Gene Expression Analyses Support Fallopian Tube Epithelium as the Cell of Origin of Epithelial Ovarian Cancer
Abstract
:1. Introduction
2. Results and Discussion
2.1. Gene Selection
2.2. Donor Sample Characteristics
2.3. Comparative Gene Expression Profiles in EOC Compared to ON or NAT
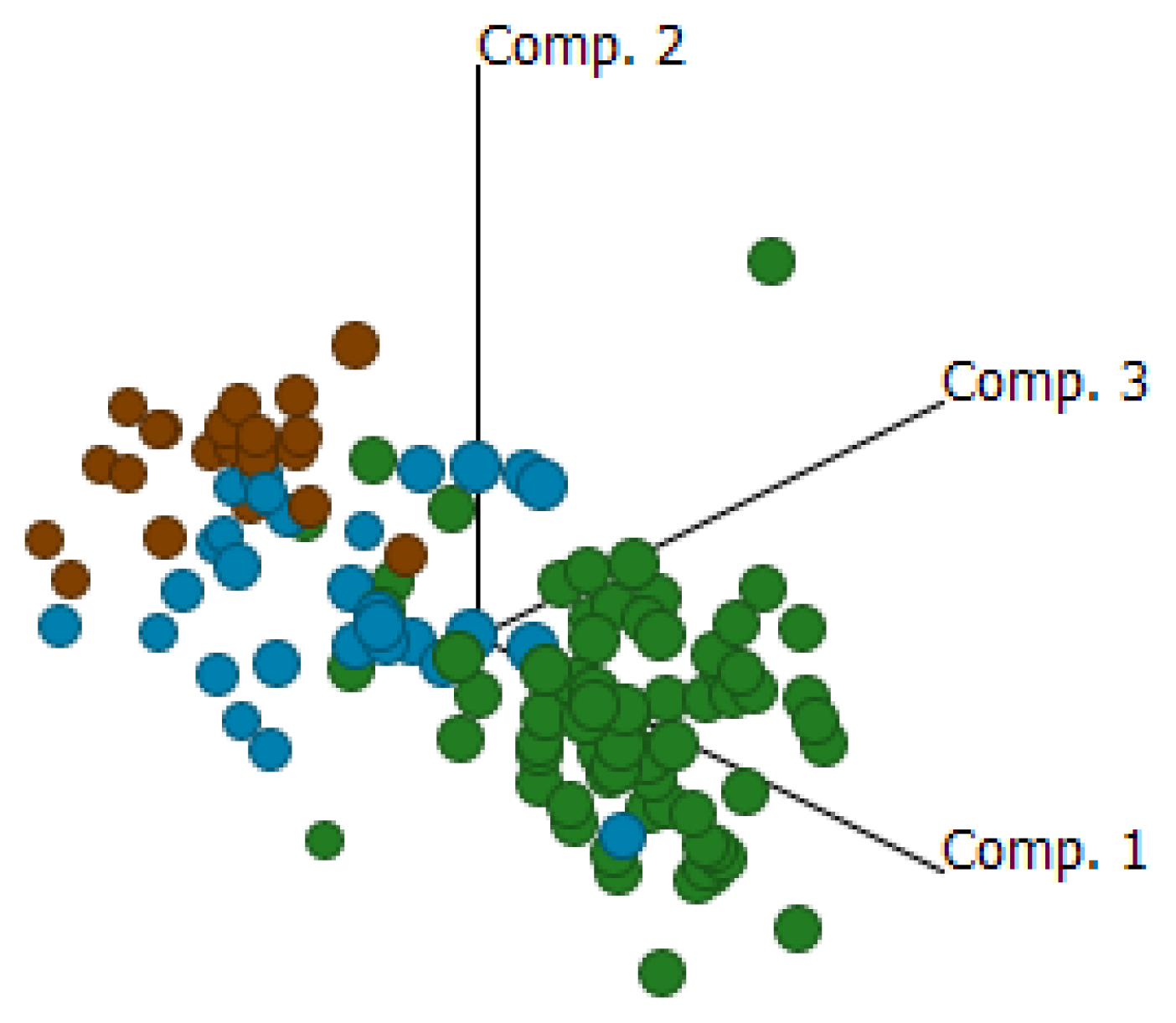
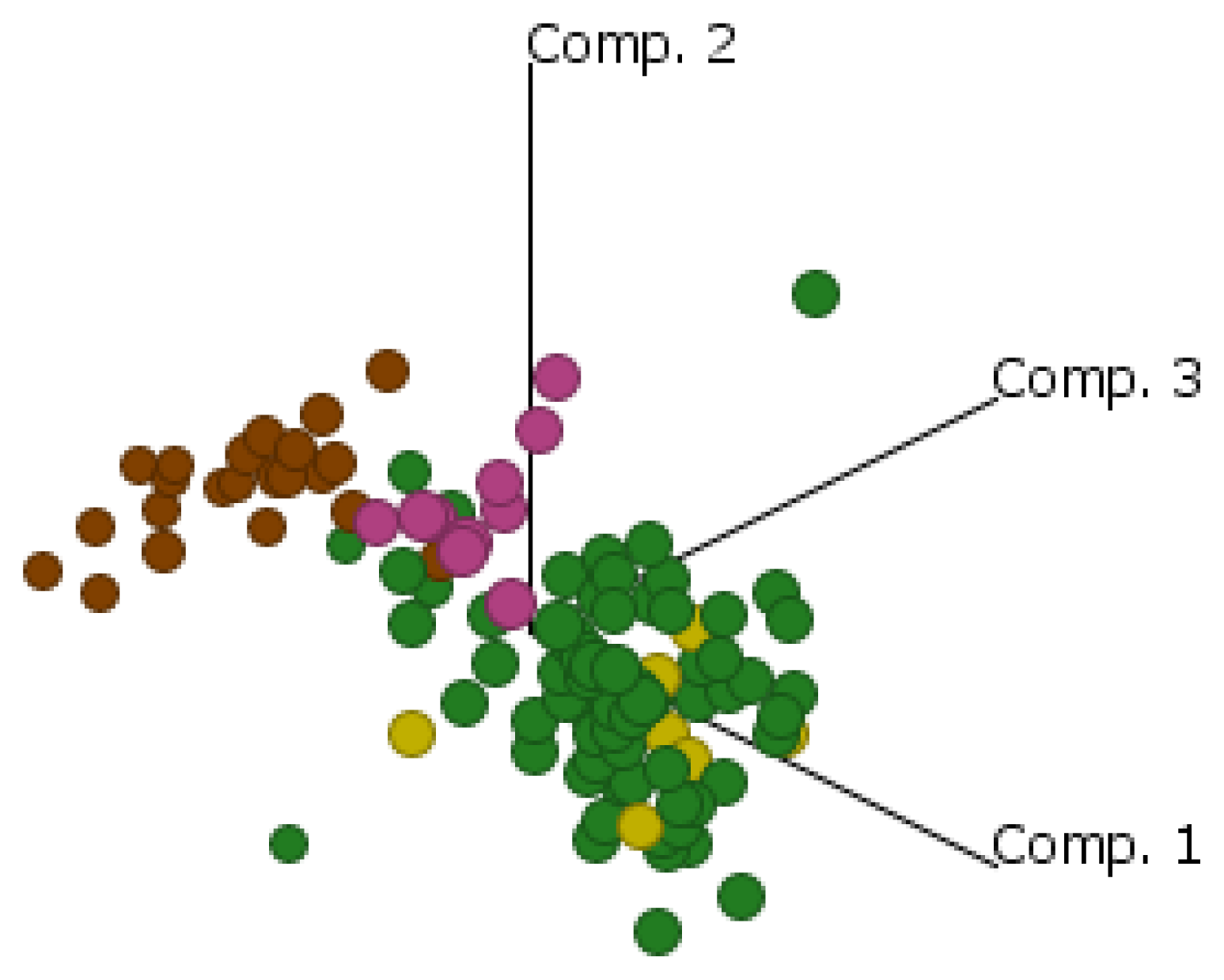
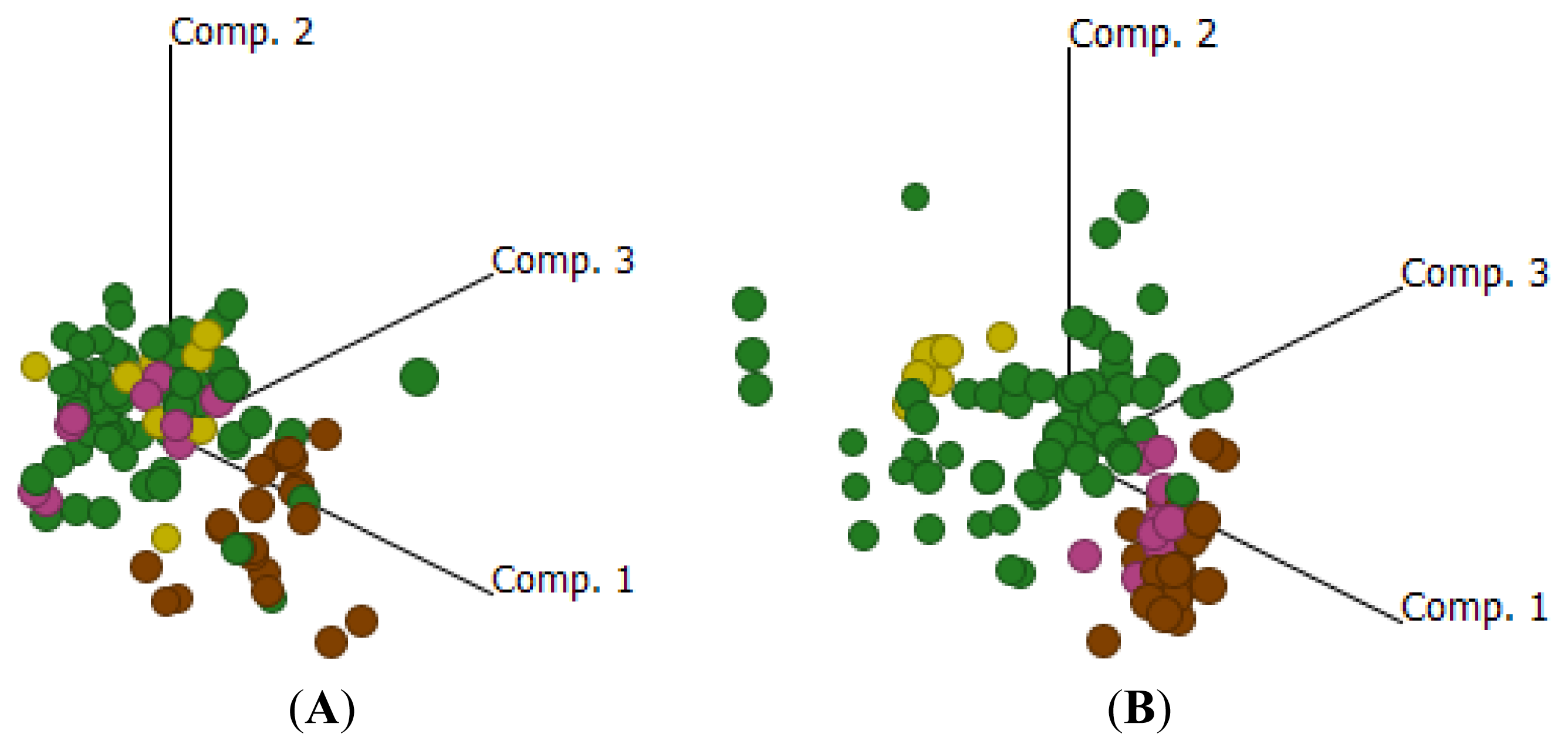
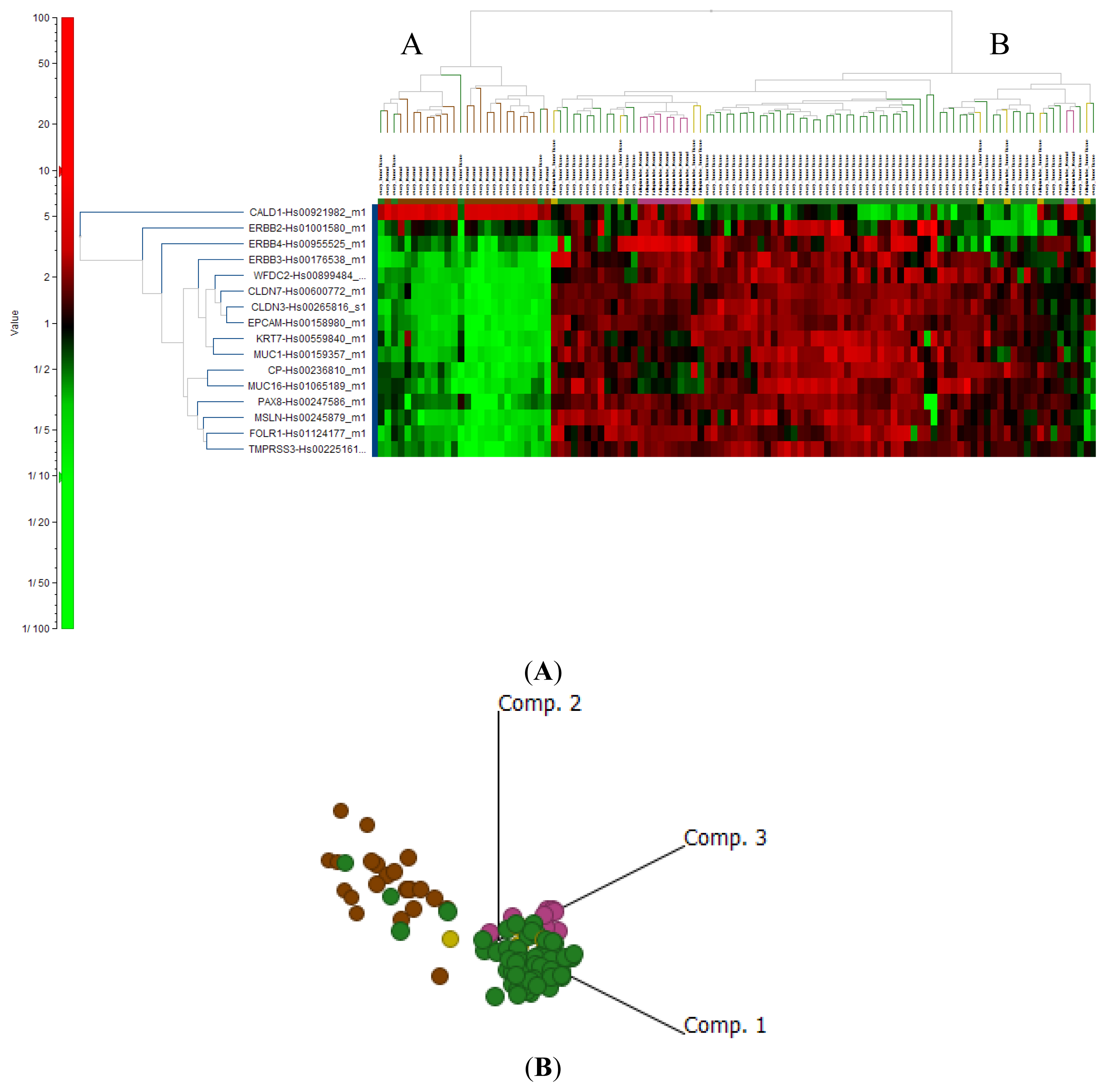
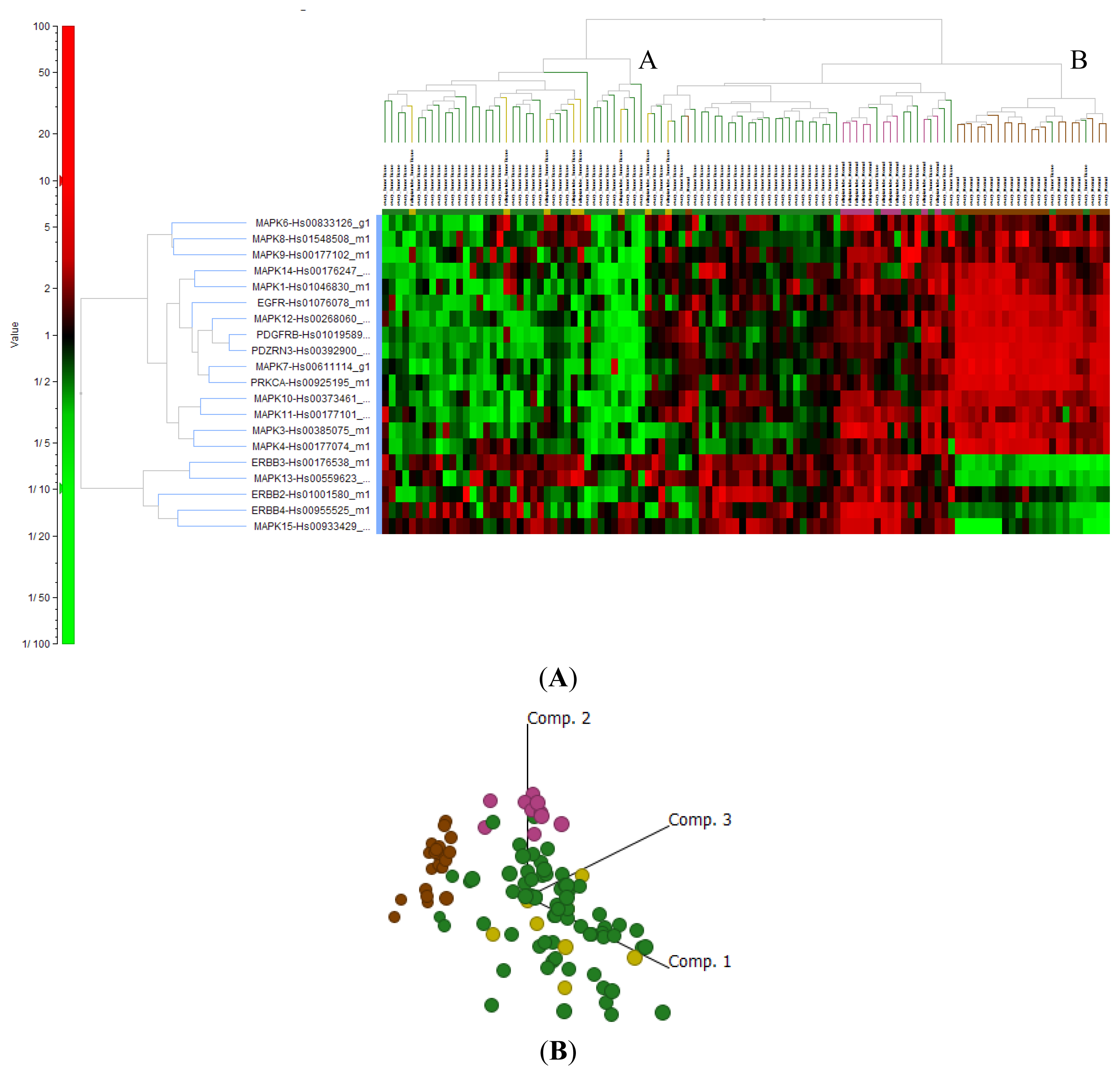
2.4. Cluster Analysis Reveals Strong Similarities between EOC and Fallopian Tube (FN)
2.4.1. Folate Transport and Regulation
2.4.2. One-Carbon Cycle Metabolism, DNA Replication/Repair
2.4.3. Known Markers of Ovarian Cancer
2.4.4. Signal Transduction Genes
2.4.5. Steroid Receptors
3. Experimental Section
3.1. Sample Description
3.2. RNA Isolation Method and Gene Expression Profiling Using Affymetrix Human Exon Array ST 2.0
3.3. Gene Expression Profiling Using Custom Taqman Low Density Array
4. Conclusions
Supplementary Information
ijms-14-13687-s001.pdfAcknowledgments
Conflict of Interest
References
- Siegel, R.; Naishadham, D.; Jemal, A. Cancer statistics, 2012. CA 2012, 62, 10–29. [Google Scholar]
- Fleming, J.S.; Beaugie, C.R.; Haviv, I.; Chenevix-Trench, G.; Tan, O.L. Incessant ovulation, inflammation and epithelial ovarian carcinogenesis: Revisiting old hypotheses. Mol. Cell Endocrinol 2006, 247, 4–21. [Google Scholar]
- Cramer, D.W. The epidemiology of endometrial and ovarian cancer. Hematol. Oncol. Clin. N. Am 2012, 26, 1–12. [Google Scholar]
- Sarojini, S.; Tamir, A.; Lim, H.; Li, S.; Zhang, S.; Goy, A.; Pecora, A.; Suh, K.S. Early detection biomarkers for ovarian cancer. J. Oncol. 2012. [Google Scholar] [CrossRef]
- Davidson, B. The diagnostic and molecular characteristics of malignant mesothelioma and ovarian/peritoneal serous carcinoma. Cytopathology 2011, 22, 5–21. [Google Scholar]
- Kalli, K.R.; Oberg, A.L.; Keeney, G.L.; Christianson, T.J.; Low, P.S.; Knutson, K.L.; Hartmann, L.C. Folate receptor alpha as a tumor target in epithelial ovarian cancer. Gynecol. Oncol 2008, 108, 619–626. [Google Scholar]
- Kelemen, L.E. The role of folate receptor alpha in cancer development, progression and treatment: Cause, consequence or innocent bystander? Int. J. Cancer 2006, 119, 243–250. [Google Scholar]
- Spannuth, W.A.; Sood, A.K.; Coleman, R.L. Farletuzumab in epithelial ovarian carcinoma. Expert Opin. Biol. Ther 2010, 10, 431–437. [Google Scholar]
- Kelemen, L.E.; Sellers, T.A.; Vierkant, R.A.; Harnack, L.; Cerhan, J.R. Association of folate and alcohol with risk of ovarian cancer in a prospective study of postmenopausal women. Cancer Causes Control 2004, 15, 1085–1093. [Google Scholar]
- Kelemen, L.E.; Sellers, T.A.; Schildkraut, J.M.; Cunningham, J.M.; Vierkant, R.A.; Pankratz, V.S.; Fredericksen, Z.S.; Gadre, M.K.; Rider, D.N.; Liebow, M.; et al. Genetic variation in the one-carbon transfer pathway and ovarian cancer risk. Cancer Res 2008, 68, 2498–2506. [Google Scholar]
- Liu, J.J.; Ward, R.L. Folate and one-carbon metabolism and its impact on aberrant DNA methylation in cancer. Adv. Genet 2010, 71, 79–121. [Google Scholar]
- Kotsopoulos, J.; Hecht, J.L.; Marotti, J.D.; Kelemen, L.E.; Tworoger, S.S. Relationship between dietary and supplemental intake of folate, methionine, vitamin B6 and folate receptor alpha expression in ovarian tumors. Int. J. Cancer 2010, 126, 2191–2198. [Google Scholar]
- Feeley, K.M.; Wells, M. Precursor lesions of ovarian epithelial malignancy. Histopathology 2001, 38, 87–95. [Google Scholar]
- Kim, J.; Coffey, D.M.; Creighton, C.J.; Yu, Z.; Hawkins, S.M.; Matzuk, M.M. High-grade serous ovarian cancer arises from fallopian tube in a mouse model. Proc. Natl. Acad. Sci. USA 2012, 109, 3921–3926. [Google Scholar]
- Kurman, R.J.; Shih, I. The origin and pathogenesis of epithelial ovarian cancer: A proposed unifying theory. Am. J. Surg. Pathol 2010, 34, 433–443. [Google Scholar]
- Collins, I.M.; Domchek, S.M.; Huntsman, D.G.; Mitchell, G. The tubal hypothesis of ovarian cancer: Caution needed. Lancet Oncol 2011, 12, 1089–1091. [Google Scholar]
- Li, J.; Fadare, O.; Xiang, L.; Kong, B.; Zheng, W. Ovarian serous carcinoma: Recent concepts on its origin and carcinogenesis. J. Hematol. Oncol. 2012, 5. [Google Scholar] [CrossRef]
- Fadare, O.; Zheng, W. Insights into endometrial serous carcinogenesis and progression. Int. J. Clin. Exp. Pathol 2009, 2, 411–432. [Google Scholar]
- Piek, J.M.; Kenemans, P.; Verheijen, R.H. Intraperitoneal serous adenocarcinoma: A critical appraisal of three hypotheses on its cause. Am. J. Obstet. Gynecol 2004, 191, 718–732. [Google Scholar]
- O’Shannessy, D.J.; Somers, E.B.; Smale, R.; Fu, Y.S. Expression of folate receptor-α (FRA) in gynecologic malignancies and its relationship to the tumor type. Int. J. Gynecol. Pathol 2013, 32, 258–268. [Google Scholar]
- Gubbels, J.A.; Belisle, J.; Onda, M.; Rancourt, C.; Migneault, M.; Ho, M.; Bera, T.K.; Connor, J.; Sathyanarayana, B.K.; Lee, B.; et al. Mesothelin-MUC16 binding is a high affinity, N-glycan dependent interaction that facilitates peritoneal metastasis of ovarian tumors. Mol. Cancer 2006, 5. [Google Scholar] [CrossRef]
- Hassan, R.; Ebel, W.; Routhier, E.L.; Patel, R.; Kline, J.B.; Zhang, J.; Chao, Q.; Jacob, S.; Turchin, H.; Gibbs, L.; et al. Preclinical evaluation of MORAb-009, a chimeric antibody targeting tumor-associated mesothelin. Cancer Immun 2007, 7, 20. [Google Scholar]
- Hellstrom, I.; Hellstrom, K.E. Two novel biomarkers, mesothelin and HE4, for diagnosis of ovarian carcinoma. Expert Opin. Med. Diagn 2011, 5, 227–240. [Google Scholar]
- Schummer, M.; Drescher, C.; Forrest, R.; Gough, S.; Thorpe, J.; Hellstrom, I.; Hellstrom, K.E.; Urban, N. Evaluation of ovarian cancer remission markers HE4, MMP7 and Mesothelin by comparison to the established marker CA125. Gynecol. Oncol 2012, 125, 65–69. [Google Scholar]
- Rump, A.; Marikawa, Y.; Tanaka, M.; Minami, S.; Umesaki, N.; Takeuchi, M.; Miyajima, A. Binding of ovanian cancer antigen CA125/MUC16 to mesothelin mediates cell adhesion. J. Biol. Chem 2004, 279, 9190–9198. [Google Scholar]
- Anastasi, E.; Marchei, G.G.; Viggiani, V.; Gennarini, G.; Frati, L.; Reale, M.G. HE4: A new potential early biomarker for the recurrence of ovarian cancer. Tumour Biol 2010, 31, 113–119. [Google Scholar]
- Hellstrom, I.; Raycraft, J.; Hayden-Ledbetter, M.; Ledbetter, J.A.; Schummer, M.; McIntosh, M.; Drescher, C.; Urban, N.; Hellstrom, K.E. The HE4 (WFDC2) protein is a biomarker for ovarian carcinoma. Cancer Res 2003, 63, 3695–3700. [Google Scholar]
- Drapkin, R.; von Horsten, H.H.; Lin, Y.; Mok, S.C.; Crum, C.P.; Welch, W.R.; Hecht, J.L. Human epididymis protein 4 (HE4) is a secreted glycoprotein that is overexpressed by serous and endometrioid ovarian carcinomas. Cancer Res 2005, 65, 2162–2169. [Google Scholar]
- Spizzo, G.; Fong, D.; Wurm, M.; Ensinger, C.; Obrist, P.; Hofer, C.; Mazzoleni, G.; Gastl, G.; Went, P. EpCAM expression in primary tumour tissues and metastases: An immunohistochemical analysis. J. Clin. Pathol 2011, 64, 415–420. [Google Scholar]
- Chandran, U.R.; Dhir, R.; Ma, C.; Michalopoulos, G.; Becich, M.; Gilbertson, J. Differences in gene expression in prostate cancer, normal appearing prostate tissue adjacent to cancer and prostate tissue from cancer free organ donors. BMC Cancer 2005, 5. [Google Scholar] [CrossRef] [Green Version]
- Shen, F.; Wu, M.; Ross, J.F.; Miller, D.; Ratnam, M. Folate receptor type gamma is primarily a secretory protein due to lack of an efficient signal for glycosyl-phosphatidylinositol modification: Protein characterization and cell type specificity. Biochemistry 1995, 34, 5660–5665. [Google Scholar]
- Friso, S.; Choi, S.W. Gene-nutrient interactions and DNA methylation. J. Nutr 2002, 132, 2382S–2387S. [Google Scholar]
- Jacob, R.A. Folate, DNA methylation, and gene expression: Factors of nature and nurture. Am. J. Clin. Nutr. 2000, 72, 903–904. [Google Scholar]
- Stover, P.J. Polymorphisms in 1-carbon metabolism, epigenetics and folate-related pathologies. J. Nutrigenet. Nutrigenomics 2011, 4, 293–305. [Google Scholar]
- Laury, A.R.; Perets, R.; Piao, H.; Krane, J.F.; Barletta, J.A.; French, C.; Chirieac, L.R.; Lis, R.; Loda, M.; Hornick, J.L.; et al. A comprehensive analysis of PAX8 expression in human epithelial tumors. Am. J. Surg. Pathol 2011, 35, 816–826. [Google Scholar]
- Laury, A.R.; Hornick, J.L.; Perets, R.; Krane, J.F.; Corson, J.; Drapkin, R.; Hirsch, M.S. PAX8 reliably distinguishes ovarian serous tumors from malignant mesothelioma. Am. J. Surg. Pathol 2010, 34, 627–635. [Google Scholar]
- Ji, H.; Isacson, C.; Seidman, J.D.; Kurman, R.J.; Ronnett, B.M. Cytokeratins 7 and 20, Dpc4, and MUC5AC in the distinction of metastatic mucinous carcinomas in the ovary from primary ovarian mucinous tumors: Dpc4 assists in identifying metastatic pancreatic carcinomas. Int. J. Gynecol. Pathol 2002, 21, 391–400. [Google Scholar]
- Nayak, S.B.; Bhat, V.R.; Mayya, S.S. Serum copper, ceruloplasmin and thiobarbituric acid reactive substance status in patients with ovarian cancer. Indian J. Physiol. Pharmacol 2004, 48, 486–488. [Google Scholar]
- Shyian, M.; Gryshkova, V.; Kostianets, O.; Gorshkov, V.; Gogolev, Y.; Goncharuk, I.; Nespryadko, S.; Vorobjova, L.; Filonenko, V.; Kiyamova, R. Quantitative analysis of SLC34A2 expression in different types of ovarian tumors. Exp. Oncol 2011, 33, 94–98. [Google Scholar]
- Sawasaki, T.; Shigemasa, K.; Gu, L.; Beard, J.B.; O’Brien, T.J. The transmembrane protease serine (TMPRSS3/TADG-12) D variant: A potential candidate for diagnosis and therapeutic intervention in ovarian cancer. Tumour Biol 2004, 25, 141–148. [Google Scholar]
- Scoccia, B.; Lee, Y.M.; Niederberger, C.; Ilekis, J.V. Expression of the ErbB family of receptors in ovarian cancer. J. Soc. Gynecol. Investig 1998, 5, 161–165. [Google Scholar]
- Sivakumaran, S.; Zhang, J.; Kelley, K.M.; Gonit, M.; Hao, H.; Ratnam, M. Androgen activation of the folate receptor α gene through partial tethering of the androgen receptor by C/EBPα. J. Steroid Biochem. Mol. Biol 2010, 122, 333–340. [Google Scholar]
- Kelley, K.M.; Rowan, B.G.; Ratnam, M. Modulation of the folate receptor alpha gene by the estrogen receptor: Mechanism and implications in tumor targeting. Cancer Res 2003, 63, 2820–2828. [Google Scholar]
- Butler, M.S.; Ricciardelli, C.; Tilley, W.D.; Hickey, T.E. Androgen receptor protein levels are significantly reduced in serous ovarian carcinomas compared with benign or borderline disease but are not altered by cancer stage or metastatic progression. Horm. Cancer 2013, 4, 154–164. [Google Scholar]
- Lau, K.-M.; Mok, S.C.; Ho, S.-M. Expression of human estrogen receptor-α and -β, progesterone receptor, and androgen receptor mRNA in normal and malignant ovarian epithelial cells. Proc. Natl. Acad. Sci. USA 1999, 96, 5722–5727. [Google Scholar]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) method. Methods 2001, 25, 402–408. [Google Scholar]
- Eisen, M.B.; Spellman, P.T.; Brown, P.O.; Botstein, D. Cluster analysis and display of genome-wide expression patterns. Proc. Natl. Acad. Sci. USA 1998, 95, 14863–14868. [Google Scholar]
- Yeung, K.Y.; Ruzzo, W.L. Principal component analysis for clustering gene expression data. Bioinformatics 2001, 17, 763–774. [Google Scholar]

| Gene Categories | Sample Size |
|---|---|
| Folate Transporters/Receptors | 6 |
| One-Carbon Metabolism and folate homeostasis | 19 |
| Known Markers of Ovarian Cancer | 13 |
| Signal Transduction | 19 |
| Steroid Receptors | 3 |
| Miscellaneous Genes | 30 |
| Endogenous Controls | 6 |
| Total | 96 |
| Variable | Normal ovary, N (%) | Epithelial ovarian cancer, N (%) | Fallopian tube normal, N (%) | Fallopian tube tumor, N (%) |
|---|---|---|---|---|
| Tumor histology | ||||
| Normal | 22 | 9 | ||
| Normal Adjacent Tissue | 30 | NA | ||
| Serous Adenocarcinoma | 68 | 8 | ||
| Age (Mean, SD) | 62.5, 12.3 | 57.7, 10.3 | 52.4, 12.6 | 60.1, 4.9 |
| Race | ||||
| White/caucasian | 51 | 68 | 1 | 8 |
| Black/African American | 1 | 0 | 1 | 0 |
| Native American/Alaskan | 0 | 0 | 0 | 0 |
| Unspecified | 0 | 0 | 7 | 0 |
| Tumor grade1,2 | ||||
| Grade 1 | 17 (25.0) | 1 (12.5) | ||
| Grade 2 | 18 (26.4) | 0 (0) | ||
| Grade 3 | 23 (33.8) | 7 (87.5) | ||
| Unspecified | 10 (14.7) | 0 (0) | ||
| Tumor stage1,3 | ||||
| Stage I | 19 (27.9) | 3 (17.6) | ||
| Stage II | 6 (11.7) | 1 (5.9) | ||
| Stage III | 27 (39.7) | 4 (23.5) | ||
| Stage IV | 8 (11.8) | 0 (0) | ||
© 2013 by the authors; licensee MDPI, Basel, Switzerland This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
O'Shannessy, D.J.; Jackson, S.M.; Twine, N.C.; Hoffman, B.E.; Dezso, Z.; Agoulnik, S.I.; Somers, E.B. Gene Expression Analyses Support Fallopian Tube Epithelium as the Cell of Origin of Epithelial Ovarian Cancer. Int. J. Mol. Sci. 2013, 14, 13687-13703. https://doi.org/10.3390/ijms140713687
O'Shannessy DJ, Jackson SM, Twine NC, Hoffman BE, Dezso Z, Agoulnik SI, Somers EB. Gene Expression Analyses Support Fallopian Tube Epithelium as the Cell of Origin of Epithelial Ovarian Cancer. International Journal of Molecular Sciences. 2013; 14(7):13687-13703. https://doi.org/10.3390/ijms140713687
Chicago/Turabian StyleO'Shannessy, Daniel J., Stephen M. Jackson, Natalie C. Twine, Bryan E. Hoffman, Zoltan Dezso, Sergei I. Agoulnik, and Elizabeth B. Somers. 2013. "Gene Expression Analyses Support Fallopian Tube Epithelium as the Cell of Origin of Epithelial Ovarian Cancer" International Journal of Molecular Sciences 14, no. 7: 13687-13703. https://doi.org/10.3390/ijms140713687
APA StyleO'Shannessy, D. J., Jackson, S. M., Twine, N. C., Hoffman, B. E., Dezso, Z., Agoulnik, S. I., & Somers, E. B. (2013). Gene Expression Analyses Support Fallopian Tube Epithelium as the Cell of Origin of Epithelial Ovarian Cancer. International Journal of Molecular Sciences, 14(7), 13687-13703. https://doi.org/10.3390/ijms140713687




