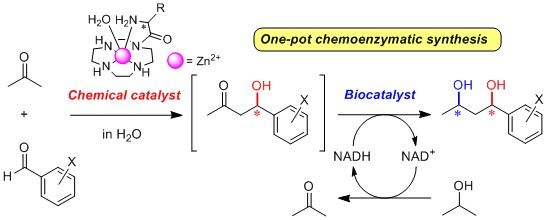
Design and Synthesis of Chiral Zn2+ Complexes Mimicking Natural Aldolases for Catalytic C–C Bond Forming Reactions in Aqueous Solution
Abstract
Share and Cite
Itoh, S.; Sonoike, S.; Kitamura, M.; Aoki, S. Design and Synthesis of Chiral Zn2+ Complexes Mimicking Natural Aldolases for Catalytic C–C Bond Forming Reactions in Aqueous Solution. Int. J. Mol. Sci. 2014, 15, 2087-2118. https://doi.org/10.3390/ijms15022087
Itoh S, Sonoike S, Kitamura M, Aoki S. Design and Synthesis of Chiral Zn2+ Complexes Mimicking Natural Aldolases for Catalytic C–C Bond Forming Reactions in Aqueous Solution. International Journal of Molecular Sciences. 2014; 15(2):2087-2118. https://doi.org/10.3390/ijms15022087
Chicago/Turabian StyleItoh, Susumu, Shotaro Sonoike, Masanori Kitamura, and Shin Aoki. 2014. "Design and Synthesis of Chiral Zn2+ Complexes Mimicking Natural Aldolases for Catalytic C–C Bond Forming Reactions in Aqueous Solution" International Journal of Molecular Sciences 15, no. 2: 2087-2118. https://doi.org/10.3390/ijms15022087
APA StyleItoh, S., Sonoike, S., Kitamura, M., & Aoki, S. (2014). Design and Synthesis of Chiral Zn2+ Complexes Mimicking Natural Aldolases for Catalytic C–C Bond Forming Reactions in Aqueous Solution. International Journal of Molecular Sciences, 15(2), 2087-2118. https://doi.org/10.3390/ijms15022087