Prognostic Value of Tumor Markers, NSE, CA125 and SCC, in Operable NSCLC Patients
Abstract
:1. Introduction
2. Results and Discussion
2.1. Patients’ Characteristics
2.2. Tumor Markers and Patients’ Characteristics
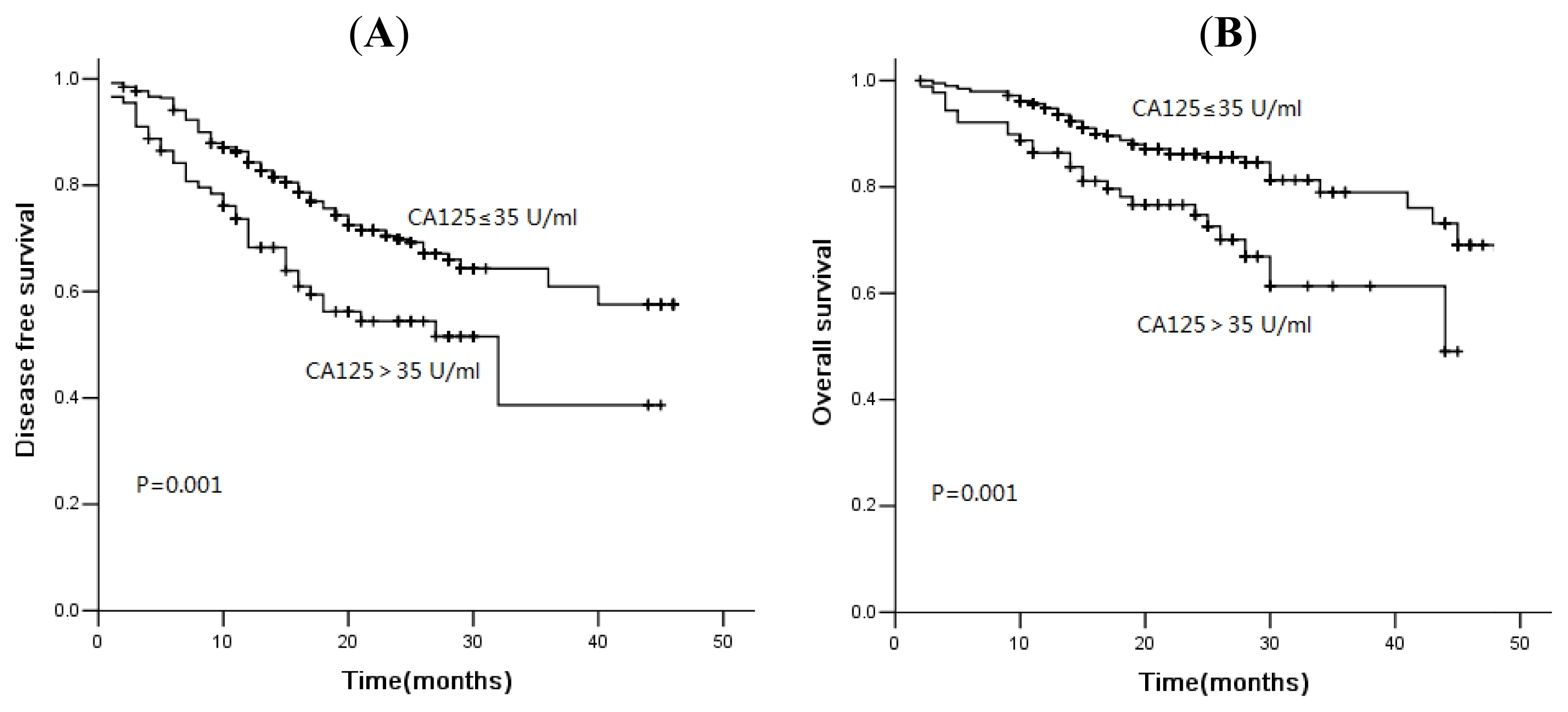
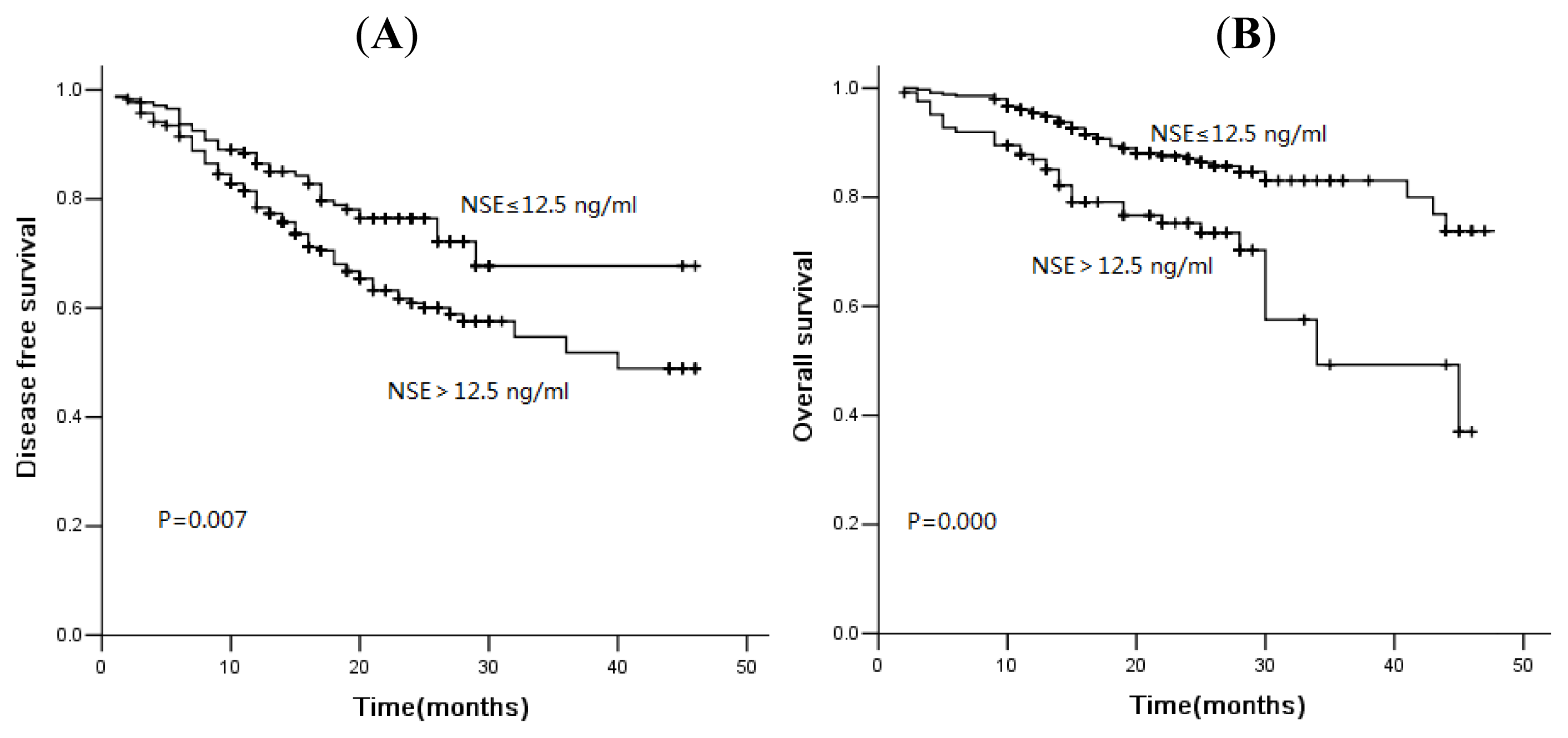
2.3. Association of Tumor Markers with Disease-Free Survival and Overall Survival
2.4. Discussion
3. Experimental Section
3.1. Patients and Treatment
3.2. Tumor Markers Measurement
3.3. Follow-Up
3.4. Statistical Analysis
4. Conclusions
Conflict of Interest
References
- Siegel, R.; Naishadham, D.; Jemal, A. Cancer statistics, 2012. CA Cancer J. Clin 2012, 62, 10–29. [Google Scholar]
- Govindan, R.; Bogart, J.; Vokes, E.E. Locally advanced non-small cell lung cancer: The past, present, and future. J. Thorac. Oncol 2008, 3, 917–928. [Google Scholar]
- Miller, Y.E. Pathogenesis of lung cancer: 100 year report. Am. J. Respir. Cell Mol. Biol 2005, 33, 216–223. [Google Scholar]
- Barak, V.; Holdenrieder, S.; Nisman, B.; Stieber, P. Relevance of circulating biomarkers for the therapy monitoring and follow-up investigations in patients with non-small cell lung cancer. Cancer Biomark 2010, 6, 191–196. [Google Scholar]
- Holdenrieder, S.; Nagel, D.; Stieber, P. Estimation of prognosis by circulating biomarkers in patients with non-small cell lung cancer. Cancer Biomark 2010, 6, 179–190. [Google Scholar]
- Jorgensen, L.G.; Hansen, H.H.; Cooper, E.H. Neuron specific enolase, carcinoembryonic antigen and lactate dehydrogenase as indicators of disease activity in small cell lung cancer. Eur. J. Cancer Clin. Oncol 1989, 25, 123–128. [Google Scholar]
- Petrovic, M.; Baskic, D.; Bankovic, D.; Ilic, N. Neuroendocrine differentiation as an indicator of chemosensitivity and prognosis in nonsmall cell lung cancer. Biomarkers 2011, 16, 311–320. [Google Scholar]
- Wang, Y.; Tang, D.; Sui, A.; Jiao, W.; Luo, Y.; Wang, M.; Yang, R.; Wang, Z.; Shen, Y. Prognostic significance of NSE mRNA in advanced NSCLC treated with gefitinib. Clin. Transl. Oncol 2013, 15, 384–390. [Google Scholar]
- Nagele, F.; Petru, E.; Medl, M.; Kainz, C.; Graf, A.H.; Sevelda, P. Preoperative CA 125: An independent prognostic factor in patients with stage I epithelial ovarian cancer. Obstet Gynecol 1995, 86, 259–264. [Google Scholar]
- Bast, R.C., Jr; Badgwell, D.; Lu, Z.; Marquez, R.; Rosen, D.; Liu, J.; Baggerly, K.A.; Atkinson, E.N.; Skates, S.; Zhang, Z.; et al. New tumor markers: CA125 and beyond. Int. J. Gynecol. Cancer 2005, 15, 274–281. [Google Scholar]
- Cedres, S.; Nunez, I.; Longo, M.; Martinez, P.; Checa, E.; Torrejón, D.; Felip, E. Serum tumor markers CEA, CYFRA21–1, and CA-125 are associated with worse prognosis in advanced non-small-cell lung cancer (NSCLC). Clin. Lung Cancer 2011, 12, 172–179. [Google Scholar]
- Kato, H.; Morioka, H.; Tsutsui, H.; Aramaki, S.; Torigoe, T. Value of tumor-antigen (TA-4) of squamous cell carcinoma in predicting the extent of cervical cancer. Cancer 1982, 50, 1294–1296. [Google Scholar]
- Kagohashi, K.; Satoh, H.; Ishikawa, H.; Ohtsuka, M.; Sekizawa, K. A re-evaluation of squamous cell carcinoma antigen (SCC) as a serum marker for non-small cell lung cancer. Med. Oncol 2008, 25, 187–189. [Google Scholar]
- Niklinski, J.; Furman, M.; Laudanski, J.; Kozlowski, M. Evaluation of squamous cell carcinoma antigen (SCC-Ag) in the diagnosis and follow-up of patients with non-small cell lung carcinoma. Neoplasma 1992, 39, 279–282. [Google Scholar]
- Moro, D.; Villemain, D.; Vuillez, J.P.; Delord, C.A.; Brambilla, C. CEA, CYFRA21–1 and SCC in non-small cell lung cancer. Lung Cancer 1995, 13, 169–176. [Google Scholar]
- Schneider, J.; Philipp, M.; Salewski, L.; Velcovsky, H.G. Pro-gastrin-releasing peptide (ProGRP) and neuron specific enolase (NSE) in therapy control of patients with small-cell lung cancer. Clin. Lab 2003, 49, 35–42. [Google Scholar]
- Pujol, J.L.; Quantin, X.; Jacot, W.; Boher, J.M.; Grenier, J.; Lamy, P.J. Neuroendocrine and cytokeratin serum markers as prognostic determinants of small cell lung cancer. Lung Cancer 2003, 39, 131–138. [Google Scholar]
- Pujol, J.L.; Boher, J.M.; Grenier, J.; Quantin, X. Cyfra 21–1, neuron specific enolase and prognosis of non-small cell lung cancer: Prospective study in 621 patients. Lung Cancer 2001, 31, 221–231. [Google Scholar]
- Barlesi, F.; Gimenez, C.; Torre, J.P.; Doddoli, C.; Mancini, J.; Greillier, L.; Roux, F.; Kleisbauer, J.P. Prognostic value of combination of Cyfra 21–1, CEA and NSE in patients with advanced non-small cell lung cancer. Respir. Med 2004, 98, 357–362. [Google Scholar]
- Nisman, B.; Heching, N.; Biran, H.; Barak, V.; Peretz, T. The prognostic significance of circulating neuroendocrine markers chromogranin a, pro-gastrin-releasing peptide and neuron-specific enolase in patients with advanced non-small-cell lung cancer. Tumour. Biol 2006, 27, 8–16. [Google Scholar]
- Jacot, W.; Quantin, X.; Boher, J.M.; Andre, F.; Moreau, L.; Gainet, M.; Depierre, A.; Quoix, E.; Chevalier, T.L.; Pujol, J.L. Association d’Enseignement et de Recherche des Internes en Oncologie. Brain metastases at the time of presentation of non-small cell lung cancer: A multi-centric AERIO analysis of prognostic factors. Br. J. Cancer 2001, 84, 903–909. [Google Scholar]
- Reinmuth, N.; Brandt, B.; Semik, M.; et al. Prognostic impact of Cyfra21–1 and other serum markers in completely resected non-small cell lung cancer. Lung Cancer 2002, 36, 265–270. [Google Scholar]
- Diez, M.; Torres, A.; Maestro, M.L.; Ortega, M.D.; Gómez, A.; Pollán, M.; Lopez, J.A.; Picardo, A.; Hernando, F.; Balibrea, J.L. Prediction of survival and recurrence by serum and cytosolic levels of CEA, CA125 and SCC antigens in resectable non-small-cell lung cancer. Br. J. Cancer 1996, 73, 1248–1254. [Google Scholar]
- Diez, M.; Gomez, A.; Hernando, F.; Ortega, M.D.; Gómez, A.; Pollán, M.; Lopez, J.A.; Picardo, A.; Hernando, F.; Balibrea, J.L. Serum CEA, CA125, and SCC antigens and tumor recurrence in resectable non-small cell lung cancer. Int. J. Biol. Markers 1995, 10, 5–10. [Google Scholar]
- Hatzakis, K.D.; Froudarakis, M.E.; Bouros, D.; Tzanakis, N.; Karkavitsas, N.; Siafakas, N.M. Prognostic value of serum tumor markers in patients with lung cancer. Respiration 2002, 69, 25–29. [Google Scholar]
- Gaspar, M.J.; Diez, M.; Rodriguez, A.; Ratia, T.; Martin, D.A.; Galvan, M.; Granell, J.; Coca, C. Clinical value of CEA and CA125 regarding relapse and metastasis in resectable non-small cell lung cancer. Anticancer Res 2003, 23, 3427–3432. [Google Scholar]
- Nuñez, G.R.; Ito, C.; Del Giglio, A. Increased serum CA-125 levels in patients with lung cancer post thoracotomy. South Med. J 2009, 102, 427–428. [Google Scholar]
- Foa, P.; Fornier, M.; Miceli, R.; Seregni, E.; Santambrogio, L.; Nosotti, M.; Massaron, S.; Cataldo, I.; Oldani, S.; Iurlo, A.; et al. Preoperative CEA, NSE, SCC, TPA and CYFRA 21.1 serum levels as prognostic indicators in resected non-small cell lung cancer. Int. J. Biol. Markers 1999, 14, 92–98. [Google Scholar]
- Mizuguchi, S.; Nishiyama, N.; Iwata, T.; Nishida, T.; Izumi, N.; Tsukioka, T.; Inoue, K.; Uenishi, T.; Wakasa, K.; Suehiro, S. Serum Sialyl Lewis x and cytokeratin 19 fragment as predictive factors for recurrence in patients with stage I non-small cell lung cancer. Lung Cancer 2007, 58, 369–375. [Google Scholar]
- Foa, P.; Fornier, M.; Miceli, R.; Seregni, E.; Santambrogio, L.; Nosotti, M.; Cataldo, I.; Sala, M.; Caldiera, S.; Bombardieri, E. Tumour markers CEA, NSE, SCC, TPA and CYFRA 21.1 in resectable non-small cell lung cancer. Anticancer Res 1999, 19, 3613–3618. [Google Scholar]
- Li, X.; Asmitananda, T.; Gao, L.; Seregni, E.; Santambrogio, L.; Nosotti, M.; Cataldo, I.; Sala, M.; Caldiera, S.; Bombardieri, E. Biomarkers in the lung cancer diagnosis: A clinical perspective. Neoplasma 2012, 59, 500–507. [Google Scholar]
- Chiu, C.H.; Shih, Y.N.; Tsai, C.M.; Liou, J.L.; Chen, Y.M.; Perng, R.P. Serum tumor markers as predictors for survival in advanced non-small cell lung cancer patients treated with gefitinib. Lung Cancer 2007, 57, 213–221. [Google Scholar]
- Ma, S.; Shen, L.; Qian, N.; Chen, K. The prognostic values of CA125, CA19.9, NSE, AND SCC for stage I NSCLC are limited. Cancer Biomark. 2011–2012, 10, 155–162. [Google Scholar]
- Tomita, M.; Shimizu, T.; Ayabe, T.; Onitsuka, T. Maximum SUV on positron emission tomography and serum CEA level as prognostic factors after curative resection for non-small cell lung cancer. Asia Pac. J. Clin. Oncol 2012, 8, 244–247. [Google Scholar]
- Grunnet, M.; Sorensen, J.B. Carcinoembryonic antigen (CEA) as tumor marker in lung cancer. Lung Cancer 2012, 76, 138–143. [Google Scholar]
- Cho, W.C. Potentially useful biomarkers for the diagnosis, treatment and prognosis of lung cancer. Biomed. Pharmacother 2007, 61, 515–519. [Google Scholar]



| Variables | Patient n (%) | NSE level | p | CA125 level | p | SCC level | p | |||
|---|---|---|---|---|---|---|---|---|---|---|
| Normal | High | Normal | High | Normal | High | 0.000 | ||||
| Sex | 0.752 | 0.652 | 246 (67.6) | 118 (62.4) | ||||||
| Male | 364 (75.7) | 131 (36.0) | 233 (64.0) | 295 (81.0) | 69 (19.0) | 110 (94.0) | 7 (6.0) | |||
| Female | 117 (24.3) | 44 (37.6) | 73 (62.4) | 97 (82.9) | 20 (17.1) | |||||
| Age | 0.206 | 0.092 | 0.917 | |||||||
| <65 | 333 (69.2) | 115 (34.5) | 218 (65.5) | 278 (83.5) | 55 (16.5) | 246 (73.9) | 87 (26.1) | |||
| ≥65 | 148 (30.8) | 60 (40.5) | 88 (59.5) | 114 (77.0) | 34 (23.0) | 110 (74.3) | 38 (25.7) | |||
| Smoking | 0.643 | 0.640 | 0.000 | |||||||
| Never | 145 (30.1) | 55 (37.9) | 90 (62.1) | 120 (82.8) | 25 (17.2) | 130 (89.7) | 15 (10.3) | |||
| Ever/Current | 336 (69.9) | 120 (35.7) | 216 (64.3) | 272 (81.0) | 64 (19.0) | 226 (67.3) | 110 (32.7) | |||
| Alcohol | 0.403 | 0.104 | 0.001 | |||||||
| Never | 249 (48.8) | 95 (38.2) | 154 (61.8) | 196 (78.7) | 53 (21.3) | 200 (80.3) | 49 (19.7) | |||
| Ever/Current | 232 (48.2) | 80 (34.5) | 152 (65.5) | 196 (84.5) | 36 (15.5) | 156 (67.2) | 76 (32.8) | |||
| Histologic type | 0.835 | 0.762 | 0.000 | |||||||
| Squamous cell | 236 (49.1) | 84 (35.6) | 152 (64.4) | 195 (82.6) | 41 (17.4) | 128 (54.2) | 108 (45.8) | |||
| Adenocarcinoma | 221 (45.9) | 81 (36.7) | 140 (63.3) | 177 (80.1) | 44 (19.9) | 209 (94.6) | 12 (5.4) | |||
| Others | 24 (5.0) | 10 (41.7) | 14 (58.3) | 20 (83.3) | 4 (16.7) | 19 (79.2) | 5 (20.8) | |||
| Differentiation | 0.495 | 0.021 | 0.001 | |||||||
| Well | 27 (5.6) | 12 (44.4) | 15 (55.6) | 25 (92.6) | 2 (7.4) | 22 (81.5) | 5 (18.5) | |||
| Moderate | 248 (51.6) | 93 (37.5) | 155 (62.5) | 210 (84.7) | 38 (15.3) | 166 (66.9) | 82 (33.1) | |||
| Poor | 206 (42.8) | 70 (34.0) | 136 (66.0) | 157 (76.2) | 49 (23.8) | 168 (81.6) | 38 (18.41) | |||
| T stage | 0.000 | 0.001 | 0.039 | |||||||
| T1 | 47 (9.8) | 24 (51.1) | 23 (48.9) | 43 (91.5) | 4 (8.5) | 41 (87.2) | 6 (12.8) | |||
| T2 | 342 (71.1) | 130 (38.0) | 212 (62.0) | 285 (83.3) | 57 (16.7) | 255 (74.6) | 87 (25.4) | |||
| T3 | 57 (11.9) | 6 (10.5) | 51 (89.5) | 43 (75.14) | 14 (24.6) | 36 (63.2) | 21 (36.8) | |||
| T4 | 35 (7.3) | 15 (42.9) | 20 (57.1) | 21 (60.0) | 14 (40.0) | 24 (68.6) | 11 (31.4) | |||
| N stage | 0.360 | 0.004 | 0.223 | |||||||
| N0 | 251 (52.2) | 97 (38.6) | 154 (61.4) | 218 (86.9) | 33 (13.1) | 194 (77.3) | 57 (22.7) | |||
| N1 | 128 (26.6) | 40 (31.3) | 88 (68.8) | 100 (78.1) | 28 (21.9) | 91 (71.1) | 37 (28.9) | |||
| N2 | 102 (21.2) | 38 (37.3) | 64 (62.7) | 74 (72.5) | 28 (27.5) | 71 (69.6) | 31 (30.4) | |||
| Clinical stage | 0.092 | 0.000 | 0.189 | |||||||
| I | 217 (45.1) | 90 (41.5) | 127 (58.5) | 191 (88.0) | 26 (12.0) | 171 (78.8) | 46 (21.2) | |||
| II | 120 (24.9) | 36 (30.0) | 84 (70.0) | 97 (80.8) | 23 (19.2) | 84 (70.0) | 36 (30.0) | |||
| IIIa | 109 (22.7) | 34 (31.2) | 75 (68.8) | 83 (76.1) | 26 (23.9) | 77 (70.6) | 32 (29.4) | |||
| IIIb | 35 (7.3) | 15 (42.9) | 20 (57.1) | 21 (60.0) | 14 (40.0) | 24 (68.6) | 11 (31.4) | |||
| End point | Parameter | HR | 95% CI | p |
|---|---|---|---|---|
| DFS | Sex: Male vs. Female | 1.005 | 0.528–1.914 | 0.988 |
| Age: <65 Y vs. ≥65 Y | 1.379 | 0.971–1.959 | 0.072 | |
| DFS | Smoking: Ever vs. Never | 1.030 | 0.573–1.850 | 0.922 |
| DFS | Clinical stage: I, II vs. III | 1.298 | 1.093–1.542 | 0.003 |
| DFS | NSE level: <12.5 ng/mL vs. ≥12.5 ng/mL | 1.609 | 1.110–2.333 | 0.012 |
| DFS | CA125 level: <35 U/mL vs. ≥35 U/mL | 1.857 | 1.121–2.407 | 0.006 |
| DFS | SCC level: <1.5 ng/mL vs. ≥1.5 ng/m | 1.236 | 0.805–1.896 | 0.333 |
| OS | Sex: Male vs. Female | 0.820 | 0.308–2.180 | 0.690 |
| Age: <65 Y vs. ≥65 Y | 1.676 | 1.051–2.673 | 0.030 | |
| OS | Smoking: Ever vs. Never | 1.111 | 0.474–2.604 | 0.808 |
| OS | Clinical stage: I, II vs. III | 1.377 | 1.089–1.743 | 0.003 |
| OS | NSE level: <12.5 ng/mL vs. ≥12.5 ng/mL | 1.907 | 1.148–3.169 | 0.013 |
| OS | CA125 level: <35 U/mL vs. ≥35 U/mL | 2.042 | 1.290–3.225 | 0.005 |
| OS | SCC level: <1.5 ng/mL vs. ≥1.5 ng/mL | 1.303 | 0.788–2.157 | 0.303 |
| End point | Parameter | HR | 95% CI | p |
|---|---|---|---|---|
| DFS | Sex: Male vs. Female | 1.527 | 0.687–3.393 | 0.299 |
| Age: <65 Y vs. ≥65 Y | 1.135 | 0.659–1.955 | 0.648 | |
| DFS | Smoking: Ever vs. Never | 1.764 | 0.786–3.959 | 0.169 |
| DFS | Clinical stage: I, II vs. III | 2.154 | 1.256–3.695 | 0.005 |
| DFS | NSE level: <12.5 ng/mL vs. ≥12.5 ng/mL | 1.205 | 0.725–2.004 | 0.471 |
| DFS | CA125 level: <35 U/mL vs. ≥35 U/mL | 1.459 | 0.841–2.531 | 0.179 |
| DFS | SCC level: <1.5 ng/mL vs. ≥1.5 ng/m | 4.067 | 1.639–10.091 | 0.002 |
| OS | Sex: Male vs. Female | 1.609 | 0.395–6.552 | 0.507 |
| Age: <65 Y vs. ≥65 Y | 2.242 | 0.908–5.533 | 0.080 | |
| OS | Smoking: Ever vs. Never | 1.857 | 0.443–7.779 | 0.397 |
| OS | Clinical stage: I, II vs. III | 2.515 | 1.105–5.723 | 0.028 |
| OS | NSE level: <12.5 ng/mL vs. ≥12.5 ng/mL | 2.007 | 0.804–5.012 | 0.136 |
| OS | CA125 level: <35 U/mL vs. ≥35 U/mL | 0.715 | 0.300–1.706 | 0.450 |
| OS | SCC level: <1.5 ng/mL vs. ≥1.5 ng/mL | 6.909 | 2.167–22.026 | 0.001 |
© 2013 by the authors; licensee MDPI, Basel, Switzerland This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license ( http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Yu, D.; Du, K.; Liu, T.; Chen, G. Prognostic Value of Tumor Markers, NSE, CA125 and SCC, in Operable NSCLC Patients. Int. J. Mol. Sci. 2013, 14, 11145-11156. https://doi.org/10.3390/ijms140611145
Yu D, Du K, Liu T, Chen G. Prognostic Value of Tumor Markers, NSE, CA125 and SCC, in Operable NSCLC Patients. International Journal of Molecular Sciences. 2013; 14(6):11145-11156. https://doi.org/10.3390/ijms140611145
Chicago/Turabian StyleYu, Dangfan, Kaiqi Du, Taifeng Liu, and Guojun Chen. 2013. "Prognostic Value of Tumor Markers, NSE, CA125 and SCC, in Operable NSCLC Patients" International Journal of Molecular Sciences 14, no. 6: 11145-11156. https://doi.org/10.3390/ijms140611145




