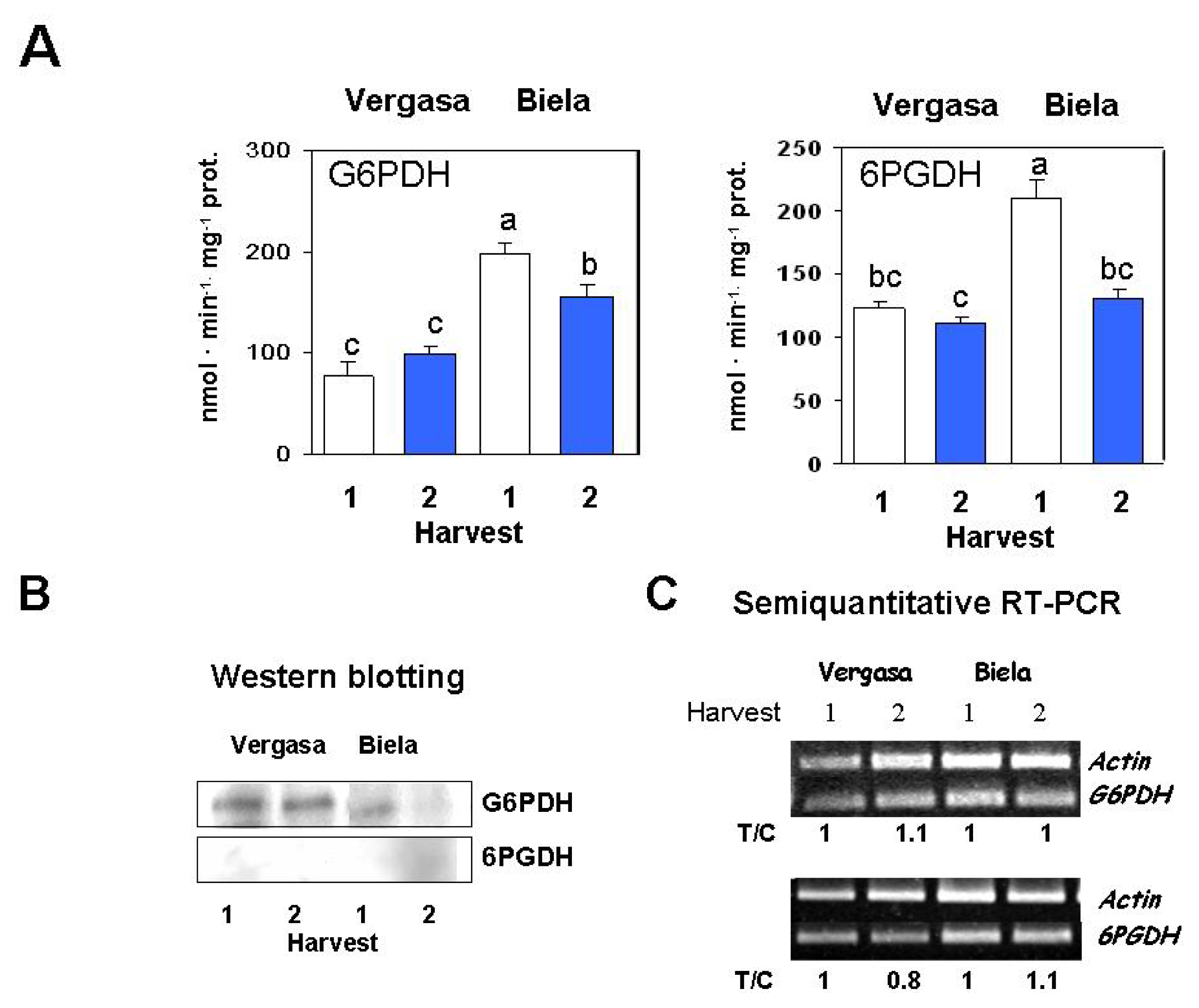
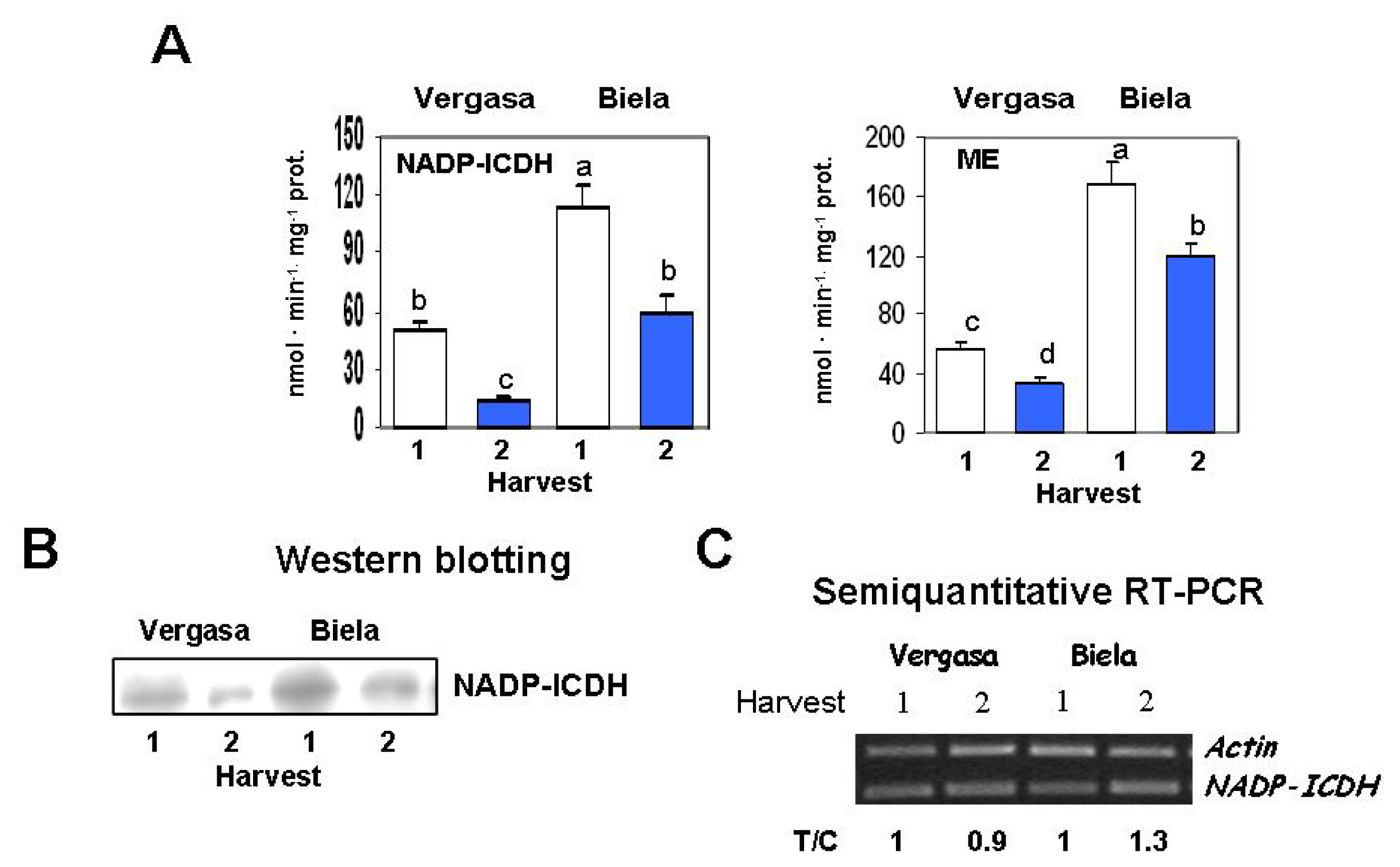
2. Results
3. Discussion
4. Experimental Section
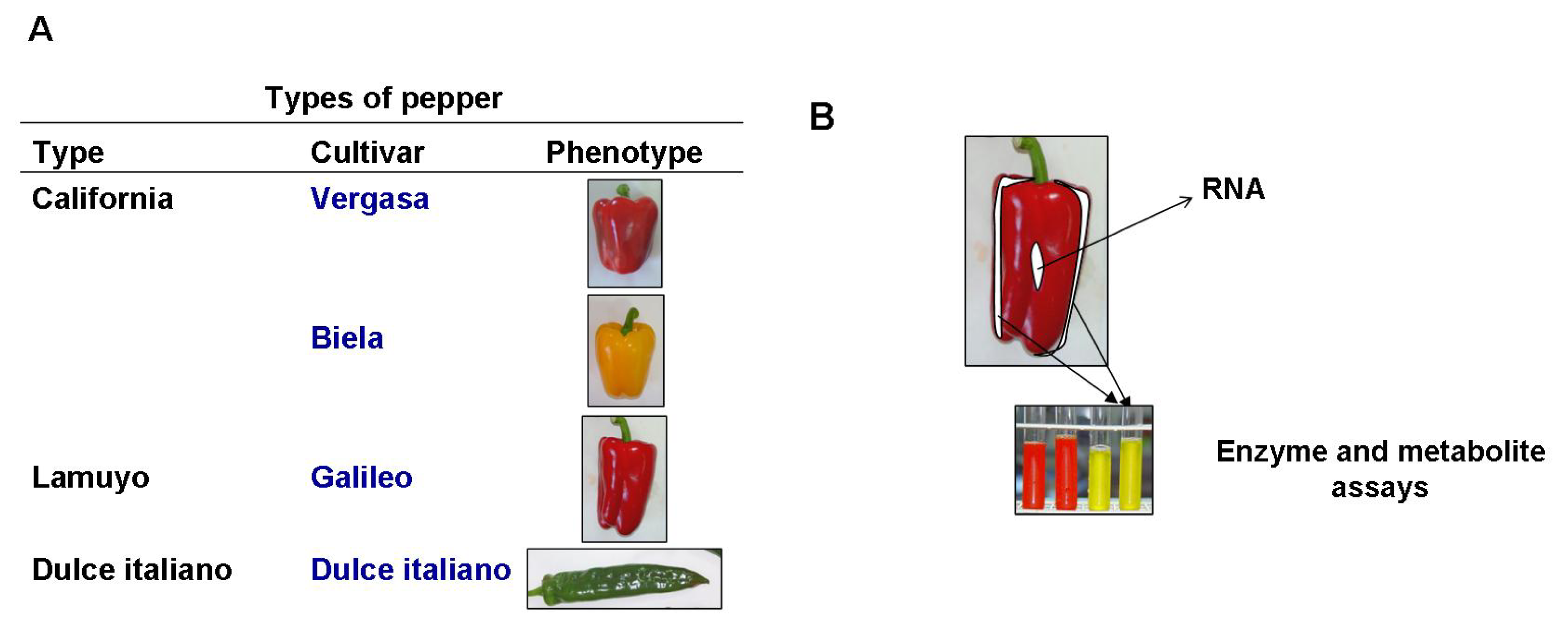
4.1. Plant Material and Growth Conditions
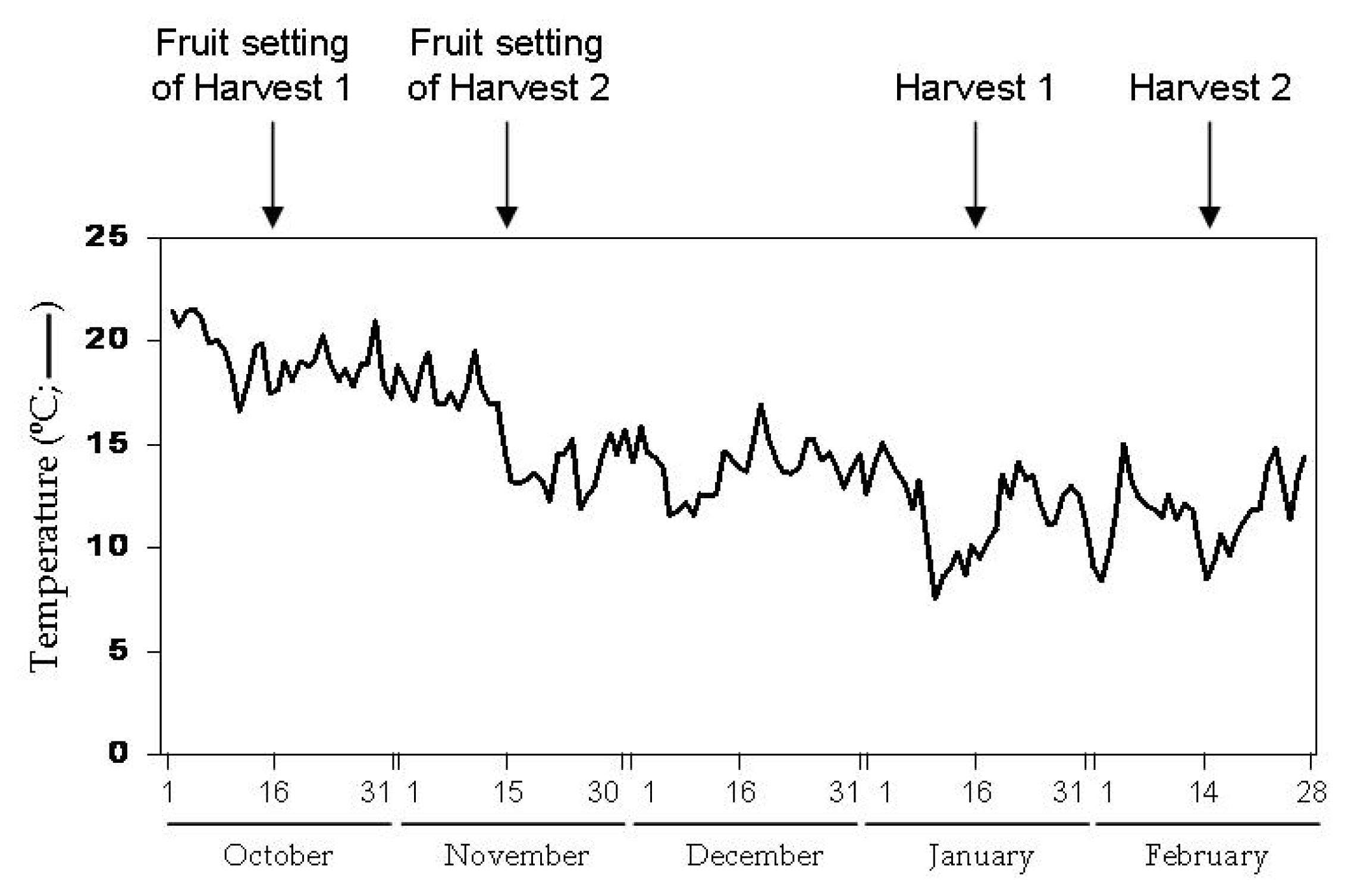
4.2. Experimental Design
4.3. Preparation of Crude Extracts
4.4. Enzyme Activities
4.5. Western Blotting
4.6. RNA Isolation and Semiquantitative RT-PCR
4.7. Determination of Total Ascorbate Content and Total Antioxidant Activity
4.8. Lipid Peroxidation and Protein Oxidation
4.9. Statistical Analysis
5. Conclusions
Acknowledgments
Conflict of Interest
References
- Howard, L.R.; Talcott, S.T.; Brenes, C.H.; Villalon, B. Changes in phytochemical and antioxidant activity of selected pepper cultivars (Capsicum species) as influenced by maturity. J. Agric. Food Chem 2000, 48, 1713–1720. [Google Scholar]
- Mateos, R.M. Pepper Antioxidants: Biochemical and Molecular Study of the Fruit Ripening and the Response to Abiotic Stress. Ph.D. Thesis, University of Granada, Spain, 20 June 2006. [Google Scholar]
- Palma, J.M.; Corpas, F.J.; del Río, L.A. Plant Vitamin Antioxidants and Their Influence on the Human Diet. In Fruit and Vegetable Consumption and Health; Papareschi, A., Eppolito, H., Eds.; Nova Science Publishers, Inc: New York, NY, USA, 2009; pp. 127–138. [Google Scholar]
- Palma, J.M.; Jiménez, A.; Corpas, F.J.; Mateos, R.M.; Martí, M.C.; Sevilla, F.; del Río, L.A. Role of ascorbate on the fruit physiology of pepper (Capsicum annuum L.). Funct. Plant Sci. Biotech 2011, 5, 56–61. [Google Scholar]
- Martí, M.C.; Camejo, D.; Vallejo, F.; Romojaro, F.; Bacarizo, S.; Palma, J.M.; Sevilla, F.; Jiménez, A. Influence of fruit ripening stage and harvest period on the antioxidant content of sweet pepper cultivars. Plant Foods Hum. Nutr 2011, 66, 416–423. [Google Scholar]
- Wall, M.M.; Waddell, C.A.; Bosland, P.W. Variation in beta-carotene and total carotenoid content in fruits of Capsicum. HortScience 2001, 36, 746–749. [Google Scholar]
- Yanishlieva, N.V.; Marinova, E.; Pokorny, J. Natural antioxidants from herbs and spices. Eur. J. Lipid Sci. Technol 2006, 108, 776–793. [Google Scholar]
- Liu, Y.B.; Nair, M.G. Non-pungent functional food components in the water extracts of hot peppers. Food Chem 2010, 122, 731–736. [Google Scholar]
- O’Sullivan, L.; Jiwan, M.A.; Daly, T.; O’Brien, N.M.; Aherne, S.A. Bioaccessibility, uptake, and transport of carotenoids from peppers (Capsicum spp.) using the coupled in vitro digestion and human intestinal caco-2 cell model. J. Agric. Food Chem 2010, 58, 5374–5379. [Google Scholar]
- Song, W.; Derito, C.M.; Liu, M.K.; He, X.; Dong, M.; Liu, R.H. Cellular antioxidant activity of common vegetables. J. Agric. Food Chem 2010, 58, 6621–6629. [Google Scholar]
- Basu, S.K.; De, A.K. Capsicum: Historical and Botanical Perspectives. In Capsicum. The Genus Capsicum; De, A.K., Ed.; Taylor & Francis: London, UK, 2003; pp. 1–15. [Google Scholar]
- Camara, B.; Hugueney, P.; Bouvier, F.; Kuntz, M.; Moneager, R. Biochemistry and molecular biology of chromoplast development. Int. Rev. Cytol 1995, 163, 175–247. [Google Scholar]
- Markus, F.; Daood, H.G.; Kapitány, J.; Biacs, P.A. Change in the carotenoid and antioxidant content of spice red pepper (paprika) as a function of ripening and some technological factors. J. Agric. Food Chem 1999, 47, 100–107. [Google Scholar]
- Manirakiza, P.; Covaci, A.; Schepens, P. Pungency Principles in Capsicum—Analytical Determinations and Toxicology. In Capsicum. The Genus Capsicum; De, A.K., Ed.; Taylor & Francis: London, UK, 2003; pp. 71–86. [Google Scholar]
- Palma, J.M.; Corpas, F.J.; del Río, L.A. Proteomics as an approach to the understanding of the molecular physiology of fruit development and ripening. J. Proteomic 2011, 74, 1230–1243. [Google Scholar]
- Mercado, J.A.; Reid, M.S.; Valpuesta, V.; Quesada, M.A. Metabolic changes and susceptibility to chilling stress in Capsicum annuum plants grown at suboptimal temperature. Aust. J. Plant Physiol 1997, 24, 759–767. [Google Scholar]
- Polowick, P.L.; Sawhney, V.K. Temperature effects on male fertility and flower and fruit development in Capsicum annuum L. Sci. Horticult 1985, 25, 117–127. [Google Scholar]
- Pressman, E.; Moshkovitch, H.; Rosenfeld, K.; Shaked, R.; Gamliel, B.; Aloni, B. Influence of low night temperatures on sweet pepper flower quality and the effect of repeated pollinations, with viable pollen, on fruit setting. J. Horticult. Sci. Biotechnol 1998, 73, 131–136. [Google Scholar]
- Pressman, E.; Shaked, R.; Firon, N. Exposing pepper plants to high day temperatures prevents the adverse low night temperature symptoms. Physiol. Plant 2006, 126, 618–626. [Google Scholar]
- Shaked, R.; Rosenfeld, K.; Pressman, E. The effect of low night temperatures on carbohydrates metabolism in developing pollen grains of pepper in relation to their number and functioning. Sci. Horticult 2004, 102, 29–36. [Google Scholar]
- Airaki, M.; Leterrier, M.; Mateos, R.M.; Valderrama, R.; Chaki, M.; Barroso, J.B.; del Río, L.A.; Palma, J.M.; Corpas, F.J. Metabolism of reactive oxygen species and reactive nitrogen species in pepper (Capsicum annuum L.) plants under low temperature stress. Plant Cell Environ 2012, 35, 281–295. [Google Scholar]
- Del Río, L.A.; Pastori, G.M.; Palma, J.M.; Sandalio, L.M.; Sevilla, F.; Corpas, F.J.; Jiménez, A.; López-Huertas, E.; Hernandez, J.A. The activated oxygen role of peroxisomes in senescence. Plant Physiol 1998, 116, 1195–1200. [Google Scholar]
- Apel, K.; Hirt, H. Reactive oxygen species: Metabolism, oxidative stress, and signal transduction. Annu. Rev. Plant Biol 2004, 55, 373–399. [Google Scholar]
- Bhattacharjee, S. Reactive oxygen species and oxidative burst: Roles in stress, senescence and signal transduction in plants. Curr. Sci 2005, 89, 1113–11121. [Google Scholar]
- Foyer, C.H.; Noctor, G. Oxidant and antioxidant signalling in plants: A re-evaluation of the concept of oxidative stress in a physiological context. Plant Cell Environ 2005, 28, 1056–1071. [Google Scholar]
- Puppo, A.; Groten, K.; Bastian, F.; Carzaniga, R.; Soussi, M.; Lucas, M.M.; de Felipe, M.R.; Harrison, J.; Vanacker, H.; Foyer, C.H. Legume nodule senescnece: Roles for redox and hormone signalling in the orchestration of the natural aging process. New Phytol 2005, 165, 683–701. [Google Scholar]
- Gechev, T.S.; van Breusegem, F.; Stone, J.M.; Denev, I.; Laloi, C. Reactive oxygen species as signals that modulate plant stress responses and programmed cell death. Bioessays 2006, 28, 1091–1101. [Google Scholar]
- Suzuki, N.; Mittler, R. Reactive oxygen species and temperature stresses: A delicate balance between signaling and destruction. Physiol. Plant 2006, 126, 45–51. [Google Scholar]
- del Río, L.A.; Puppo, A. Peroxisomes as a Celular Source of ROS Signal Molecules. In Reactive Oxygen Species in Plant Signaling; del Río, L.A.; Puppo, A. Springer-Verlag: Berlin & Heidelberg, Germany, 2009; pp. 95–111. [Google Scholar]
- Del Río, L.A.; Puppo, A. Reactive Oxygen Species in Plant Signaling; Springer: Heidelberg, Germany, 2009. [Google Scholar]
- Miller, G.; Suzuki, N.; Ciftci-Yilmaz, S.; Mittler, R. Reactive oxygen species homeostasis and signalling during drought and salinity stresses. Plant Cell Environ 2010, 33, 453–467. [Google Scholar]
- McCarthy, I.; Gómez, M.; del Río, L.A.; Palma, J.M. Role of peroxisomes in the oxidative injury induced by the auxin herbicide 2,4-d in leaves of pea plants. Biol. Plant 2011, 55, 485–492. [Google Scholar]
- Halliwell, B.; Gutteridge, J.M.C. Free Radicals in Biology and Medicine; Oxford University Press: Oxford, UK, 2007. [Google Scholar]
- Gómez, J.M.; Hernández, J.A.; Jiménez, A.; del Río, L.A.; Sevilla, F. Differential response of antioxidative enzymes of chloroplasts and mitochondria to long-term NaCl stress of pea plants. Free Radic. Res 1999, 31, S11–S18. [Google Scholar]
- Mittler, R. Oxidative stress, antioxidants and stress tolerance. Trends Plant Sci 2002, 7, 405–410. [Google Scholar]
- Jiménez, A.; Romojaro, F.; Llanos, M.R.; Gómez, J.M.; León, A.; Sevilla, F. Antioxidant systems and their relationship with the response of pepper fruits to storage at 20 °C. J. Agric. Food Chem 2003, 51, 6293–6299. [Google Scholar]
- Mittova, V.; Guy, M.; Tal, M.; Volokita, M. Salinity up-regulates the antioxidative system in root mitochondria and peroxisomes of the wild salt-tolerant tomato species Lycopersicon pennellii. J. Exp. Bot 2003, 55, 1105–1113. [Google Scholar]
- Vanacker, H.; Sandalio, L.M.; Jiménez, A.; Palma, J.M.; Corpas, F.J.; Masaguer, V.; Gomez, M.; Sevilla, F.; Leterrier, M.; Foyer, C.H.; et al. Roles for redox regulation in leaf senescence of pea plants grown on different sources of nitrogen nutrition. J. Exp. Bot 2006, 57, 1735–1745. [Google Scholar]
- Noctor, G.; Foyer, C.H. Ascorbate and glutathione: Keeping active oxygen under control. Ann. Rev. Plant Physiol. Plant Mol. Biol 1998, 49, 249–279. [Google Scholar]
- Van Breusegem, F.; Bailey-Serres, J.; Mittler, R. Unraveling the tapestry of networks involving reactive oxygen species in plants. Plant Physiol 2008, 147, 978–984. [Google Scholar]
- Foyer, C.H.; Noctor, G. Ascorbate and glutathione: The heart of the redox hub. Plant Physiol 2011, 155, 2–18. [Google Scholar]
- Anjum, N.A.; Umar, S.; Chan, M.T. Ascorbate-Glutathione Pathway and Stress Tolerance in Plants; Springer: Dordrecht, The Netherlands, 2011. [Google Scholar]
- Reumann, S.; Corpas, F.J. The Peroxisomal Ascorbate-Glutathione Pathways: Molecular Identification and Insights into Its Essential Role Ander Environmental Stress Conditions. In Ascorbate-Glutathione Pathway and Stress Tolerance in Plants; Anjum, N.A., Umar, S., Chan, M.T., Eds.; Springer: Dordrecht, The Netherlands, 2011; pp. 387–404. [Google Scholar]
- Corpas, F.J.; Barroso, J.B.; Sandalio, L.M.; Distefano, S.; Palma, J.M.; Lupiáñez, J.A.; del Río, L.A. A dehydrogenase-mediated recycling system of NADPH in plant peroxisomes. Biochem. J 1998, 330, 777–784. [Google Scholar]
- León, A.M.; Palma, J.M.; Corpas, F.J.; Gómez, M.; Romero-Puertas, M.C.; Chatterjee, D.; Mateos, R.M.; del Río, L.A.; Sandalio, L.M. Antioxidative enzymes in cultivars of pepper plants with different sensitivity to cadmium. Plant Physiol. Biochem 2002, 40, 813–820. [Google Scholar]
- Noctor, G.; Queval, G.; Gakiere, B. NAD(P) synthesis and pyridine nucleotide cycling in plants and their potential importance in stress conditions. J. Exp. Bot 2006, 57, 1603–1620. [Google Scholar]
- Valderrama, R.; Corpas, F.J.; Carreras, A.; Gómez-Rodríguez, M.V.; Chaki, M.; Pedrajas, J.R.; Fernández-Ocaña, A.; del Río, L.A.; Barroso, J.B. The dehydrogenase mediated recycling of NADPH is a key antioxidant system against salt-induced oxidative stress in olive plants. Plant Cell Environ 2006, 29, 1449–1459. [Google Scholar]
- Pascual, I.; Azcona, I.; Aguirreolea, J.; Morales, F.; Corpas, F.J.; Palma, J.M.; Rellán-Álvarez, R.; Sánchez-Díaz, M. Growth, yield, and fruit quality of pepper plants amended with two sanitized sewage sludges. J. Agric. Food Chem 2010, 58, 6951–6959. [Google Scholar]
- Jiménez, A.; Gómez, J.M.; Navarro, E.; Sevilla, F. Changes in the antioxidative systems in mitochondria during ripening of pepper fruits. Plant Physiol. Biochem 2002, 40, 515–520. [Google Scholar]
- Martínez, S.; López, M.; González-Raurich, M.; Álvarez, A.B. Effects of ripening stage and procesing systems on vitamin C content in sweet peppers (Capsicum annuum L.). Int. Food Sci. Nutr 2005, 56, 45–51. [Google Scholar]
- Mateos, R.M.; León, A.M.; Sandalio, L.M.; Gómez, M.; del Río, L.A.; Palma, J.M. Peroxisomes from pepper fruits (Capsicum annuum L): Purification, characterization and antioxidant activity. J. Plant Physiol 2003, 160, 1507–1516. [Google Scholar]
- Mateos, R.M.; Bonilla-Valverde, D.; del Río, L.A.; Palma, J.M.; Corpas, F.J. NADP-dehydrogenases from pepper fruits: Effect of maturation. Physiol. Plant 2009, 135, 130–139. [Google Scholar]
- Kevers, C.; Falkowski, M.; Tabart, J.; Defraigne, J.O.; Dommes, J.; Pincemail, J. Evolution of antioxidant capacity during storage of selected fruits and vegetables. J. Sci. Food Agric 2007, 55, 8596–8603. [Google Scholar]
- Matsufuji, H.; Ishikawa, K.; Nunomura, O.; Chino, M.; Takeda, M. Antioxidant content of different coloured sweet peppers, white, green, yellow, orange, and red (Capsicum annuum L.). Int. J. Food Sci. Tech 2007, 42, 1482–1488. [Google Scholar]
- Martí, M.C.; Camejo, D.; Olmos, E.; Sandalio, L.M.; Fernández-García, N.; Jiménez, A.; Sevilla, F. Characterisation and changes in the antioxidant system of chloroplasts and chromoplasts isolated from green and mature pepper fruits. Plant Biol 2009, 11, 613–624. [Google Scholar]
- Mariko, N.; Hassimoto, A.; Genovese, M.I.; Lajolo, F.M. Antioxidant capacity of Brazilian fruit, vegetables and commercially-frozen fruit pulps. J. Food Comp. Anal 2009, 22, 394–396. [Google Scholar]
- Smirnoff, N.; Pallanca, J.E. Ascorbate metabolism in relation to oxidative stress. Biochem. Soc. Trans 1996, 24, 472–478. [Google Scholar]
- Foyer, C.H. The Role of Ascorbic Acid in Defence Networks and Signalling in Plants. In Vitamin C: Functions and Biochemistry in Animals and Plants; Asard, H., May, J.M., Smirnoff, N., Eds.; Bios Scientific Publishers: Oxford, UK, 2004; pp. 65–82. [Google Scholar]
- Tabata, K.; Takaoka, T.; Esaka, M. Gene expression of ascorbic acid-related enzymes in tobacco. Phytochemistry 2002, 61, 631–635. [Google Scholar]
- Pateraki, I.; Sanmartin, M.; Kalamaki, M.S.; Gerasopoulos, B.; Kanellis, A.K. Molecular characterization and expression studies during melon fruit development and ripening of l-galactono-1,4-lactone dehydrogenase. J. Exp. Bot 2004, 55, 1623–1633. [Google Scholar]
- Leterrier, M.; Barroso, J.B.; Palma, J.M.; Corpas, F.J. Cytosolic NADP-isocitrate dehydrogenase in Arabidopsis leaves and roots. Biol. Plant 2012, 56, 705–710. [Google Scholar]
- Noctor, G. Metabolic signalling in defence and stress: The central roles of soluble redox couples. Plant Cell Environ 2006, 29, 409–425. [Google Scholar]
- Corpas, F.J.; Barroso, J.B.; Sandalio, L.M.; Palma, J.M.; Lupiáñez, J.A.; del Río, L.A. Peroxisomal NADP-dependent isocitrate dehydrogenase: Characterization and activity regulation during natural senescence. Plant Physiol 1999, 121, 921–928. [Google Scholar]
- Leterrier, M.; del Río, L.A.; Corpas, F.J. Cytosolic NADP-isocitrate dehydrogenase of pea plants: Genomic clone characterization and functional analysis under abiotic stress conditions. Free Radic. Res 2007, 41, 191–199. [Google Scholar]
- Levine, R.L.; Williams, J.A.; Stadtman, E.R.; Shacter, E. Carbonyl assays for determination of oxidatively modified proteins. Methods Enzymol 1991, 233, 346–363. [Google Scholar]
- Jaleel, C.A.; Riadh, K.; Gopi, R.; Manivannan, P.; Ines, J.; Al-Juburi, H.; Chang-Xing, Z.; Hong-Bo, S. Antioxidant defense responses: Physiological plasticity in higher plants under abiotic constraints. Acta Physiol. Plant 2009, 31, 427–436. [Google Scholar]
- Møller, I.A.; Sweetlove, L.J. ROS signaling-specificity is required. Trends Plant Sci 2010, 15, 370–374. [Google Scholar]
- Hayat, S.; Khalique, G.; Irfan, M.; Wani, A.S.; Tripathi, B.N.; Ahmad, A. Physiological changes induced by chromium stress in plants: An overview. Protoplasma 2012, 249, 599–611. [Google Scholar]
- Aebi, H. Catalase in vitro. Methods Enzymol 1984, 105, 121–126. [Google Scholar]
- McCord, J.M.; Fridovich, I. Superoxide dismutase: An enzymic function for erythrocuprein. J. Biol. Chem 1969, 244, 6049–6055. [Google Scholar]
- Beauchamp, C.; Fridovich, I. Superoxide dismutase: Improved assays and an assay applicable to acrylamide gels. Anal. Biochem 1971, 44, 276–287. [Google Scholar]
- Hossain, M.A.; Asada, K. Inactivation of ascorbate peroxidase in spinach chloroplasts on dark addition of hydrogen peroxide: Its protection by ascorbate. Plant Cell Physiol 1984, 25, 1285–1295. [Google Scholar]
- Mittler, R.; Zilinskas, B. Detection of ascorbate peroxidase activity in native gels by inhibition of the ascorbate-dependent reduction of nitroblue tetrazolium. Anal. Biochem 1993, 212, 540–546. [Google Scholar]
- Edwards, E.A.; Rawsthorne, S.; Mullineaux, P.M. Subcellular distribution of multiple forms of glutathione reductase in leaves of pea (Pisum sativum L.). Planta 1990, 180, 278–284. [Google Scholar]
- Ôba, K.; Fukui, M.; Imai, Y.; Iriyama, S.; Nogami, K. l-Galactono-γ-lactone dehydrogenase. Partial characterization, induction of activity and role in the synthesis of ascorbic acid in wounded white potato tuber tissue. Plant Cell Physiol 1994, 35, 473–478. [Google Scholar]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem 1976, 72, 248–254. [Google Scholar]
- Rodríguez-Serrano, M.; Romero-Puertas, M.C.; Pastori, G.M.; Corpas, F.J.; Sandalio, L.M.; del Río, L.A.; Palma, J.M. Peroxisomal membrane manganese superoxide dismutase: Characterization of the isozyme from watermelon (Citrullus lanatus Schrad.) cotyledons. J. Exp. Bot 2007, 58, 2417–2427. [Google Scholar]
- Corpas, F.J.; Fernández-Ocaña, A.; Carreras, A.; Valderrama, R.; Luque, F.; Esteban, F.J.; Rodríguez-Serrano, M.; Chaki, M.; Pedrajas, J.R.; Sandalio, L.M.; et al. The expression of different superoxide dismutase forms is cell-type dependent in olive (Olea europaea L.) leaves. Plant Cell Physiol 2006, 47, 984–994. [Google Scholar]
- Bueno, P.; Varela, J.; Giménez-Gallego, G.; del Río, L.A. Peroxisomal copper, zinc superoxide dismutase. Characterization of the isoenzyme from watermelon cotyledons. Plant Physiol 1995, 108, 1151–1160. [Google Scholar]
- Krepinsky, K.; Plaumann, M.; Martin, W.; Schnarrenberger, C. Purification and cloning of chloroplast 6-phosphogluconate dehydrogenase from spinach. Cyanobacterial genes for chloroplast and cytosolic isoenzymes encoded in eukaryotic chromosomes. Eur. J. Biochem 2001, 268, 2678–2686. [Google Scholar]
- Chen, R. Plant NADP-dependent isocitrate dehydrogenases are predominantly localized in the cytosol. Planta 1998, 207, 280–285. [Google Scholar]
- Sambrook, J.; Russell, D.W. Molecular Cloning. A Laboratory Manual, 3th ed; Cold Spring Harbor Laboratory Press: Cold Spring Harbor, NY, USA, 2001. [Google Scholar]
- Marone, M.; Mozzetti, S.; De Ritis, D.; Pierelli, L.; Scambia, G. Semiquantitative RT-PCR analysis to assess the expression levels of multiple transcripts from the same sample. Biol. Proc. Online 2001, 3, 19–25. [Google Scholar] [Green Version]
- Jiménez, A.; Hernández, J.A.; del Río, L.A.; Sevilla, F. Evidence for the presence of the ascorbate-glutathione cycle in mitochondria and peroxisomes of pea leaves. Plant Physiol 1997, 114, 275–284. [Google Scholar]
- Castillo, F.J.; Greppin, H. Extracellular ascorbic acid and enzyme activities related to ascorbic acid metabolism in Sedum album L. leaves after ozone exposure. Environ. Exp. Bot 1988, 28, 231–238. [Google Scholar]
- Miller, N.J.; Rice-Evans, C.A.; Davies, M.J.; Gopinathan, V.; Milner, A. A novel method for measuring antioxidant capacity and its application to monitoring the antioxidant status in premature neonates. Clin. Sci 1993, 84, 407–412. [Google Scholar]
- Miller, N.J.; Diplock, A.T.; Rice-Evans, C.A. Evaluation of the total antioxidant activity as a marker of the deterioration of apple juice on storage. J. Agric. Food Chem 1995, 43, 1794–1801. [Google Scholar]
- Buege, J.A.; Aust, S.D. Microsomal lipid peroxidation. Methods Enzymol 1978, 52, 302–310. [Google Scholar]
- Levine, A.; Tenhaken, R.; Dixon, R.; Lamb, C. H2O2 from the oxidative burst orchestrates the plant hypersensitive disease resistance response. Cell 1994, 79, 583–593. [Google Scholar]

| Vergasa | Biela | |||
|---|---|---|---|---|
| Harvest | 1 | 2 | 1 | 2 |
| ASC + DHA (μg g−1 fruit) | 1,118 ± 55 | 1,079 ± 160 | 1,267 ± 112 | 1,225 ± 223 |
| ASC/DHA | 1.41 | 0.61 | 1.36 | 0.59 |
| TAA (μM) | 3,656 ± 285 | 4,155 ± 125 | 4,410 ± 150 | 3,920 ± 127 |
| Protein concentration (μg/mL) | ||
|---|---|---|
| Cultivar | Harvest 1 | Harvest 2 |
| Vergasa | 340 ± 61 | 523 ± 76 |
| Biela | 144 ± 35 | 274 ± 55 |
| Enzyme | Oligonucleotide Sequences 5′ to 3′ | Genebank Accession No |
|---|---|---|
| Catalase | F: GATTTCTTCTCTTTCCTCC R: CGATGTTCCTATTCAATACC | AF227952 |
| Ascorbate peroxidase (cyt.) | F: TGTGCTCCTCTTATGCTCC R: CTCAAAACCAGAACGCTCC | X81376 |
| Glutathione reductase | F: TTTGGTTTATGGAGCTGCC R: CAGTGGGAGTTGCTTTCTG | AY547351 * |
| Galactono-γ-lactone dehydrogenase | F: TTACTCTTCAGAACTTTGC R: GGATTGCATGTCACAACCAC | AY547352 * |
| Mn-superoxide dismutase | F: CATGCAGCTTCATCACCAGA R: ATAACAAGGCGCTTCAGCTC | AF036936 |
| Fe-superoxide dismutase | F: CATCACAGGACCTATGTCG R: GGTGTTTTCACAACTACAAGC | AY173123 * |
| CuZn-superoxide dismutase | F: TGTTAGTGGCACCATCCTCT R: GGCCGATAATACCACAAGCA | AF009734 |
| Actin | F: ACTCTTAATCAATCCCTCC R: GCACTGTATGACTGACACC | AY572427 * |
© 2013 by the authors; licensee MDPI, Basel, Switzerland This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Mateos, R.M.; Jiménez, A.; Román, P.; Romojaro, F.; Bacarizo, S.; Leterrier, M.; Gómez, M.; Sevilla, F.; Del Río, L.A.; Corpas, F.J.; et al. Antioxidant Systems from Pepper (Capsicum annuum L.): Involvement in the Response to Temperature Changes in Ripe Fruits. Int. J. Mol. Sci. 2013, 14, 9556-9580. https://doi.org/10.3390/ijms14059556
Mateos RM, Jiménez A, Román P, Romojaro F, Bacarizo S, Leterrier M, Gómez M, Sevilla F, Del Río LA, Corpas FJ, et al. Antioxidant Systems from Pepper (Capsicum annuum L.): Involvement in the Response to Temperature Changes in Ripe Fruits. International Journal of Molecular Sciences. 2013; 14(5):9556-9580. https://doi.org/10.3390/ijms14059556
Chicago/Turabian StyleMateos, Rosa M., Ana Jiménez, Paloma Román, Félix Romojaro, Sierra Bacarizo, Marina Leterrier, Manuel Gómez, Francisca Sevilla, Luis A. Del Río, Francisco J. Corpas, and et al. 2013. "Antioxidant Systems from Pepper (Capsicum annuum L.): Involvement in the Response to Temperature Changes in Ripe Fruits" International Journal of Molecular Sciences 14, no. 5: 9556-9580. https://doi.org/10.3390/ijms14059556
APA StyleMateos, R. M., Jiménez, A., Román, P., Romojaro, F., Bacarizo, S., Leterrier, M., Gómez, M., Sevilla, F., Del Río, L. A., Corpas, F. J., & Palma, J. M. (2013). Antioxidant Systems from Pepper (Capsicum annuum L.): Involvement in the Response to Temperature Changes in Ripe Fruits. International Journal of Molecular Sciences, 14(5), 9556-9580. https://doi.org/10.3390/ijms14059556








