Competing Intramolecular vs. Intermolecular Hydrogen Bonds in Solution
Abstract
:1. Introduction


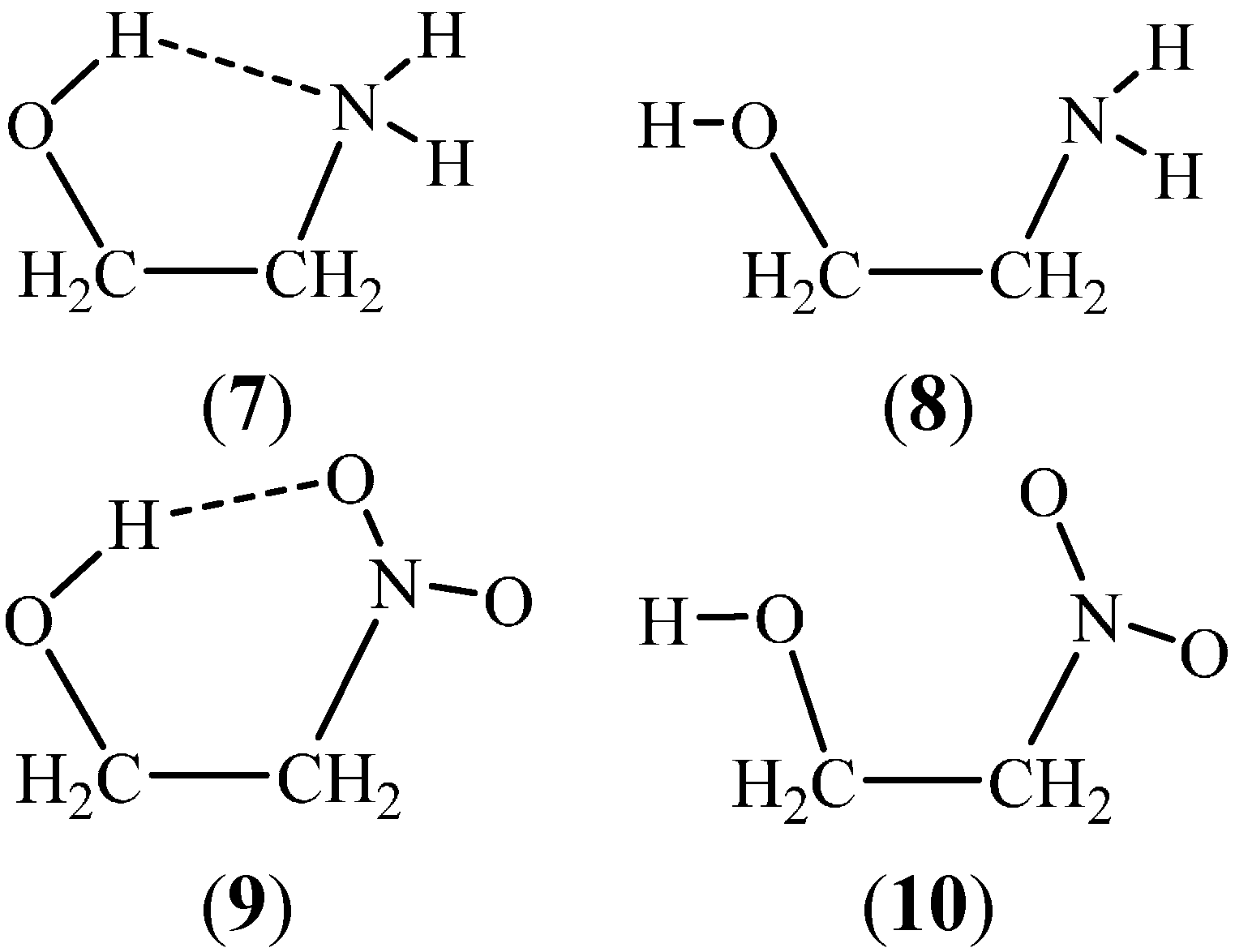
2. Methodology
2.1. Experimental Methods
2.2. Geometry Optimization
2.2.1. General Problems
2.2.2. Special Problems
2.3. Free Energy Calculations

2.4. Dimeric Solutes

3. Conformational Equilibria
3.1. 3-Member and 4-Member Rings
3.2. 5-Member Rings
3.2.1. 1,2-Disubstituted Ethanes and Derivatives

| Structures | Theor. Calc. | Gas-Phase | Aqueous Solution | |||||
|---|---|---|---|---|---|---|---|---|
| Theor. Ref. a | Exp. Ref. b | Eint c | ZPE/Gth d | Cont. Solv. e | MC/FEP f | MD/FEP g | Exp. Ref. | |
| 1,2-Ethanediol | [137,138,139,140] | [141,142,144] | ||||||
| [42,43] | MP2/6-31G* | + h | OPLS | |||||
| [44] | MP2/cc-pvtz + CCSD(T) corr. | + | SMx | |||||
| [143] | GROMOS | |||||||
| [145] | Amber | |||||||
| 2-Aminoethanol neutral | [154] | [158,159] | ||||||
| [64] | IEFPCM/CBS | + | IEFPCM | OPLS | ||||
| [145] | Amber | |||||||
| [155] | Amber | |||||||
| [156] | own FF i | |||||||
| Protonated | [158,159] | |||||||
| [89] | IEFPCM/CBS | + | IEFPCM | OPLS | ||||
| Ethylenediamine neutral | [160] | [158] | ||||||
| [145] | Amber | |||||||
| Monocation | [89] | IEFPCM/B97D | + | IEFPCM | OPLS | [158] | ||
| aug-cc-pvtz | ||||||||
| Dication ethylenedime | ||||||||
| [161] | OPLS | [158] | ||||||
3.2.2. α-Substituted Carboxylic Acids

3.2.3. Ortho Phenols and Naphthols
3.3. 6-Member Rings
3.3.1. β-Substituted Ethylamines

| Structures | Theor. Calc. | Gas-Phase | Aqueous Solution | |||||
|---|---|---|---|---|---|---|---|---|
| Theor. Ref. | Exp. Ref. | Eint | ZPE/Gth | Cont. Solv. | MC/FEP | MD/FEP | Exp. Ref. | |
| Histamine neutral | [194] | |||||||
| [196] | MP2/6-31G | SCRF | [197] | |||||
| [198] | MP2/6-311++G** | + | OPLS | |||||
| [199] | B3LYP/6-311G** | SCRF | ||||||
| [200] | HF/6-31G* | PCM | ||||||
| [201] | MP2/augccpvtz | + | MST b | |||||
| Protonated | [195] | |||||||
| [196] | MP2/6-31G | SCRF | [197] | |||||
| [198] | MP2/6-311++G** | + | OPLS | |||||
| [200] | HF/6-31G* | PCM | ||||||
| [202] | HF/6-31G* | Amber | ||||||
| Tyramine neutral | [203] | |||||||
| [123,204] | MP2/6-31G* | PCM | OPLS | |||||
| Zwitterion | [123,204] | B3LYP/6-311++G** | PCM | OPLS | [204] | |||
| Dopamine neutral | [205] | |||||||
| [206] | AM1 | SM1 c | [204,207] | |||||
| [123] | B3LYP/6-311++G** | PCM | OPLS | |||||
| Dopamine zwitterion | ||||||||
| [123,204] | B3LYP/6-311++G** | PCM | OPLS | [204] | ||||
| Protonated | [208] | |||||||
| [208] | AM1 | SM1 | [204,207] | |||||
| [209] | HF/6-31G* | PCM | OPLS | |||||
| Anionic | [206] | AM1 | SM1 | [207] | ||||
| Norepinephrine neutral | [210] | |||||||
| [211] | MP2/6-31G* | PCM | [207] | |||||
| Protonated | [192] | MP2/6-31G* | + | OPLS | [192,207] | |||
| [211] | MP2/6-31G* | PCM | ||||||
| Epinephrine neutral | [212] | |||||||
| Protonated | [213] | B3LYP/6-311++G** | + | IEFPCM d | [204,207] | |||
| Serotonin neutral | [214] | [204] | ||||||
| Protonated | [215] | |||||||
| [216] | MP2/6-31G* | + | IEFPCM | OPLS | [192,204] | |||
| [217] | MP2/6-31G* | + | IEFPCM | |||||
3.3.2. β-OH Carboxylic Acids
3.3.3. β-NH2 Carboxylic Acids
3.3.4. Cyclic Enols
3.4. 7-Member Rings
γ-OH and γ-NH2 Carboxylic Acids
3.5. Acid-Base Complexes
4. Concluding Remarks
Acknowledgments
Conflicts of Interest
References
- Arunan, E.; Desiraju, G.R.; Klein, R.A.; Sadlej, J.; Scheiner, S.; Alkorta, I.; Clary, D.C.; Crabtree, R.H.; Dannenberg, J.J.; Hobza, P.; et al. Definition of the hydrogen bond (IUPAC Recommendations 2011). Pure Appl. Chem. 2011, 83, 1637–1641. [Google Scholar]
- Grabowski, S.J. Theoretical studies of strong hydrogen bonds. Annu. Rep. Prog. Chem. 2006, 102, 131–165. [Google Scholar] [CrossRef]
- Bader, R.F.W. Atoms in Molecules: A Quantum Theory; Oxford University Press: New York, NY, USA, 1990. [Google Scholar]
- Popelier, P.L.A. Atoms in Molecules: An Introduction; Prentice Hall: Harlow, UK, 1999. [Google Scholar]
- Koch, U.; Popelier, P.L.A. Characterization of C–H–O hydrogen bonds on the basis of the charge density. J. Phys. Chem. 1995, 99, 9747–9754. [Google Scholar] [CrossRef]
- Klein, R.A. Ab initio conformational studies on diols and binary diol-water systems using DFT methods. Intramolecular hydrogen bonding and 1:1 complex formation with water. J. Comput. Chem. 2002, 23, 585–599. [Google Scholar]
- Mandado, M.; Graňa, A.M.; Mosquera, R.A. Do 1,2-ethanediol and 1,2-dihydroxybenzene present intramolecular hydrogen bond? Phys. Chem. Chem. Phys. 2004, 6, 4391–4396. [Google Scholar] [CrossRef]
- Vinogradov, S.N.; Linnell, R.H. Hydrogen Bonding; Van Nostrand Reinhold: New York, NY, USA, 1971. [Google Scholar]
- Bondi, A. Van der Waals volumes and radii. J. Phys. Chem. 1964, 68, 441–451. [Google Scholar] [CrossRef]
- Weinhold, F.; Klein, R.A. What is a hydrogen bond? Mutually consistent theoretical and experimental criteria for characterizing H-bonding interactions. Mol. Phys. 2012, 110, 565–579. [Google Scholar] [CrossRef]
- Contreras-García, J.; Yang, W.; Johnson, E.R. Analysis of hydrogen-bond interaction potentials from the electron density: Integration of noncovalent interaction regions. J. Phys. Chem. A 2011, 115, 12983–12990. [Google Scholar] [CrossRef] [PubMed]
- Carney, J.R.; Dian, B.C.; Florio, G.M.; Zwier, T.S. The role of water bridges in directing the conformational preferences of 3-indole-propionic acid and tryptamine. J. Am. Chem. Soc. 2001, 123, 5596–5597. [Google Scholar] [CrossRef] [PubMed]
- Borho, N.; Suhm, M.A.; le Barbu-Debus, K.; Zehnacker, A. Intra- vs. intermolecular hydrogen bonding: Dimers of α-hydroxyesters with methanol. Phys. Chem. Chem. Phys. 2006, 8, 4449–4460. [Google Scholar] [CrossRef] [PubMed]
- Le Barbu-Debus, K.; Guchhait, N.; Zehnacker-Rentien, A. Electronic and infrared spectroscopy of jet-cooled (±)-cis-1-amino-indan-2-ol hydrates. Phys. Chem. Chem. Phys. 2007, 9, 4465–4471. [Google Scholar] [CrossRef] [PubMed]
- Nagy, P.I. Are the intramolecular O–H…F and O–H…Cl hydrogen bonds maintained in solution? A theoretical study. J. Phys. Chem. A 2013, 117, 2812–2826. [Google Scholar] [CrossRef] [PubMed]
- Senent, M.L.; Niño, A.; Muñoz-Caro, C.; Smeyers, Y.G.; Domínguez-Gómez, R.; Orza, J.M. Theoretical study of the effect of hydrogen-bonding on the stability and vibrational spectrum of isolated 2,2,2-trifluoroethanol and its molecular complexes. J. Phys. Chem. A 2002, 106, 10673–10680. [Google Scholar] [CrossRef]
- Silverstein, R.M.; Webster, F.X.; Kiemle, D.J. Spectrometric Identification of Organic Compounds, 7th ed.; John Wiley and Sons, Inc.: New York, NY, USA, 2005. [Google Scholar]
- Hobza, P.; Havlas, Z. Blue-shifting in hydrogen bonds. Chem. Rev. 2000, 100, 4253–4264. [Google Scholar] [CrossRef] [PubMed]
- Reed, A.E.; Curtiss, L.A.; Weinhold, F. Intermolecular interactions from a natural bond orbital, donor-acceptor viewpoint. Chem. Rev. 1988, 88, 899–926. [Google Scholar] [CrossRef]
- Jorgensen, W.L.; Chandrasekhar, J.; Madura, J.D.; Impey, R.W.; Klein, M.L. Comparison of simple potential functions for simulating liquid water. J. Chem. Phys. 1983, 79, 926–935. [Google Scholar] [CrossRef]
- Nagy, P.I.; Dunn, W.J., III; Nicholas, J.B. Investigations on the convergence rate of the thermodynamic parameters from Monte Carlo simulations of aqueous solutions of methanol and methylamine. J. Chem. Phys. 1989, 91, 3707–3715. [Google Scholar] [CrossRef]
- Nagy, P.I. Theoretical calculations on the conformational/tautomeric equilibria for small molecules in solution. Biochem. Pharmacol. 2013. [Google Scholar] [CrossRef]
- Zwier, T.S. Laser spectroscopy of jet-cooled biomolecules and their water-containing clusters: Water bridges and molecular conformation. J. Phys. Chem. A 2001, 105, 8827–8839. [Google Scholar] [CrossRef]
- Marstokk, K.-M.; Møllendal, H. Microwave spectrum, conformation, intramolecular hydrogen bonding and ab initio calculations for ethylene glycol vinyl ether. Acta Chem. Scand. 1995, 49, 728–733. [Google Scholar] [CrossRef]
- Marstokk, K.-M.; Møllendal, H. Microwave spectrum, conformation, intramolecular hydrogen bonding and ab initio calculations for 2-nitroethanol. Acta Chem. Scand. 1996, 50, 505–511. [Google Scholar] [CrossRef]
- Møllendal, H.; Samdal, S.; Guillemin, J.-C. Microwave spectrum and intramolecular hydrogen bonding of 2-isocyanoethanol (HOCH2CH2N≡C). J. Phys. Chem. A 2014, 118, 3120–3127. [Google Scholar] [CrossRef] [PubMed]
- Dyllick-Brenzinger, C.E.; Bauder, A.; Günthard, H.-H. The substitution structure, barrier to internal rotation and low frequency vibrations of pyruvic acid. Chem. Phys. 1977, 23, 195–206. [Google Scholar] [CrossRef]
- Derissen, J.L. A reinvestigation of the molecular structure of acetic acid monomer and dimer by gas electron diffraction. J. Mol. Struct. 1971, 7, 67–80. [Google Scholar] [CrossRef]
- Huang, J.; Hedberg, K. Conformational analysis. 13. 2-Fluoroethanol. An investigation of the molecular structure and conformational composition at 20, 156, and 240 degree. Estimate of the anti-gauche energy difference. J. Am. Chem. Soc. 1989, 111, 6909–6913. [Google Scholar] [CrossRef]
- Kazerouni, M.R.; Hedberg, L.; Hedberg, K. Conformational analysis. 21. Ethane-1,2-diol. An electron-diffraction investigation, augmented by rotational constants and ab initio calculations, of the molecular structure, conformational composition, SQM vibrational force field, and anti-gauche energy difference with implications for internal hydrogen bonding. J. Am. Chem. Soc. 1997, 119, 8324–8331. [Google Scholar] [CrossRef]
- Belova, N.V.; Oberhammer, H.; Trang, N.H.; Girichev, G.V. Tautomeric properties and gas-phase structure of acetylacetone. J. Org. Chem. 2014, 79, 5412–5419. [Google Scholar] [CrossRef] [PubMed]
- Nagy, P.I.; Maheshwari, A.; Kim, Y.W.; Messer, W.J., Jr. Theoretical and experimental studies of the isomeric protonation in solution for a prototype aliphatic ring containing two nitrogens. J. Phys. Chem. B 2010, 114, 349–360. [Google Scholar] [CrossRef] [PubMed]
- Grimme, S. Semiempirical GGA-type density functional constructed with a long-range dispersion correction. J. Comput. Chem. 2006, 27, 1787–1799. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Truhlar, D.G. Applications and validations of the Minnesota density functionals. Chem. Phys. Lett. 2011, 502, 1–13. [Google Scholar] [CrossRef]
- Peterson, K.A. Gaussian basis sets exhibiting systematic convergence to the complete basis set limit. Annu. Rep. Comput. Chem. 2007, 3, 195–206. [Google Scholar]
- Purvis, G.D.; Bartlett, R.J. A full coupled-cluster singles and doubles model: the inclusion of the disconnected triples. J. Chem. Phys. 1982, 76, 1910–1918. [Google Scholar] [CrossRef]
- Raghavachari, K.; Trucks, G.W.; Pople, J.A.; Head-Gordon, M. A fifth-order perturbation comparison of electron correlation theory. Chem. Phys. Lett. 1989, 157, 479–483. [Google Scholar] [CrossRef]
- Hobza, P. Theoretical studies of hydrogen bonding. Annu. Rep. Prog. Chem. Sect. C 2004, 100, 3–27. [Google Scholar] [CrossRef]
- McQuarrie, D.A. Statistical Mechanics; University Science Books: Sausalito, CA, USA, 2000. [Google Scholar]
- De Prince, A.E., III; Mazziotti, D.A. Isomerization of nitrosomethane to formaldoxime: Energies, geometries, and frequencies from the parametric variational two-electron reduced-density-matrix method. J. Chem. Phys. 2010, 133, 034112. [Google Scholar]
- Gu, Q.; Trindle, C.; Knee, J.L. Frequency shifts of an intramolecular hydrogen bond as a measure of intermolecular hydrogen bond strengths. J. Chem. Phys. 2012, 137, 091101. [Google Scholar] [CrossRef]
- Nagy, P.I.; Dunn, W.J., III; Alagona, G.; Ghio, C. Theoretical calculations on 1,2-ethanediol. Gauche-trans equilibrium in gas-phase and aqueous solution. J. Am. Chem. Soc. 1991, 113, 6719–6729. [Google Scholar]
- Nagy, P.I.; Dunn, W.J., III; Alagona, G.; Ghio, C. Theoretical calculations on 1,2-ethanediol. 2. Equilibrium of the gauche conformers with and without an intramolecular hydrogen bond in aqueous solution. J. Am. Chem. Soc. 1992, 114, 4752–4758. [Google Scholar]
- Cramer, C.J.; Truhlar, D.G. Quantum chemical conformational analysis of 1,2-ethanediol: Correlation and solvation effects on the tendency to form internal hydrogen bonds in the gas phase and in aqueous solution. J. Am. Chem. Soc. 1994, 116, 3892–3900. [Google Scholar] [CrossRef]
- Nagy, P.I.; Dunn, W.J, III; Alagona, G.; Ghio, C. Theoretical studies of the 2- and 4-hydroxybenzoic acids with competing hydrogen bonds in the gas phase and aqueous solution. J. Phys. Chem. 1993, 97, 4628–4642. [Google Scholar]
- Florio, G.M.; Zwier, T.S.; Myshakin, E.M.; Jordan, K.D.; Sibert, E.L., III. Theoretical modeling of the OH stretch infrared spectrum of carboxylic acid dimers based on first-principles anharmonic couplings. J. Chem. Phys. 2003, 118, 1735–1746. [Google Scholar] [CrossRef]
- Chen, H.-Y.; Cheng, Y.-L.; Takahashi, K. Theoretical calculation of the OH vibrational overtone spectra of 1,5-pentanediol and 1,6-hexanediol. J. Phys. Chem. A 2011, 115, 14315–14324. [Google Scholar] [CrossRef] [PubMed]
- Nagy, P.I.; Flock, M.; Ramek, M. Theoretical studies on the conformational equilibria of the γ-hydroxybutyric acid analysis in the gas phase and in solution. J. Phys. Chem. A 1997, 101, 5942–5948. [Google Scholar] [CrossRef]
- Suenram, R.D.; Pajski, J.J.; Neill, J.L.; Pate, B.H.; Tubergen, M.J. Rotational Studies in the Hydroxybutiric System; Ohio State University: Columbus, OH, USA, 2007. [Google Scholar]
- Blanco, S.; López, J.C.; Mata, S.; Alonso, J.L. Conformations of γ-aminobutyric aid (GABA): The role of the n→π* interaction. Angew. Chem. Int. Ed. 2010, 49, 9187–9192. [Google Scholar] [CrossRef]
- Miertus, S.; Scrocco, E.; Tomasi, J. Electrostatic interaction of a solute with a continuum. A direct utilization of ab intio molecular potentials for the prevision of solvent effects. Chem. Phys. 1981, 55, 117–129. [Google Scholar]
- Tomasi, J.; Persico, M. Molecular interactions in solution. An overview of methods based on continuous distributions of the solvent. Chem. Rev. 1994, 94, 2027–2094. [Google Scholar] [CrossRef]
- Klamt, A.; Schüürmann, G. COSMO: A new approach to dielectric screening in solvents with explicit expressions for the screening energy and its gradient. J. Chem. Soc. Perkin Trans. 1993, 2, 799–805. [Google Scholar] [CrossRef]
- Klamt, A. Conductor-like screening model for real solvents: A new approach to the quantitative calculation of solvation phenomena. J. Phys. Chem. 1995, 99, 2224–2235. [Google Scholar] [CrossRef]
- Barone, V.; Cossi, M. Quantum calculation of molecular energies and energy gradients in solution by a conductor solvent model. J. Phys. Chem. 1998, 102, 1995–2001. [Google Scholar] [CrossRef]
- Cossi, M.; Rega, N.; Scalmani, G.; Barone, V. Energies, structures, and electric properties of molecules in solution with the C-PCM solvation model. J. Comput. Chem. 2003, 24, 669–681. [Google Scholar] [CrossRef] [PubMed]
- Soteras, I.; Curutchet, C.; Bidon-Chanel, A.; Orozco, M.; Luque, F.J. Extension of the MST model to the IEF formalism: HF and B3LYP parametrizations. J. Mol. Struct. Theochem 2005, 727, 29–40. [Google Scholar] [CrossRef]
- Cramer, C.J.; Truhlar, D.G. A universal approach to solvation modeling. Acc. Chem. Res. 2008, 41, 760–768. [Google Scholar] [CrossRef] [PubMed]
- Marenich, A.V.; Cramer, C.J.; Truhlar, D.G. Universal solvation model based on solute electron density and on a continuum model of the solvent defined by the bulk dielectric constant and atomic surface tensions. J. Phys. Chem. B 2009, 113, 6378–6396. [Google Scholar] [CrossRef] [PubMed]
- Klamt, A.; Jonas, V.; Bürger, T.; Lohrenz, J.C.W. Refinement and parametrization of COSMO-RS. J. Phys. Chem. A 1998, 102, 5074–5085. [Google Scholar] [CrossRef]
- Cramer, C.J.; Truhlar, D.G. Implicit solvation models: Equilibria, structure, spectra, and dynamics. Chem. Rev. 1999, 99, 2161–2200. [Google Scholar] [CrossRef] [PubMed]
- Orozco, M.; Luque, F.J. Theoretical methods for the description of the solvent effect in biomolecular systems. Chem. Rev. 2000, 100, 4187–4225. [Google Scholar] [CrossRef] [PubMed]
- Tomasi, J.; Mennucci, B.; Cammi, R. Quantum mechanical continuum solvation models. Chem. Rev. 2005, 105, 2999–3094. [Google Scholar] [CrossRef] [PubMed]
- Nagy, P.I. Theoretical studies of the solvent effect on the conformation of the HO–C–C–X (X = F, NH2, NO2) moiety with competing intra- and intermolecular hydrogen bonds. J. Phys. Chem. A 2012, 116, 7726–7741. [Google Scholar] [CrossRef] [PubMed]
- Wiberg, K.B.; Clifford, S.; Jorgensen, W.L.; Frisch, M.J. Origin of the inversion of the acidity order for haloacetic acids on going from the gas phase to solution. J. Phys. Chem. A 2000, 114, 7625–7628. [Google Scholar] [CrossRef]
- Nagy, P.I.; Alagona, G.; Ghio, C. Theoretical investigation of tautomeric equilibria for isonicotinic acid, 4-pyridone, and acetylacetone in vacuo and in solution. J. Chem. Theory Comput. 2007, 3, 1249–1266. [Google Scholar] [CrossRef]
- Snoek, L.C.; van Mourik, T.; ÇarÇabal, P.; Simons, J.P. Neurotransmitters in the gas phase: Hydrated noradrenaline. Phys. Chem. Chem. Phys. 2003, 5, 4518–4526. [Google Scholar] [CrossRef]
- Van Mourik, T. The shape of neurotransmitters in the gas phase: A theoretical study of adrenaline, pseudoadrenaline, and hydrated adrenaline. Phys. Chem. Chem. Phys. 2004, 6, 2827–2837. [Google Scholar] [CrossRef]
- LeGreve, T.A.; James, W.J., III; Zwier, T.S. Solvent effects on the conformational preferences of serotonin: Serotonin-(H2O)n, n = 1, 2. J. Phys. Chem. A 2009, 113, 399–410. [Google Scholar]
- Fricke, H.; Schwing, K.; Gerlach, A.; Unterberg, C.; Gerhards, M. Investigations of the water clusters of the protected amino acid Ac-Phe-OMe by applying IR/UV double resonance spectroscopy: Microsolvation of the backbone. Phys. Chem. Chem. Phys. 2010, 12, 3511–3521. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Sanz, A.A.; Cabaleiro-Lago, E.M.; Rodríguez-Otero, J. Cation…π interaction and microhydration effects in complexes formed by pyrrolidinium cation and aromatic species in amino acid side chains. Org. Biomol. Chem. 2014, 12, 2938–2949. [Google Scholar] [CrossRef] [PubMed]
- Gerhards, M.; Kleinermanns, K. Structure and vibrations of phenol (H2O)2. J. Chem. Phys. 1995, 103, 7392–7340. [Google Scholar] [CrossRef]
- Shukla, M.K.; Leszczynski, J. Interaction of water molecules with cytosine tautomers: An excited-state quantum chemical investigation. J. Phys. Chem. A 2002, 106, 11338–11346. [Google Scholar] [CrossRef]
- Rejnek, J.; Hanus, M.; Kabeláč, M.; Ryjáček, F.; Hobza, P. Correlated ab initio study of nucleic acid bases and their tautomers in the gas phase, in a microhydrated environment and in aqueous solution. Part 4. Uracil and thymine. Phys. Chem. Chem. Phys. 2005, 7, 2006–2017. [Google Scholar] [CrossRef] [PubMed]
- Rudić, S.; Xie, H.B.; Gerber, R.B.; Simons, J.P. Protonated sugars: Vibrational spectroscopy and conformational structure of protonated O-methyl α-d-galactopyranoside. Mol. Phys. 2012, 110, 1609–1615. [Google Scholar] [CrossRef]
- Crittenden, D.L.; Chebib, M.; Jordan, M.J.T. Stabilization of zwitterions in solution: γ-Aminobutyric acid (GABA). J. Phys. Chem. A 2004, 108, 203–211. [Google Scholar] [CrossRef]
- D’Cunha, C.; Morozov, A.N.; Chatfield, D.C. Theoretical study of HOCl-catalyzed keto-enol tautomerization of β-cyclopentanedione in an explicit water environment. J. Phys. Chem. A 2013, 117, 8437–8448. [Google Scholar] [CrossRef] [PubMed]
- Lu, B.-M.; Jin, X.-Y.; Tang, J.; Bi, S.-P. DFT studies of the Al–O Raman vibrational frequencies for aquated aluminimu species. J. Mol. Struct. 2010, 982, 9–15. [Google Scholar] [CrossRef]
- Nagy, P.I. The syn-anti equilibrium for the –COOH group reinvestigated. Theoretical conformation analysis for acetic acid in the gas phase and in solution. Comput. Theor. Chem. 2013, 1022, 59–69. [Google Scholar]
- Beveridge, D.L.; DiCapua, F.M. Free energy via molecular simulation: Applications to chemical and biomolecular systems. Annu. Rev. Biophys. Biophys. Chem. 1989, 18, 431–492. [Google Scholar] [CrossRef] [PubMed]
- Kollman, P.A. Free energy calculations: Applications to chemical and biochemical phenomena. Chem. Rev. 1993, 93, 2395–2417. [Google Scholar] [CrossRef]
- Metropolis, N.; Rosenbluth, A.W.; Rosenbluth, M.N.; Teller, A.H.; Teller, E. Equation of state calculations by fast computing machines. J. Chem. Phys. 1953, 21, 1087–1092. [Google Scholar] [CrossRef]
- Torrie, G.M.; Valleau, J.P. Nonphysical sampling distributions in Monte Carlo free-energy estimation: Umbrella sampling. J. Comput. Phys. 1977, 23, 187–199. [Google Scholar] [CrossRef]
- Owicki, J.C.; Scheraga, H.A. Preferential sampling near solutes in Monte Carlo calculations on dilute solutions. Chem. Phys. Lett. 1977, 47, 600–602. [Google Scholar] [CrossRef]
- Jorgensen, W.L.; Maxwell, D.S.; Tirado-Rives, J. Development and testing of the OPLS all-atom force field on conformational energetics and properties of organic liquids. J. Am. Chem. Soc. 1996, 118, 11225–11236. [Google Scholar] [CrossRef]
- Rizzo, R.C.; Jorgensen, W.L. OPLS all-atom model for amines: Resolution of the amine hydration problem. J. Am. Chem. Soc. 1999, 121, 4827–4836. [Google Scholar] [CrossRef]
- Case, D.A.; Babin, V.; Berryman, J.T.; Betz, R.M.; Cai, Q.; Cerutti, D.S.; Cheatham, T.E., III; Darden, T.A.; Duke, R.E.; Gohlke, H.; et al. AMBER 14. In Amber 14 Reference Manual; University of California: San Francisco, CA, USA, 2014. [Google Scholar]
- MacKerell, A.D., Jr.; Bashford, D.; Bellott, M.; Dunbrack, R.L., Jr.; Evanseck, J.D.; Field, M,J.; Fischer, S.; Gao, J.; Guo, H.; Ha, S.; et al. All-atom empirical potential for molecular modeling and dynamics studies of proteins. J. Phys. Chem. B 1998, 102, 3586–3616. [Google Scholar] [CrossRef] [PubMed]
- Nagy, P.I. Theoretical study of the gauche-trans equilibrium with and without an intramolecular hydrogen bond for +H3NCH2CH2X systems (X = OH, NH2, COO−) in solution. Phys. Chem. Chem. Phys. 2012, 14, 13955–13962. [Google Scholar] [CrossRef] [PubMed]
- Singh, U.C.; Kollman, P.A. An approach to computing electrostatic charges for molecules. J. Comput. Chem. 1984, 5, 129–145. [Google Scholar] [CrossRef]
- Bayly, C.I.; Cieplak, P.; Cornell, W.; Kollman, P.A. A well-behaved electrostatic potential based method using charge restraints for deriving atomic charges: The RESP model. J. Phys. Chem. 1993, 97, 10269–10280. [Google Scholar] [CrossRef]
- Breneman, C.M.; Wiberg, K.B. Determining atom-centered monopoles from molecular electrostatic potentials. The need for high sampling density in formamide conformational analysis. J. Comput. Chem. 1990, 11, 361–373. [Google Scholar] [CrossRef]
- Zwanzig, R.W. High-temperature equation of state by a perturbation method. I. Nonpolar gases. J. Chem. Phys. 1954, 22, 1420–1426. [Google Scholar] [CrossRef]
- Jorgensen, W.L.; Ravimohan, C. Monte Carlo simulation of differences in free energies of hydration. J. Chem. Phys. 1985, 83, 3050–3054. [Google Scholar] [CrossRef]
- Jorgensen, W.L.; Briggs, J.M.; Contreras, L.M. Relative partition coefficients for organic solutes from fluid simulations. J. Phys. Chem. A 1990, 94, 1683–1686. [Google Scholar] [CrossRef]
- Jorgensen, W.L. BOSS, Version 4.8; Biochemical and Organic Simulation System User’s Manual; Yale University: New Haven, CT, USA, 2007. [Google Scholar]
- Jorgensen, W.L.; Madura, J.D. Quantum and statistical mechanical studies of liquids. 25. Solvation and conformation of methanol in water. J. Am. Chem. Soc. 1983, 105, 1407–1413. [Google Scholar] [CrossRef]
- Jorgensen, W.L.; Swenson, C.J. Optimized intermolecular potential functions for amides and peptides. Hydration of amides. J. Am. Chem. Soc. 1985, 107, 1489–1496. [Google Scholar] [CrossRef]
- Jorgensen, W.L.; Gao, J. Monte Carlo simulations of the hydration of ammonium and carboxylate ions. J. Phys. Chem. 1986, 90, 2174–2182. [Google Scholar] [CrossRef]
- Rick, S.W.; Stuart, S.J.; Berne, B.J. Dynamical fluctuating charge force field: Application to liquid water. J. Chem. Phys. 1994, 101, 6141–6157. [Google Scholar] [CrossRef]
- Rick, S.W.; Berne, B.J. Dynamical fluctuating charge force fields: The aqueous solvation of amides. J. Am. Chem. Soc. 1996, 118, 672–679. [Google Scholar] [CrossRef]
- Patel, S.; Brooks, C.L., III. CHARMM fluctuating charge force field for proteins: I parameterization and application to bulk organic liquid simulations. J. Comput. Chem. 2004, 25, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Patel, S.; MacKerell, A.D., Jr.; Brooks, C.L., III. CHARMM fluctuating charge force field for proteins: II protein/solvent properties from molecular dynamics simulations using a nonadditive electrostatic model. J. Comput. Chem. 2004, 25, 1504–1514. [Google Scholar] [CrossRef] [PubMed]
- Ponder, J.W.; Wu, C.; Ren, P.; Pande, V.S.; Chodera, J.D.; Schnieders, M.J.; Haque, I.; Mobley, D.L.; Lambrecht, D.S.; DiStasio, R.A., Jr.; et al. Current status of the AMOEBA polarizable force field. J. Phys. Chem. B 2010, 114, 2549–2564. [Google Scholar] [CrossRef] [PubMed]
- Woods, C.J.; Manby, F.R.; Mulholland, A.J. An efficient method for the calculation of quantum mechanics/molecular mechanics free energies. J. Chem. Phys. 2008, 128, 014109. [Google Scholar] [CrossRef]
- Ranaghan, K.E.; Mulholland, A.J. Investigations of enzyme-catalysed reactions with combined quantum mechanics/molecular mechanics (QM/MM) methods. Int. Rev. Phys. Chem. 2010, 29, 65–133. [Google Scholar] [CrossRef]
- Car, R.; Parinello, M. Unified approach for molecular dynamics and density-functional theory. Phys. Rev. Lett. 1985, 55, 2471–2474. [Google Scholar] [CrossRef] [PubMed]
- Lerner, R.G.; Dailey, B.P.; Friend, J.P. Microwave spectrum and structure of formic acid. J. Chem. Phys. 1957, 26, 680–684. [Google Scholar] [CrossRef]
- Turi, L. Ab initio molecular orbital analysis of dimers of cis-formic acid. Implications for condensed phases. J. Phys. Chem. 1996, 100, 11285–11291. [Google Scholar] [CrossRef]
- Zielke, P.; Suhm, M.A. Raman jet spectroscopy of formic acid dimers: Low frequency vibrational dynamics and beyond. Phys. Chem. Chem. Phys. 2007, 9, 4528–4534. [Google Scholar] [CrossRef] [PubMed]
- Matylitsky, V.V.; Riehn, C.; Gelin, M.F.; Brutschy, B. The formic acid dimer (HCOOH)2 probed by time-resolved structure selective spectroscopy. J. Chem. Phys. 2003, 119, 10553–10562. [Google Scholar] [CrossRef]
- Balabin, R.M. Polar (acyclic) isomer of formic acid dimer: Gas-phase Raman spectroscopy study and thermodynamic parameters. J. Phys. Chem. A 2009, 113, 4910–4918. [Google Scholar] [CrossRef] [PubMed]
- Derissen, J.L. An investigation of the structure of propionic acid monomer and dimer by gas electron diffraction. J. Mol. Struct. 1971, 7, 81–88. [Google Scholar] [CrossRef]
- Emmeluth, C.; Suhm, M.A. A chemical approach towards the spectroscopy of carboxylic acid dimer isomerism. Phys. Chem. Chem. Phys. 2003, 5, 3094–3099. [Google Scholar] [CrossRef]
- Nagy, P.I.; Erhardt, P.W. Theoretical studies of salt-bridge formation by amino acid side chains in low and medium polarity environments. J. Phys. Chem. B 2010, 114, 16436–16442. [Google Scholar] [CrossRef] [PubMed]
- Nagy, P.I.; Erhardt, P.W. On the interaction of aliphatic amines and ammonium ions with carboxylic acids in solution and in receptor pockets. J. Phys. Chem. B 2012, 116, 5425–5436. [Google Scholar] [CrossRef] [PubMed]
- Nagy, P.I.; Sarver, J.G. Theoretical conformation analysis for chain systems with two conjugated double bonds in the gas phase and in solution. Comput. Theor. Chem. 2014, 1033, 43–51. [Google Scholar] [CrossRef]
- Long, J.A.; Harris, N.J.; Lammertsma, K. Formaldehyde oxime ↔ nitrosomethane tautomerism. J. Org. Chem. 2001, 66, 6762–6767. [Google Scholar] [CrossRef] [PubMed]
- Alkorta, I.; Elguero, J. 1,2-Proton shifts in pyrazole and related systems: A computational study of 1,5-sigmatropic migrations of hydrogen and related phenomena. J. Chem. Soc. Perkin Trans. 1998, 2, 2497–2503. [Google Scholar] [CrossRef]
- Rice, C.A.; Borho, N.; Suhm, M.A. Dimerization of pyrazole in slit jet expansions. Z. Phys. Chem. 2005, 219, 379–388. [Google Scholar] [CrossRef]
- Tsuchida, N.; Yamabe, S. Reaction paths of tautomerization between hydroxypyridines and pyridones. J. Phys. Chem. A 2005, 109, 1974–1980. [Google Scholar] [CrossRef] [PubMed]
- Nagy, P.I.; Takács-Novák, K. Theoretical and experimental studies of the zwitterion ↔ neutral form equilibrium of ampholytes in pure solvents and mixtures. J. Am. Chem. Soc. 1997, 119, 4999–5006. [Google Scholar] [CrossRef]
- Nagy, P.I.; Völgyi, G.; Takács-Novák, K. Tautomeric and conformational equilibria of tyramine and dopamine in aqueous solution. Mol. Phys. 2005, 103, 1589–1601. [Google Scholar] [CrossRef]
- Pranata, J. Relative basicities of carboxylate lone pairs in aqueous solution. J. Comput. Chem. 1993, 14, 685–690. [Google Scholar] [CrossRef]
- Lütgens, M.; Friedriszik, F.; Lochbrunner, S. Direct observation of the cyclic dimer in liquid acetic acid by probing the C=O vibration with ultrafast coherent Raman spectroscopy. Phys. Chem. Chem. Phys. 2014, 16, 18010–18016. [Google Scholar] [CrossRef] [PubMed]
- Yamabe, S.; Tsuchida, N. A computational study of interactions between acetic acid and water molecules. J. Comput. Chem. 2003, 24, 939–947. [Google Scholar] [CrossRef] [PubMed]
- Ciccotti, G.; Ferrario, M.; Hynes, J.Y.; Kapral, R. Constrained molecular dynamics and the mean potential for an ion pair in a polar solvent. Chem. Phys. Lett. 1989, 129, 241–251. [Google Scholar]
- Chen, J.; Brooks, C.L., III; Scheraga, H.A. Revisiting the carboxylic acid dimers in aqueous solution: Interplay of hydrogen bonding, hydrophobic interactions, and entropy. J. Phys. Chem. B 2008, 112, 242–249. [Google Scholar] [CrossRef] [PubMed]
- Chocholoušová, J.; Vacek, J.; Hobza, P. Acetic acid dimer in the gas phase, nonpolar solvent, microhydrated environment, and dilute and concentrated acetic acid: Ab initio quantum chemical and molecular dynamics simulations. J. Phys. Chem. A 2003, 107, 3086–3092. [Google Scholar] [CrossRef]
- Pašalić, H.; Tunega, D.; Aquino, A.J.A.; Haberhauer, G.; Gerzabek, M.H.; Lischka, H. The stability of the acetic acid dimer in microhydrated environments and in aqueous solution. Phys. Chem. Chem. Phys. 2012, 14, 4162–4170. [Google Scholar] [CrossRef] [PubMed]
- Elstner, M.; Frauenheim, Th.; Kaxiras, E.; Seifert, G.; Suhai, S. A self-consistent charge density-functional based tight-binding scheme for large biomolecules. Phys. Stat. Sol. B 2000, 217, 357–376. [Google Scholar] [CrossRef]
- D’Amico, F.; Bencivenga, F.; Gessini, A.; Masciovecchio, C. Temperature dependence of hydrogen-bond dynamics in acetic acid-water solutions. J. Phys. Chem. B 2010, 114, 10628–10633. [Google Scholar] [CrossRef] [PubMed]
- Kao, J.; Radom, L. Conformations, stabilities, and charge distributions in 2- and 3- monosubstituted thiophenes. An ab initio molecular orbital study. J. Am. Chem. Soc. 1979, 101, 311–318. [Google Scholar] [CrossRef]
- Woodcock, S.; Green, D.V.S.; Vincent, M.A.; Hillier, I.H.; Guest, M.F.; Sherwood, P. Tautomeric equilibria in 3- and 5-hydroxyisoxazole in the gas phase and in aqueous solution: A test of molecular dynamics and continuum models of solvation. J. Chem. Soc. Perkin Trans. 1992, 2, 2151–2154. [Google Scholar] [CrossRef]
- Gould, I.R.; Hillier, I.H. Modelling of tautomeric equilibria of 5-hydroxy-isoxazole in aqueous solution. J. Chem. Soc. Perkin Trans. 1993, 2, 1771–1773. [Google Scholar] [CrossRef]
- Cramer, C.J.; Truhlar, D.G. Correlation and solvation effects on heterocyclic equilibria in aqueous solution. J. Am. Chem. Soc. 1993, 115, 8810–8817. [Google Scholar] [CrossRef]
- Bastiensen, O. Intra-molecular hydrogen bonds in ethylene glycol, glycerol, and ethylene chlorohydrine. Acta Chem. Scand. 1949, 3, 415–421. [Google Scholar] [CrossRef]
- Caminati, W.; Corbelli, G. Conformation of ethylene glycol from the rotational spectra of the nontunneling O-monodeuterated species. J. Mol. Spectrosc. 1981, 90, 572–578. [Google Scholar] [CrossRef]
- Frei, H.; Ha, T.-K.; Meyer, R.; Günthard, H.H. Ethylene glycol: Infrared spectra, ab initio calculations, vibrational analysis and conformations of 5 matrix isolated isotope modifications. Chem. Phys. 1977, 25, 271–208. [Google Scholar] [CrossRef]
- Takeuchi, H.; Tasumi, M. Infrared-induced conformational isomerization of ethylene glycol in a low-temperature argon matrix. Chem. Phys. 1983, 77, 21–34. [Google Scholar] [CrossRef]
- Krueger, P.J.; Mettee, H.D. Spectroscopic studies of alcohols. Part VII. Intramolecular hydrogen bonds in ethylene glycol and 2-methoxyethanol. J. Mol. Spectrosc. 1965, 18, 131–140. [Google Scholar] [CrossRef]
- Pachler, K.G.R.; Wessels, P.L. Rotational isomerism X. A nuclear magnetic resonance study of 2-fluoro-ethanol and ethylene glycol. J. Mol. Struct. 1970, 6, 471–478. [Google Scholar] [CrossRef]
- Hooft, R.W.W.; van Eijck, B.P.; Kroon, J. Use of molecular dynamics in conformational analysis. Glycol. A model study. J. Chem. Phys. 1992, 97, 3639–3646. [Google Scholar] [CrossRef]
- Petterson, K.A.; Stein, R.S.; Drake, M.D.; Roberts, J.D. An NMR investigation of the importance of intramolecular hydrogen bonding in determining the conformational equilibrium of ethylene glycol in solution. Magn. Reson. Chem. 2005, 43, 225–230. [Google Scholar] [CrossRef] [PubMed]
- Gubskaya, A.G.; Kusalik, P.G. Molecular dynamics simulation study of ethylene glycol, ethylenediamine, and 2-aminoethanol. 2. Structure in aqueous solutions. J. Phys. Chem. A 2004, 108, 7165–7178. [Google Scholar] [CrossRef]
- Bubb, W.A. NMR spectroscopy in the study of carbohydrates: Characterizing the structural complexity. Concept. Magn. Reson. Part A 2003, 19, 1–19. [Google Scholar] [CrossRef]
- Jeong, K.; Byun, B.J.; Kang, Y.K. Conformational preferences of glycerol in the gas phase and in water. Bull. Korean Chem. Soc. 2012, 33, 917–923. [Google Scholar] [CrossRef]
- Callam, C.S.; Singer, S.J.; Lowary, T.L.; Hadad, C.M. Computational analysis of the potential energy surfaces of glycerol in the gas and aqueous phases: Effects of level of theory, basis set, and solvation on strongly intramolecularly hydrogen-bonded systems. J. Am. Chem. Soc. 2001, 123, 11743–11754. [Google Scholar] [CrossRef] [PubMed]
- Van Koningsveld, H. A conformational study on glycerol in a D2O solution by means of 220 Mc PMR data. Recl. Trav. Chim. Pays-Bas. 1970, 89, 801–812. [Google Scholar] [CrossRef]
- Egorov, A.V.; Lyubartsev, A.P.; Laaksonen, A. Molecular dynamics simulation study of glycerol-water liquid mixtures. J. Phys. Chem. B 2011, 115, 14572–14581. [Google Scholar] [CrossRef] [PubMed]
- Nagy, P.I.; Erhardt, P.W. Ab initio study of hydrogen-bond formation between cyclic ethers and selected amino acid side chains. J. Phys. Chem. A 2006, 110, 13923–13932. [Google Scholar] [CrossRef] [PubMed]
- Gil, F.P.S.C.; Fausto, R.; Amorim da Costa, A.M.; Teixeira-Dias, J.J.C. Structures and vibrational spectra of CH3OCH2CH2OH: The hydrogen-bonded conformers. J. Chem. Soc. Faraday Trans. 1994, 90, 689–695. [Google Scholar] [CrossRef]
- Tafazzoli, M.; Jalili, S. Study of association of 2-methoxyethanol in aqueous phase. Theor. Chem. Acc. 2001, 106, 194–198. [Google Scholar] [CrossRef]
- Penn, R.E.; Curl, R.F., Jr. Microwave spectrum of 2-aminoethanol: Structural effects of the hydrogen bond. J. Chem. Phys. 1971, 55, 651–658. [Google Scholar] [CrossRef]
- Da Silva, E.F.; Kuznetsova, T.; Kvamme, B.; Merz, K.M., Jr. Molecular dynamics study of ethanolamine as a pure liquid and in aqueous solution. J. Phys. Chem. B 2007, 111, 3695–3703. [Google Scholar] [CrossRef] [PubMed]
- López-Rendón, R.; Mora, M.A.; Alejandre, J.; Tuckerman, M.E. Molecular dynamics simulations of aqueous solutions of ethanolamines. J. Phys. Chem. B 2006, 110, 14652–14658. [Google Scholar] [CrossRef] [PubMed]
- Haufa, K.Z.; Czarnecki, M.A. Molecular structure and hydrogen bonding of 2-aminoethanol, 1-amino-2-propanol, 3-amino-1-propanol, and binary mixtures with water studied by Fourier transform near-infrared spectroscopy and density functional theory calculations. Appl. Spectrosc. 2010, 64, 351–358. [Google Scholar] [CrossRef] [PubMed]
- Omura, Y.; Shimanouchi, T. Raman spectra and rotational isomerism of ethylenediammonium and monoethanolammonium ions in aqueous solution. J. Mol. Spectrosc. 1975, 55, 430–434. [Google Scholar] [CrossRef]
- Smith, T.D.; Gerken, J.B.; Jog, P.V.; Roberts, J.D. Conformational equilibria of ethanolamine and its hydrochloride in solution. Org. Lett. 2007, 9, 4555–4557. [Google Scholar] [CrossRef] [PubMed]
- Marstokk, K.-M.; Møllendal, H. Microwave spectrum, conformational equilibrium, intramolecular hydrogen bonding, inversion tunneling, dipole moments and centrifugal distortion of ethylenediamine. J. Mol. Struct. 1978, 49, 221–237. [Google Scholar] [CrossRef]
- Boudon, S.; Wipff, G. Conformational analysis of protonated ethylenediamine in the gas phase and in water. J. Mol. Struct. Theochem 1991, 228, 61–70. [Google Scholar] [CrossRef]
- Azrak, R.G.; Wilson, E.B. Microwave spectra and intramolecular hydrogen bonding in the 2-haloethanols: Molecular structure and quadrupole coupling constants for 2-chloroethanol and 2-bromoethanol. J. Chem. Phys. 1970, 52, 5299–5316. [Google Scholar] [CrossRef]
- Roccatano, D.; Colombo, G.; Fioroni, M.; Mark, A.E. Mechanism by which 2,2,2 trifluoroethanol-water mixtures stabilize secondary-structure formation in peptides: A molecular dynamics study. PNAS 2002, 99, 12179–12184. [Google Scholar] [CrossRef] [PubMed]
- Molinaro, A.; de Castro, C.; Lanzetta, R.; Manzo, E.; Parrilli, M. Solvent effect on the isomeric equilibrium of carbohydrates: The superior ability of 2,2,2-trifluoroethanol for intramolecular hydrogen bond stabilization. J. Am. Chem. Soc. 2001, 123, 12605–12610. [Google Scholar] [CrossRef] [PubMed]
- Livingston, R.L.; Vaughan, G. An electron diffraction investigation of the molecular structure of trifluoroethanol. J. Am. Chem. Soc. 1956, 78, 2711–2714. [Google Scholar] [CrossRef]
- Xu, L.-H.; Fraser, G.T.; Lovas, F.J.; Suenram, R.D.; Gillies, C.W.; Warner, H.E.; Gillies, J.Z. The microwave spectrum and OH internal rotation dynamics of gauche-2,2,2-trifluoroethanol. J. Chem. Phys. 1995, 103, 9541–9548. [Google Scholar] [CrossRef]
- Durig, J.R.; Larsen, R.A. Torsional vibrations and barriers to internal rotation for ethanol and 2,2,2-trifluoroethanol. J. Mol. Struct. 1990, 238, 195–222. [Google Scholar] [CrossRef]
- Radnai, T.; Ishiguro, S.; Ohtaki, H. Intramolecular and liquid structure of 2,2,2,-trifluoroethanol by X-ray diffration. J. Solut. Chem. 1989, 18, 771–783. [Google Scholar] [CrossRef]
- Bakó, I.; Radnai, T.; Funel, M.C.B. Investigation of structure of liquid 2,2,2-trifluoroethanol: Neutron diffraction, molecular dynamics, and ab initio quantum chemical study. J. Chem. Phys. 2004, 121, 12472–12480. [Google Scholar] [CrossRef] [PubMed]
- Borba, A.; Gomez-Zavaglia, A.; Lapinski, L.; Fausto, R. Rotational isomers of lactic acid: First experimental observation of higher energy forms. Phys. Chem. Chem. Phys. 2004, 6, 2101–2108. [Google Scholar] [CrossRef]
- Yang, X.; Orlova, G.; Zhou, X.J.; Leung, K.T. A DFT study on the radical, monomer and dimer of α-keto pyruvic acid: Equilibrium structures and vibrational analysis of stable conformers. Chem. Phys. Lett. 2003, 380, 34–41. [Google Scholar] [CrossRef]
- Chermahini, A.N.; Mahdavian, M.; Teimouri, A. Theoretical studies of hydrogen bond interactions in fluoroacetic acid dimer. Bull. Korean Chem. Soc. 2010, 31, 941–948. [Google Scholar] [CrossRef]
- Kasalová, V.; Allen, W.D.; Schaffer, H.F., III; Czinki, E.; Császár, A.G. Molecular structures of the two most stable conformers of free glycine. J. Comput. Chem. 2007, 28, 1373–1383. [Google Scholar] [CrossRef] [PubMed]
- Nagaoka, M.; Okuyama-Yoshida, N.; Yamabe, T. Origin of the transition state on the free energy surface: Intramolecular proton transfer reaction of glycine in aqueous solution. J. Phys. Chem. A 1998, 102, 8202–8208. [Google Scholar] [CrossRef]
- Tuñón, I.; Silla, E.; Millot, C.; Martins-Costa, M.T.C.; Ruiz-López, M.F. Intramolecular proton transfer of glycine in aqueous solution using quantum mechanics-molecular mechanics simulations. J. Phys. Chem. A 1998, 102, 8673–8678. [Google Scholar] [CrossRef]
- Lunazzi, L.; Parisi, F.; Macciantelli, D. Conformational studies by dynamic nuclear magnetic resonance spectroscopy. Part 27. Kinetics and mechanism of annular tautomerism in isomeric triazoles. J. Chem. Soc. Perkin Trans. 1984, 2, 1025–1028. [Google Scholar] [CrossRef]
- Tortonda, F.R.; Silla, E.; Tuñón, I.; Rinaldi, D.; Ruiz-López, M.F. Intramolecular proton transfer of serine in aqueous solution. Mechanism and energetics. Theor. Chem. Acc. 2000, 104, 89–95. [Google Scholar] [CrossRef]
- Tortonda, F.R.; Pascal-Ahuir, J.L.; Silla, E.; Tuñón, I. Why is glycine a zwitterion in aqueous solution? A theoretical study of solvent stabilizing factors. Chem. Phys. Lett. 1996, 260, 21–26. [Google Scholar] [CrossRef]
- Gronert, S.; O’Hair, R.A.J. Ab initio studies of amino acid conformations. 1. The conformers of alanine, serine, and cystein. J. Am. Chem. Soc. 1995, 117, 2071–2081. [Google Scholar] [CrossRef]
- Blanco, S.; Sanz, M.E.; López, J.C.; Alonso, J.L. Revealing the multiple structures of serine. Proc. Natl. Acad. Sci. USA 2007, 104, 20183–20188. [Google Scholar] [CrossRef] [PubMed]
- Simperler, A.; Lampert, H.; Mikenda, W. Intramolecular interactions in ortho-substituted phenols: Survey of DFT-B3LYP calculated data. J. Mol. Struct. 1998, 448, 191–199. [Google Scholar] [CrossRef]
- Schreiber, V.M. Some effects of intramolecular hydrogen bonding on vibrational spectra. J. Mol. Struct. 1989, 197, 73–85. [Google Scholar] [CrossRef]
- Silvi, B.; Kryachko, E.S.; Tishchenko, O.; Fuster, F.; Nguyen, M.T. Key properties of monohalogen substituted phenols: Interpretation in terms of the electron localization function. Mol. Phys. 2002, 100, 1659–1675. [Google Scholar] [CrossRef]
- Caminati, W.; di Bernardo, S.; Schäfer, L.; Kulp-Newton, S.Q. Investigation of the molecular structure of catechol by combined microwave spectroscopy and ab initio calculations. J. Mol. Struct. 1990, 240, 263–274. [Google Scholar] [CrossRef]
- Reynolds, C.A. Theoretical electrode potentials and conformational energies of benzoquinones and naphthoquinones in aqueous solution. J. Am. Chem. Soc. 1990, 112, 7545–7551. [Google Scholar] [CrossRef]
- Kumar, S.; Singh, S.K.; Calabrese, C.; Maris, A.; Melandri, S.; Das, A. Structure of saligenin: Microwave, UV and IR spectroscopy studies in a supersonic jet combined with quantum chemistry calculations. Phys. Chem. Chem. Phys. 2014, 16, 17163–17171. [Google Scholar] [CrossRef] [PubMed]
- Vajda, E.; Hargittai, I. Molecular structure of 2-fluorophenol and 2,6-difluorophenol from gas-phase electron diffraction. J. Phys. Chem. 1993, 97, 70–76. [Google Scholar] [CrossRef]
- Borisenko, K.B.; Bock, C.W.; Hargittai, I. Intramolecular hydrogen bonding and molecular geometry of 2-nitrophenol from a joint gas-phase electron diffraction and ab initio molecular orbital investigation. J. Phys. Chem. 1994, 98, 1442–1448. [Google Scholar] [CrossRef]
- Fiedler, P.; Böhm, S.; Kulhánek, J.; Exner, O. Acidity of ortho-substituted benzoic acids: An infrared and theoretical study of the intramolecular hydrogen bonds. Org. Biomol. Chem. 2006, 4, 2003–2011. [Google Scholar] [CrossRef] [PubMed]
- Yahagi, T.; Fujii, A.; Ebata, T.; Mikam, N. Infrared spectroscopy of the OH stretching vibrations of jet-cooled salicylic acid and its dimer in S0 and S1. J. Phys. Chem. A 2001, 105, 10673–10680. [Google Scholar] [CrossRef]
- Ivanova, G.; Enchev, V. Does tautomeric equilibria in ortho-nitrosphenols? Chem. Phys. 2001, 264, 235–244. [Google Scholar]
- Nagy, P.I.; Alagona, G.; Ghio, C.; Takács-Novák, K. Theoretical conformational analysis for neurotransmitters in the gas phase and in aqueous solution. Norepinephrine. J. Am. Chem. Soc. 2003, 125, 2770–2785. [Google Scholar] [CrossRef]
- Mitchell, S.C.; Carmichael, P.; Waring, R. Aminophenols. In Kirk-Othmer Encyclopedia of Chemical Technology; Considine, G.D., Ed.; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2001. [Google Scholar]
- Vogelsanger, B.; Godfrey, P.D.; Brown, R.D. Rotational spectra of biomolecules: Histamine. J. Am. Chem. Soc. 1991, 113, 7864–7869. [Google Scholar] [CrossRef]
- Lagutschenkov, A.; Langer, J.; Berden, G.; Oomens, J.; Dopfer, O. Infrared spectra of the protonated neurotransmitter histamine: Competition between imidazolium and ammonium isomers in the gas phase. Phys. Chem. Chem. Phys. 2011, 13, 15644–15656. [Google Scholar] [CrossRef] [PubMed]
- Karpińska, G.; Dobrowolski, J.C.; Mazurek, A.P. Tautomerism of histamine revisited. J. Mol. Struct. Theochem 1996, 369, 137–144. [Google Scholar] [CrossRef]
- Kraszni, M.; Kökösi, J.; Noszál, B. Concentration and basicity of histamine rotamers. J. Chem. Soc. Trans. 2002, 2, 914–917. [Google Scholar]
- Nagy, P.I.; Durant, J.G.; Hoss, W.P.; Smith, D.A. Theoretical analyses of the tautomeric and conformational equilibria of histamine and (α. R, β. S)-.α, β-dimethylhistamine in the gas phase and aqueous solution. J. Am. Chem. Soc. 1994, 116, 4898–4909. [Google Scholar]
- Ramírez, F.J.; Tuñón, I.; Collado, J.A.; Silla, E. Structural and vibrational study of the tautomerism of histamine free-base in solution. J. Am. Chem. Soc. 2003, 125, 2328–2340. [Google Scholar] [CrossRef] [PubMed]
- Raczyńska, E.D.; Darowska, M.; Cyrański, M.K.; Makowski, M.; Rudka, T.; Gal, J.-F.; Maria, P.-C. Ab initio study of tautomerism and of basicity center preference in histamine, from gas phase to solution–comparison with experimental data (gas phase, solution, solid state). J. Phys. Org. Chem. 2003, 16, 783–796. [Google Scholar] [CrossRef]
- Forti, F.; Cavasotto, C.N.; Orozco, M.; Barril, X.; Luque, F.J. A multilevel strategy for the exploration of the conformational flexibility of small molecules. J. Chem. Theory Comput. 2012, 8, 1808–1819. [Google Scholar] [CrossRef]
- Worth, G.A.; Richards, W.G. Calculation of the tautomer ratio of histamine in aqueous solution using free energy perturbation methods: An in-depth study. J. Am. Chem. Soc. 1994, 116, 239–250. [Google Scholar] [CrossRef]
- Melandri, S.; Maris, A. Intramolecular hydrogen bonds and conformational properties of biogenic amines: A free-jet microwave study of tyramine. Phys. Chem. Chem. Phys. 2004, 6, 2863–2866. [Google Scholar] [CrossRef]
- Nagy, P.I.; Takács-Novák, K. Tautomeric and conformational equilibria of biologically important (hydroxyphenyl)alkylamine in the gas phase and in aqueous solution. Phys. Chem. Chem. Phys. 2004, 6, 2838–2848. [Google Scholar] [CrossRef]
- Cabezas, C.; Pena, I.; Lopez, J.C.; Alonso, J.L. Seven conformers of neutral dopamine revealed in the gas phase. J. Phys. Chem. Lett. 2013, 4, 486–490. [Google Scholar] [CrossRef]
- Urban, J.J.; Cramer, C.J.; Famini, G.R. A computational study of solvent effects on the conformation of dopamine. J. Am. Chem. Soc. 1992, 114, 8226–8231. [Google Scholar] [CrossRef]
- Šolmajer, P.; Kocjan, D.; Šolmajer, T. Conformational study of catecholamines in solution. Z. Naturforsch. 1983, 38, 758–762. [Google Scholar]
- Lagutschenkov, A.; Langer, J.; Berden, G.; Oomens, J.; Dopfer, O. Infrared spectra of protonated neurotransmitters: Dopamine. Phys. Chem. Chem. Phys. 2011, 13, 2815–2813. [Google Scholar] [CrossRef] [PubMed]
- Nagy, P.I.; Alagona, G.; Ghio, C. Theoretical studies on the conformation of protonated dopamine in the gas phase and in aqueous solution. J. Am. Chem. Soc. 1999, 121, 4804–4815. [Google Scholar] [CrossRef]
- Snoek, L.C.; van Mourik, T.; Simons, J.P. Neurotransmitters in the gas phase: A computational and spectroscopic study for noradrenaline. Mol. Phys. 2003, 101, 1239–1248. [Google Scholar] [CrossRef]
- Alagona, G.; Ghio, C. Interplay of intra- and intermolecular H-bonds for the addition of a water molecule to the neutral and N-protonated forms of noradrenaline. Int. J. Q. Chem. 2002, 90, 641–656. [Google Scholar] [CrossRef]
- ÇarÇabal, P.; Snoek, L.C.; van Mourik, T. A computational and spectroscopic study of the gas-phase conformers of adrenaline. Mol. Phys. 2005, 103, 1633–1639. [Google Scholar] [CrossRef]
- Alagona, G.; Ghio, C. Competitive H-bonds in vacuo and in aqueous solution for N-protonated adrenaline and its monohydrated complexes. J. Mol. Struct. Theochem 2007, 811, 223–240. [Google Scholar] [CrossRef]
- LeGreve, T.A.; Baquero, E.E.; Zwier, T.S. Infrared and ultraviolet spectral signatures and conformational preferences of jet-cooled serotonin. J. Am. Chem. Soc. 2007, 129, 4028–4038. [Google Scholar] [CrossRef] [PubMed]
- Lagutschenkov, A.; Langer, J.; Berden, G.; Oomens, J.; Dopfer, O. Infrared spectra of the protonated neurotransmitters: Serotonin. J. Phys. Chem. A 2010, 114, 13268–13276. [Google Scholar] [CrossRef] [PubMed]
- Alagona, G.; Ghio, C.; Nagy, P.I. Theoretical conformational analysis for neurotransmitters in the gas phase and in aqueous solution. Serotonin. J. Chem. Theory Comput. 2005, 1, 801–816. [Google Scholar] [CrossRef]
- Alagona, G.; Ghio, C. Protonated serotonin conformational landscape in vacuo and in aqueous solution (IEF-PCM): Role of correlation effects and monohydration. J. Mol. Struct. Theochem 2006, 769, 123–134. [Google Scholar] [CrossRef]
- Ishiuchi, S.; Asakawa, R.; Mitsuda, H.; Miyazaki, M.; Chakraborty, S.; Fujii, M. Gas-phase spectroscopy of synephrine by laser desorption supersonic jet technique. J. Phys. Chem. A 2011, 115, 10363–10369. [Google Scholar] [CrossRef] [PubMed]
- Alonso, J.L.; Perez, C.; Sanz, M.E.; Lopez, J.C.; Blanco, S. Seven conformers of l-threonine in the gas phase: A LA-MB-FTMW study. Phys. Chem. Chem. Phys. 2009, 11, 617–627. [Google Scholar] [CrossRef] [PubMed]
- Quesada-Moreno, M.M.; Márquez-García, A.Á.; Avilés-Moreno, J.R.; López-González, J.J. Conformational landscape of l-threonine in neutral, acid and basic solutions from vibrational circular dichroism spectroscopy and quantum chemical calculations. Tetrahedron Asymmetry 2013, 24, 1537–1547. [Google Scholar] [CrossRef]
- McGlone, S.J.; Godfrey, P.D. Rotational spectrum of a neurohormone: β-Alanine. J. Am. Chem. Soc. 1995, 117, 1043–1048. [Google Scholar] [CrossRef]
- Sanz, M.E.; Lesarri, A.; Peña, M.I; Vaquero, V.; Cortijo, V.; Lopez, J.C.; Alonso, J.L. The shape of β-alanine. J. Am. Chem. Soc. 2006, 128, 3812–3817. [Google Scholar] [CrossRef]
- Southern, C.A.; Levy, D.H.; Florio, G.M.; Longarte, A.; Zwier, T.S. Electronic and infrared spectroscopy of anthranilic acid in a supersonic jet. J. Phys. Chem. A 2003, 107, 4032–4040. [Google Scholar] [CrossRef]
- Abou-Zied, O.K.; Al-Busaidi, B.Y.; Husband, J. Solvent effect on anthranilic acid spectroscopy. J. Phys. Chem. A 2014, 118, 103–109. [Google Scholar] [CrossRef] [PubMed]
- Moriyasu, M.; Kato, A.; Hashimoto, Y. Kinetic studies of fast equilibrium by means of high-performance liquid chromatography. Part 11. Keto-enol tautomerism of some β-dicarbonyl compounds. J. Chem. Soc. Perkin Trans. 1986, 4, 515–520. [Google Scholar] [CrossRef]
- Belova, N.V.; Oberhammer, H.; Girichev, G.V. Tautomeric and conformational properties of methyl acetoacetate, CH3OC(O)−CH2−C(O)CH3: Electron diffraction and quantum chemical study. J. Phys. Chem. A 2004, 108, 3593–3597. [Google Scholar] [CrossRef]
- Baughcum, S.L.; Duerst, R.W.; Rowe, W.F.; Smith, Z.; Wilson, E.B. Microwave spectroscopic study of malonaldehyde (3-hydroxy-2-propenal). 2. Structure, dipole moment, and tunneling. J. Am. Chem. Soc. 1981, 103, 6296–6303. [Google Scholar] [CrossRef]
- Seliskar, C.J.; Hoffmann, R.E. On the infrared spectrum of malonaldehyde, a tunneling hydrogen-bonded molecule. J. Mol. Spectrosc. 1982, 96, 146–155. [Google Scholar] [CrossRef]
- Bothner-By, A.A.; Harris, R.K. Conformational preferences in malondialdehyde and acetylacetaldehyde enols investigated by nuclear magnetic resonance. J. Org. Chem. 1965, 30, 254–257. [Google Scholar] [CrossRef]
- Bertz, S.H.; Dabbagh, G. NMR spectroscopy of malondialdehyde. J. Org. Chem. 1990, 66, 5161–5165. [Google Scholar] [CrossRef]
- George, P.; Bock, C.W.; Trachtman, M. An ab initio study of the geometry and energy of six planar conformers of β-hydroxyacrolein. J. Comput. Chem. 1991, 1, 373–385. [Google Scholar] [CrossRef]
- Alagona, G.; Ghio, C.; Nagy, P.I. The catalytic effect of water on the keto-enol tautomerism. Pyruvate and acetylacetone: A computational challenge. Phys. Chem. Chem. Phys. 2010, 12, 10173–10188. [Google Scholar] [CrossRef] [PubMed]
- Ishida, T.; Hirata, F.; Kato, S. Thermodynamic analysis of the solvent effect on tautomerization of acetylacetone: An ab initio approach. J. Chem. Phys. 1999, 110, 3938–3945. [Google Scholar] [CrossRef]
- Schlund, S.; Janke, E.M.B.; Weisz, K.; Engels, B. Predicting the tautomeric equilibrium of acetylacetone in solution. I. The right answer for the wrong reason? J. Comput. Chem. 2010, 31, 665–670. [Google Scholar]
- Nagy, P.I.; Fabian, W.M.F. Theoretical study of the enol imine ↔ enaminone tautomeric equilibrium in organic solvents. J. Phys. Chem. B 2006, 110, 25026–25032. [Google Scholar] [CrossRef] [PubMed]
- Ali, S.T.; Antonov, L.; Fabian, W.M.F. Phenol-quinone tautomerism in (arylazo)naphthols and the analogous Schiff bases: Benchmark Calculations. J. Phys. Chem. A 2014, 118, 778–789. [Google Scholar] [CrossRef] [PubMed]
- Sahoo, S.K.; Sharma, D.; Bera, R.K. Studies on molecular structure and tautomerism of a vitamin B6 analog with density functional theory. J. Mol. Model. 2012, 18, 1993–2001. [Google Scholar] [CrossRef] [PubMed]
- Ramek, M.; Nagy, P.I. Theoretical investigation of the neutral/zwitterionic equilibrium of the γ-aminobutyric acid (GABA) conformers in aqueous solution. J. Phys. Chem. A 2000, 104, 6844–6854. [Google Scholar] [CrossRef]
- Nagy, P.I.; Takács-Novák, K.; Ramek, M. Theoretical and experimental studies on partitions of substituted butyric acids in chloroform/water and dichloromethane/water systems. J. Phys. Chem. B 2001, 105, 5772–5781. [Google Scholar] [CrossRef]
- Liljefors, T.; Norrby, P.-O. An ab initio study of the trimethylamine-formic acid and the trimethylammonium ion-formate anion complexes, their monohydrates, and continuum solvation. J. Am. Chem. Soc. 1997, 119, 1052–1058. [Google Scholar] [CrossRef]
- Silva, P.J.; Perez, M.A.S.; Brás, N.F.; Fernandes, P.A.; Ramos, M.J. Improving the study of proton transfers between amino acid side chains in solution: Choosing appropriate DFT functionals and avoiding hidden pitfalls. Theor. Chem. Acc. 2012, 131, 1179. [Google Scholar] [CrossRef]
- Lewis, G.N. The atom and the molecule. J. Am. Chem. Soc. 1916, 38, 762–785. [Google Scholar] [CrossRef]
- Putz, M.V. Chemical action and chemical bonding. J. Mol. Struct. Theochem 2009, 900, 64–70. [Google Scholar] [CrossRef]
© 2014 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nagy, P.I. Competing Intramolecular vs. Intermolecular Hydrogen Bonds in Solution. Int. J. Mol. Sci. 2014, 15, 19562-19633. https://doi.org/10.3390/ijms151119562
Nagy PI. Competing Intramolecular vs. Intermolecular Hydrogen Bonds in Solution. International Journal of Molecular Sciences. 2014; 15(11):19562-19633. https://doi.org/10.3390/ijms151119562
Chicago/Turabian StyleNagy, Peter I. 2014. "Competing Intramolecular vs. Intermolecular Hydrogen Bonds in Solution" International Journal of Molecular Sciences 15, no. 11: 19562-19633. https://doi.org/10.3390/ijms151119562
APA StyleNagy, P. I. (2014). Competing Intramolecular vs. Intermolecular Hydrogen Bonds in Solution. International Journal of Molecular Sciences, 15(11), 19562-19633. https://doi.org/10.3390/ijms151119562




