Current Progress in Bioactive Ceramic Scaffolds for Bone Repair and Regeneration
Abstract
:1. Introduction
2. Mechanical Properties of Bioactive Ceramics
3. Materials for Strengthening and Toughening
3.1. Fiber Toughness
3.2. Whisker Toughness
3.3. Particle Toughness
4. Nanoceramic Artificial Bone
4.1. Advantages of Nanoceramic Artificial Bone
4.2. Processing Methods
5. Porous Structure and Corresponding Preparation Technique
5.1. Conventional Preparation Methods
5.2. Rapid Prototype Technology
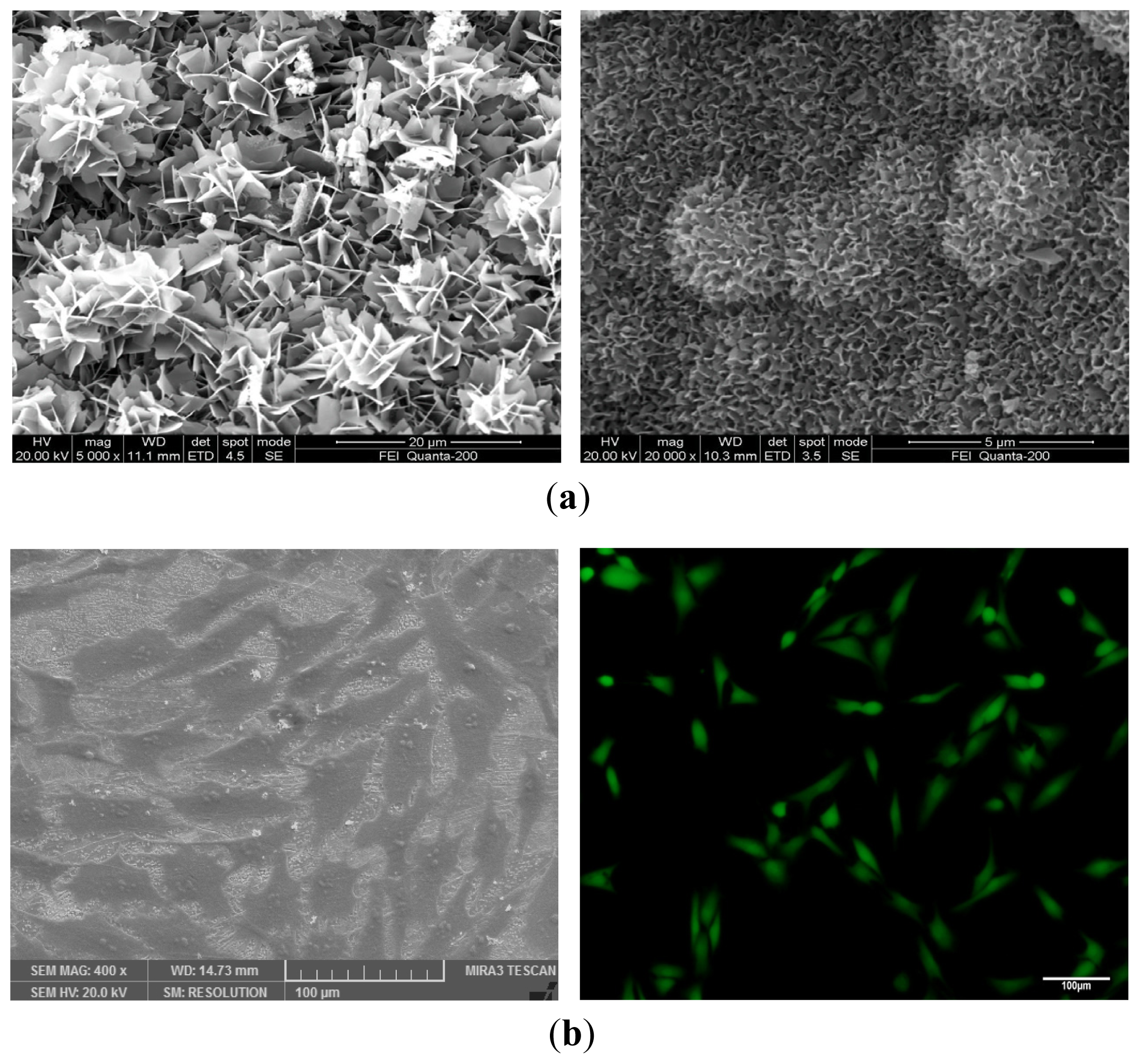
6. The Interaction between Bioactive Ceramic Scaffold and Cells
7. Ceramic-Based Composite Scaffolds
7.1. Composites with Natural Biomaterials
7.2. Composites with Metallic Materials
7.3. Composites with Biodegradable Polymers
7.4. Composites of Two or More Bioactive Ceramics
8. Novel Bioactive Ceramic Scaffold with Nano Grain and Hierarchically Porous Structure
9. Conclusions and Perspectives
Acknowledgments
Conflicts of Interest
References
- Carrington, J.L. Aging bone and cartilage: Cross-cutting issues. Biochem. Biophys. Res. Commun. 2005, 328, 700–708. [Google Scholar]
- Olshansky, S.J.; Passaro, D.J.; Hershow, R.C.; Layden, J.; Carnes, B.A.; Brody, J.; Hayflick, L.; Butler, R.N.; Allison, D.B.; Ludwig, D.S. A potential decline in life expectancy in the United States in the 21st century. N. Engl. J. Med. 2005, 352, 1138–1145. [Google Scholar]
- Ebnezar, J. Textbook of Orthopaedics; JP Medical Ltd.: London, UK, 2010. [Google Scholar]
- Cancedda, R.; Dozin, B.; Giannoni, P.; Quarto, R. Tissue engineering and cell therapy of cartilage and bone. Matrix Biol. 2003, 22, 81–91. [Google Scholar]
- Sun, K.; Tian, S.; Zhang, J.; Xia, C.; Zhang, C.; Yu, T. Anterior cruciate ligament reconstruction with BPTB autograft irradiated versus non-irradiated allograft: A prospective randomized clinical study. Knee Surg. Sports Traumatol. Arthrosc. 2009, 17, 464–474. [Google Scholar]
- Shafiei, Z.; Bigham, A.S.; Dehghani, S.N.; Nezhad, S.T. Fresh cortical autograft versus fresh cortical allograft effects on experimental bone healing in rabbits: Radiological Histopathological and Biomechanical evaluation. Cell Tissue Bank 2009, 10, 19–26. [Google Scholar]
- Younger, E.M.; Chapman, M.W. Morbidity at bone graft donor sites. J. Orthop. Trauma 1989, 3, 192–195. [Google Scholar]
- Braddock, M.; Houston, P.; Campbell, C.; Ashcroft, P. Born again bone: Tissue engineering for bone repair. Physiology 2001, 16, 208–213. [Google Scholar]
- Hench, L.L.; Polak, J.M. Third-generation biomedical materials. Science 2002, 295, 1014–1017. [Google Scholar]
- Hutmacher, D.W. Scaffolds in tissue engineering bone and cartilage. Biomaterials 2000, 21, 2529–2543. [Google Scholar]
- Swetha, M.; Sahithi, K.; Moorthi, A.; Srinivasan, N.; Ramasamy, K.; Selvamurugan, N. Biocomposites containing natural polymers and hydroxyapatite for bone tissue engineering. Int. J. Biol. Macromol. 2010, 47, 1–4. [Google Scholar]
- Tu, J.W.; Wang, H.J.; Li, H.W.; Dai, K.R.; Wang, J.Y.; Zhang, X.L. The in vivo bone formation by mesenchymal stem cells in zein scaffolds. Biomaterials 2009, 30, 4369–4376. [Google Scholar]
- Liu, F.H. Synthesis of bioceramic scaffolds for bone tissue engineering by rapid prototyping technique. J. Sol-Gel Sci. Technol. 2012, 64, 704–710. [Google Scholar]
- Zhou, H.; Lee, J. Nanoscale hydroxyapatite particles for bone tissue engineering. Acta Biomater. 2011, 7, 2769–2781. [Google Scholar]
- Mirtchi, A.A.; Lemaitre, J.; Terao, N. Calcium phosphate cements: Study of the β-tricalcium phosphate—monocalcium phosphate system. Biomaterials 1989, 10, 475–480. [Google Scholar]
- Hench, L.L. The story of Bioglass®. J. Mater. Sci. 2006, 17, 967–978. [Google Scholar]
- Liu, X.; Morra, M.; Carpi, A.; Li, B. Bioactive calcium silicate ceramics and coatings. Biomed. Pharmacother. 2008, 62, 526–529. [Google Scholar]
- Sultana, N. Biodegradable Polymer-Based Scaffolds for Bone Tissue Engineering; Springer: Berlin, Germany, 2013. [Google Scholar]
- Xu, H.H.; Quinn, J.B.; Takagi, S.; Chow, L.C. Synergistic reinforcement of in situ hardening calcium phosphate composite scaffold for bone tissue engineering. Biomaterials 2004, 25, 1029–1037. [Google Scholar]
- Rahaman, M.N. Ceramic Processing; CRC Press: Boca Raton, FL, USA, 2007. [Google Scholar]
- Kim, S.S.; Park, M.S.; Jeon, O.; Choi, C.Y.; Kim, B.S. Poly(lactide-co-glycolide)/hydroxyapatite composite scaffolds for bone tissue engineering. Biomaterials 2006, 27, 1399–1409. [Google Scholar]
- Thompson, I.; Hench, L. Mechanical properties of bioactive glasses glass-ceramics and composites. Proc. Inst. Mech. Eng. Part H 1998, 212, 127–136. [Google Scholar]
- Wu, C. Methods of improving mechanical and biomedical properties of Ca–Si-based ceramics and scaffolds. Expert Rev. Med. Devices 2009, 6, 237–241. [Google Scholar]
- Wang, J.; Shaw, L.L. Nanocrystalline hydroxyapatite with simultaneous enhancements in hardness and toughness. Biomaterials 2009, 30, 6565–6572. [Google Scholar]
- Fielding, G.A.; Bandyopadhyay, A.; Bose, S. Effects of silica and zinc oxide doping on mechanical and biological properties of 3D printed tricalcium phosphate tissue engineering scaffolds. Dent. Mater. 2012, 28, 113–122. [Google Scholar]
- Ramesh, S.; Tan, C.; Sopyan, I.; Hamdi, M.; Teng, W. Consolidation of nanocrystalline hydroxyapatite powder. Sci. Technol. Adv. Mater. 2007, 8, 124–130. [Google Scholar]
- Gerhardt, L.-C.; Boccaccini, A.R. Bioactive glass and glass-ceramic scaffolds for bone tissue engineering. Materials 2010, 3, 3867–3910. [Google Scholar]
- Becher, P.F. Microstructural design of toughened ceramics. J. Am. Ceram. Soc. 1991, 74, 255–269. [Google Scholar]
- Losquadro, W.D.; Tatum, S.A.; Allen, M.J.; Mann, K.A. Polylactide-co-glycolide fiber–reinforced calcium phosphate bone cement. Arch. Facial Plast. Surg. 2009, 11, 104–109. [Google Scholar]
- Zhang, Y.; Xu, H.H. Effects of synergistic reinforcement and absorbable fiber strength on hydroxyapatite bone cement. J. Biomed. Mater. Res. Part A 2005, 75, 832–840. [Google Scholar]
- Suping, H.; Baiyun, H.; Kechao, Z.; Zhiyou, L. Effects of coatings on the mechanical properties of carbon fiber reinforced HAP composites. Mater. Lett. 2004, 58, 3582–3585. [Google Scholar]
- Becher, P.F.; HSUEH, C.H.; Angelini, P.; Tiegs, T.N. Toughening Behavior in Whisker-Reinforced Ceramic Matrix Composites. J. Am. Ceram. Soc. 1988, 71, 1050–1061. [Google Scholar]
- Müller, F.A.; Gbureck, U.; Kasuga, T.; Mizutani, Y.; Barralet, J.E.; Lohbauer, U. Whisker-reinforced calcium phosphate cements. J. Am. Ceram. Soc. 2007, 90, 3694–3697. [Google Scholar]
- Bose, S.; Banerjee, A.; Dasgupta, S.; Bandyopadhyay, A. Synthesis processing mechanical and biological property characterization of hydroxyapatite whisker-reinforced hydroxyapatite composites. J. Am. Ceram. Soc. 2009, 92, 323–330. [Google Scholar]
- Wang, F.; Zhao, S.; Zhao, P.; Zhao, T.R.; Ren, X.H.; Chen, X.N. Hydroxyapatite whisker effect on strength of calcium phosphate bone cement. Adv. Mater. Res. 2012, 534, 30–33. [Google Scholar]
- Ahn, E.S.; Gleason, N.J.; Ying, J.Y. The effect of zirconia reinforcing agents on the microstructure and mechanical properties of hydroxyapatite-based nanocomposites. J. Am. Ceram. Soc. 2005, 88, 3374–3379. [Google Scholar]
- Wei, G.; Ma, P.X. Structure and properties of nano-hydroxyapatite/polymer composite scaffolds for bone tissue engineering. Biomaterials 2004, 25, 4749–4757. [Google Scholar]
- Gentile, P.; Chiono, V.; Boccafoschi, F.; Baino, F.; Vitale-Brovarone, C.; Vernè, E.; Barbani, N.; Ciardelli, G. Composite films of gelatin and hydroxyapatite/bioactive glass for tissue-engineering applications. J. Biomater. Sci. Polym. Ed. 2010, 21, 1207–1226. [Google Scholar]
- Gentile, P.; Mattioli-Belmonte, M.; Chiono, V.; Ferretti, C.; Baino, F.; Tonda-Turo, C.; Vitale-Brovarone, C.; Pashkuleva, I.; Reis, R.L.; Ciardelli, G. Bioactive glass/polymer composite scaffolds mimicking bone tissue. J. Biomed. Mater. Res. Part A 2012, 100, 2654–2667. [Google Scholar]
- Zhu, Y.-F.; Shi, L.; Liang, J.; Hui, D.; Lau, K.-T. Synthesis of zirconia nanoparticles on carbon nanotubes and their potential for enhancing the fracture toughness of alumina ceramics. Compos. Part B 2008, 39, 1136–1141. [Google Scholar]
- Burg, K.J.; Porter, S.; Kellam, J.F. Biomaterial developments for bone tissue engineering. Biomaterials 2000, 21, 2347–2359. [Google Scholar]
- Nel, A.; Xia, T.; Mädler, L.; Li, N. Toxic potential of materials at the nanolevel. Science 2006, 311, 622–627. [Google Scholar]
- Roco, M.C.; Williams, R.; Alivisatos, P. Nanotechnology Research Directions: IWGN Workshop Report. Vision for Nanotechnology R&D in the Next Decade; National Science and Technology Council: Washington, DC, USA, 1999. [Google Scholar]
- Meyers, M.A.; Mishra, A.; Benson, D.J. Mechanical properties of nanocrystalline materials. Prog. Mater. Sci. 2006, 51, 427–556. [Google Scholar]
- Chokshi, A. An evaluation of the grain-boundary sliding contribution to creep deformation in polycrystalline alumina. J. Mater. Sci. 1990, 25, 3221–3228. [Google Scholar]
- Thomas, S.; Chan, C.H.; Pothen, L.A.; Joy, J.; Maria, H. Natural Rubber Materials: Volume 2: Composites and Nanocomposites; Royal Society of Chemistry: London, UK, 2014; Volume 8. [Google Scholar]
- Liu, T.; Yan, Y.; Li, S. Comparative study on lattice parameters of HAP nanoparticles with those of HAP whiskers. J. Wuhan Univ. Technol.-Mater. Sci. Ed. 2008, 23, 395–398. [Google Scholar]
- Puzari, A.; Borah, J.P. Ionic self-assembly and hierarchies of polymeric structures generating nanoscale architecture: Opportunities ahead from industrial perspective. Rev. Adv. Mater. Sci. 2013, 34, 88–106. [Google Scholar]
- Chun, Y.W.; Crowder, S.W.; Mehl, S.C.; Wang, X.; Bae, H.; Sung, H.-J. Therapeutic application of nanotechnology in cardiovascular and pulmonary regeneration. Comput. Struct. Biotechnol. J. 2013, 7. [Google Scholar] [CrossRef]
- Pereira, A.L.; Veras, S.S.; Silveira, É.J.; Seabra, F.R.; Pinto, L.P.; Souza, L.B.; Freitas, R.A. The role of matrix extracellular proteins and metalloproteinases in head and neck carcinomas: An updated review. Revista Brasileira de Otorrinolaringologia 2005, 71, 81–86. [Google Scholar]
- Ding, L.; Davidchack, R.L.; Pan, J. A molecular dynamics study of sintering between nanoparticles. Comput. Mater. Sci. 2009, 45, 247–256. [Google Scholar]
- Mazaheri, M.; Zahedi, A.; Haghighatzadeh, M.; Sadrnezhaad, S. Sintering of titania nanoceramic: Densification and grain growth. Ceram. Int. 2009, 35, 685–691. [Google Scholar]
- Sanosh, K.; Chu, M.-C.; Balakrishnan, A.; Kim, T.; Cho, S.-J. Pressureless sintering of nanocrystalline hydroxyapatite at different temperatures. Met. Mater. Int. 2010, 16, 605–611. [Google Scholar]
- Sprio, S.; Tampieri, A.; Celotti, G.; Landi, E. Development of hydroxyapatite/calcium silicate composites addressed to the design of load-bearing bone scaffolds. J. Mech. Behav. Biomed. Mater. 2009, 2, 147–155. [Google Scholar]
- Gay, S.; Arostegui, S.; Lemaitre, J. Preparation and characterization of dense nanohydroxyapatite/PLLA composites. Mater. Sci. Eng. C 2009, 29, 172–177. [Google Scholar]
- Costa Oliveira, F.A.; Pascaud, P.; Domingues, J.; Marcelo, T.; Cruz Fernandes, J.; Guerra Rosa, L. Elastic properties of microwave sintered hydroxyapatite. Mater. Sci. Forum 2008, 587–588, 17–21. [Google Scholar]
- Seo, D.S.; Hwang, K.H.; Lee, J.K. Nanostructured hydroxyapatite by microwave sintering. J. Nanosci. Nanotechnol. 2008, 8, 944–948. [Google Scholar]
- Li, S.; Izui, H.; Okano, M. Densification microstructure and behavior of hydroxyapatite ceramics sintered by using spark plasma sintering. J. Eng. Mater. Technol. 2008, 130. [Google Scholar] [CrossRef]
- Zhang, J.; Cao, L.; Yang, Y.; Diao, Y.; Shen, X. Step sintering of microwave heating and microwave plasma heating for alumina ceramics. J. Mater. Sci. Technol. Shenyang 1999, 15, 419–422. [Google Scholar]
- Liu, Z.; Huang, H.; Gao, X.; Yu, H.; Zhong, X.; Zhu, J.; Zeng, D. Microstructure and property evolution of isotropic and anisotropic NdFeB magnets fabricated from nanocrystalline ribbons by spark plasma sintering and hot deformation. J. Phys. D 2011, 44, 025003. [Google Scholar]
- Yang, J.; Ouyang, H.; Wang, Y. Direct metal laser fabrication: Machine development and experimental work. Int. J. Adv. Manuf. Technol. 2010, 46, 1133–1143. [Google Scholar]
- Vinet, A.; Caine, M. Development of traction features in sprint spikes using SLS nylon sole units. Procedia Eng. 2010, 2, 2769–2774. [Google Scholar]
- Järvenpää, A.; Mäntyjärvi, K.; Merklein, M.; Hietala, M.; Karjalainen, J. Mechanical properties of laser heat treated 6 mm thick UHSS-steel. AIP Conf. Proc. 2011, 1353, 1319. [Google Scholar]
- Williams, J.M.; Adewunmi, A.; Schek, R.M.; Flanagan, C.L.; Krebsbach, P.H.; Feinberg, S.E.; Hollister, S.J.; Das, S. Bone tissue engineering using polycaprolactone scaffolds fabricated via selective laser sintering. Biomaterials 2005, 26, 4817–4827. [Google Scholar]
- Liao, H.T.; Lee, M.Y.; Tsai, W.W.; Wang, H.C.; Lu, W.C. Osteogenesis of adipose-derived stem cells on polycaprolactone–β-tricalcium phosphate scaffold fabricated via selective laser sintering and surface coating with collagen type I. J. Tissue Eng. Regen. Med. 2013. [Google Scholar] [CrossRef]
- Huyghe, J.M.; Raats, P.A.; Cowin, S.C. IUTAM Symposium on Physicochemical and Electromechanical, Interactions in Porous Media; Springer: Dordrecht, The Netherlands, 2005; Volume 125. [Google Scholar]
- Lv, R.; Zhou, W.; Shi, K.; Yang, Y.; Wang, L.; Pan, K.; Tian, C.; Ren, Z.; Fu, H. Alumina decorated TiO2 nanotubes with ordered mesoporous walls as high sensitivity of NOxgas sensors at room temperature. Nanoscale 2013, 5, 8569–8576. [Google Scholar]
- Ryan, G.; Pandit, A.; Apatsidis, D.P. Fabrication methods of porous metals for use in orthopaedic applications. Biomaterials 2006, 27, 2651–2670. [Google Scholar]
- Li, N.; Wang, R. Macroporous sol–gel bioglasses scaffold with high compressive strength porosity and specific surface area. Ceram. Int. 2012, 38, 6889–6893. [Google Scholar]
- Lv, Q.; Nair, L.; Laurencin, C.T. Fabrication characterization and in vitro evaluation of poly (lactic acid glycolic acid)/nano-hydroxyapatite composite microsphere-based scaffolds for bone tissue engineering in rotating bioreactors. J. Biomed. Mater. Res. Part A 2009, 91, 679–691. [Google Scholar]
- Krauss Juillerat, F.; Gonzenbach, U.T.; Elser, P.; Studart, A.R.; Gauckler, L.J. Microstructural Control of Self-Setting Particle-Stabilized Ceramic Foams. J. Am. Ceram. Soc. 2011, 94, 77–83. [Google Scholar]
- Espalin, D.; Arcaute, K.; Rodriguez, D.; Medina, F.; Posner, M.; Wicker, R. Fused deposition modeling of patient-specific polymethylmethacrylate implants. Rapid Prototyp. J. 2010, 16, 164–173. [Google Scholar]
- Simon, J.L.; Rekow, E.D.; Thompson, V.P.; Beam, H.; Ricci, J.L.; Parsons, J.R. MicroCT analysis of hydroxyapatite bone repair scaffolds created via three-dimensional printing for evaluating the effects of scaffold architecture on bone ingrowth. J. Biomed. Mater. Res. Part A 2008, 85, 371–377. [Google Scholar]
- Maeda, C.; Tasaki, S.; Kirihara, S. Accurate fabrication of hydroxyapatite bone models with porous scaffold structures by using stereolithography. In IOP Conference Series: Materials Science and Engineering, 2011; IOP Publishing: Bristol, UK, 2011; p. 072017. [Google Scholar]
- Simpson, R.L.; Wiria, F.E.; Amis, A.A.; Chua, C.K.; Leong, K.F.; Hansen, U.N.; Chandrasekaran, M.; Lee, M.W. Development of a 95/5 poly (l-lactide-co-glycolide)/hydroxylapatite and β-tricalcium phosphate scaffold as bone replacement material via selective laser sintering. J. Biomed. Mater. Res. Part B 2008, 84, 17–25. [Google Scholar]
- Wu, C.; Fan, W.; Zhou, Y.; Luo, Y.; Gelinsky, M.; Chang, J.; Xiao, Y. 3D-printing of highly uniform CaSiO3 ceramic scaffolds: Preparation characterization and in vivo osteogenesis. J. Mater. Chem. 2012, 22, 12288–12295. [Google Scholar]
- Bian, W.; Li, D.; Lian, Q.; Zhang, W.; Zhu, L.; Li, X.; Jin, Z. Design and fabrication of a novel porous implant with pre-set channels based on ceramic stereolithography for vascular implantation. Biofabrication 2011, 3, 034103. [Google Scholar]
- Lin, L.; Ju, S.; Cen, L.; Zhang, H.; Hu, Q. Fabrication of porous β-TCP scaffolds by combination of rapid prototyping and freeze drying technology. Proceedings of the 7th Asian-Pacific Conference on Medical and Biological Engineering, 2008, Beijing, China, 22–25 April 2008; Springer: Berlin, Germany, 2008; pp. 88–91. [Google Scholar]
- Duan, B.; Cheung, W.L.; Wang, M. Optimized fabrication of Ca–P/PHBV nanocomposite scaffolds via selective laser sintering for bone tissue engineering. Biofabrication 2011, 3, 015001. [Google Scholar]
- Meng, J.; Xiao, B.; Zhang, Y.; Liu, J.; Xue, H.; Lei, J.; Kong, H.; Huang, Y.; Jin, Z.; Gu, N. Super-paramagnetic responsive nanofibrous scaffolds under static magnetic field enhance osteogenesis for bone repair in vivo. Sci. Rep. 2013, 3. [Google Scholar] [CrossRef]
- Aderinto, J.; Blunn, G. The evaluation of a bone marrow stromal stem cell-hydroxyapatite composite graft for spinal fusion A rabbit model. J. Bone Jt. Surg. Br. Vol. 2006, 88, 368–368. [Google Scholar]
- Yeung, H.; Qin, L.; Hu, Y.Y.; Lee, K.; Cheng, J.C.; Chan, C.W.; Wong, K.; Yip, R. Bio-engineered mesenchymal stem cell-tricalcium phosphate ceramics composite augmented bone regeneration in posterior spinal fusion. Key Eng. Mater. 2007, 334, 1201–1204. [Google Scholar]
- Jo, J.H.; Lee, E.J.; Shin, D.S.; Kim, H.E.; Kim, H.W.; Koh, Y.H.; Jang, J.H. In vitro/in vivo biocompatibility and mechanical properties of bioactive glass nanofiber and poly (ε-caprolactone) composite materials. J. Biomed. Mater. Res. Part B 2009, 91, 213–220. [Google Scholar]
- Xu, S.; Lin, K.; Wang, Z.; Chang, J.; Wang, L.; Lu, J.; Ning, C. Reconstruction of calvarial defect of rabbits using porous calcium silicate bioactive ceramics. Biomaterials 2008, 29, 2588–2596. [Google Scholar]
- Silva, T.S.N.; Primo, B.T.; Júnior, S.; Novaes, A.; Machado, D.C.; Viezzer, C.; Santos, L.A. Use of calcium phosphate cement scaffolds for bone tissue engineering: In vitro study. Acta Cir. Bras. 2011, 26, 7–11. [Google Scholar]
- McCullen, S.; Zhu, Y.; Bernacki, S.; Narayan, R.; Pourdeyhimi, B.; Gorga, R.; Loboa, E. Electrospun composite poly (l-lactic acid)/tricalcium phosphate scaffolds induce proliferation and osteogenic differentiation of human adipose-derived stem cells. Biomed. Mater. 2009, 4, 035002. [Google Scholar]
- Xu, C.; Su, P.; Wang, Y.; Chen, X.; Meng, Y.; Liu, C.; Yu, X.; Yang, X.; Yu, W.; Zhang, X. A novel biomimetic composite scaffold hybridized with mesenchymal stem cells in repair of rat bone defects models. J. Biomed. Mater. Res. Part A 2010, 95, 495–503. [Google Scholar]
- Ni, S.; Chang, J. In vitro degradation bioactivity and cytocompatibility of calcium silicate dimagnesium silicate and tricalcium phosphate bioceramics. J. Biomater. Appl. 2009, 24, 139–158. [Google Scholar]
- Chiara, G.; Letizia, F.; Lorenzo, F.; Edoardo, S.; Diego, S.; Stefano, S.; Eriberto, B.; Barbara, Z. Nanostructured biomaterials for tissue engineered bone tissue reconstruction. Int. J. Mol. Sci. 2012, 13, 737–757. [Google Scholar]
- Schieker, M.; Seitz, H.; Drosse, I.; Seitz, S.; Mutschler, W. Biomaterials as scaffold for bone tissue engineering. Eur. J. Trauma 2006, 32, 114–124. [Google Scholar]
- Yoon, S.Y.; Park, H.C.; Jin, H.H.; Lee, W.K. Microstructural and mechanical properties of polymer-based scaffolds reinforced by hydroxyapatite. Mater. Sci. Forum 2007, 765–768. [Google Scholar]
- Xu, C.-Z.; Yang, W.-G.; He, X.-F.; Zhou, L.-T.; Han, X.-K.; Xu, X.-F. Vascular endothelial growth factor and nano-hydroxyapatite/collagen composite in the repair of femoral defect in rats. J. Clin. Rehabil. Tissue Eng. Res. 2011, 15. [Google Scholar] [CrossRef]
- Jingushi, S.; Urabe, K.; Okazaki, K.; Hirata, G.; Sakai, A.; Ikenoue, T.; Iwamoto, Y. Intramuscular bone induction by human recombinant bone morphogenetic protein-2 with beta-tricalcium phosphate as a carrier: In vivo bone banking for muscle-pedicle autograft. J. Orthop. Sci. 2002, 7, 490–494. [Google Scholar]
- Alvarez, K.; Nakajima, H. Metallic scaffolds for bone regeneration. Materials 2009, 2, 790–832. [Google Scholar]
- Wang, X.; Li, Y.; Hodgson, P.D.; Wen, C.E. Biomimetic modification of porous TiNbZr alloy scaffold for bone tissue engineering. Tissue Eng. Part A 2009, 16, 309–316. [Google Scholar]
- Xiong, J.; Li, Y.; Hodgson, P.D.; Wen, C.E. In vitro osteoblast-like cell proliferation on nano-hydroxyapatite coatings with different morphologies on a titanium-niobium shape memory alloy. J. Biomed. Mater. Res. Part A 2010, 95, 766–773. [Google Scholar]
- Ge, Z.; Jin, Z.; Cao, T. Manufacture of degradable polymeric scaffolds for bone regeneration. Biomed. Mater. 2008, 3, 022001. [Google Scholar]
- Zavan, B.; Vindigni, V.; Vezzù, K.; Zorzato, G.; Luni, C.; Abatangelo, G.; Elvassore, N.; Cortivo, R. Hyaluronan based porous nano-particles enriched with growth factors for the treatment of ulcers: A placebo-controlled study. J. Mater. Sci. 2009, 20, 235–247. [Google Scholar]
- Vasanthan, K.S.; Subramanian, A.; Krishnan, U.M.; Sethuraman, S. Role of biomaterials therapeutic molecules and cells for hepatic tissue engineering. Biotechnol. Adv. 2012, 30, 742–752. [Google Scholar]
- Cao, H.; Kuboyama, N. A biodegradable porous composite scaffold of PGA/β-TCP for bone tissue engineering. Bone 2010, 46, 386–395. [Google Scholar]
- Huang, Y.; Ren, J.; Chen, C.; Ren, T.; Zhou, X. Preparation and properties of poly (lactide-co-glycolide)(PLGA)/nano-hydroxyapatite (NHA) scaffolds by thermally induced phase separation and rabbit MSCs culture on scaffolds. J. Biomater. Appl. 2008, 22, 409–432. [Google Scholar]
- Ghomi, H.; Fathi, M.; Edris, H. Effect of the composition of hydroxyapatite/bioactive glass nanocomposite foams on their bioactivity and mechanical properties. Mater. Res. Bull. 2012, 47, 3523–3532. [Google Scholar]
- Lee, J.H.; Ryu, M.Y.; Baek, H.-R.; Lee, K.M.; Seo, J.-H.; Lee, H.-K. Fabrication and evaluation of porous beta-tricalcium phosphate/hydroxyapatite (60/40) composite as a bone graft extender using rat calvarial bone defect model. Sci. World J. 2013, 2013. [Google Scholar] [CrossRef]
- Lin, K.; Zhang, M.; Zhai, W.; Qu, H.; Chang, J. Fabrication and characterization of hydroxyapatite/wollastonite composite bioceramics with controllable properties for hard tissue repair. J. Am. Ceram. Soc. 2011, 94, 99–105. [Google Scholar]
- Hesaraki, S.; Safari, M.; Shokrgozar, M.A. Development of β-tricalcium phosphate/sol-gel derived bioactive glass composites: Physical mechanical and in vitro biological evaluations. J. Biomed. Mater. Res. Part B 2009, 91, 459–469. [Google Scholar]
- Shuai, C.; Gao, C.; Nie, Y.; Hu, H.; Zhou, Y.; Peng, S. Structure and properties of nano-hydroxypatite scaffolds for bone tissue engineering with a selective laser sintering system. Nanotechnology 2011, 22, 285703. [Google Scholar]
- Shuai, C.; Gao, C.; Nie, Y.; Hu, H.; Qu, H.; Peng, S. Structural design and experimental analysis of a selective laser sintering system with nano-hydroxyapatite powder. J. Biomed. Nanotechnol. 2010, 6, 370–374. [Google Scholar]
- Shuai, C.; Zhuang, J.; Hu, H.; Peng, S.; Liu, D.; Liu, J. In vitro bioactivity and degradability of β-tricalcium phosphate porous scaffold fabricated via selective laser sintering. Biotechnol. Appl. Biochem. 2013, 60, 266–273. [Google Scholar]
- Shuai, C.; Feng, P.; Zhang, L.; Gao, C.; Hu, H.; Peng, S.; Min, A. Correlation between properties and microstructure of laser sintered porous β-tricalcium phosphate bone scaffolds. Sci. Technol. Adv. Mater. 2013, 14, 055002. [Google Scholar]
- Shuai, C.; Li, P.; Liu, J.; Peng, S. Optimization of TCP/HAP ratio for better properties of calcium phosphate scaffold via selective laser sintering. Mater. Charact. 2013, 77, 23–31. [Google Scholar]


© 2014 by the authors; licensee MDPI, Basel, Switzerland This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Gao, C.; Deng, Y.; Feng, P.; Mao, Z.; Li, P.; Yang, B.; Deng, J.; Cao, Y.; Shuai, C.; Peng, S. Current Progress in Bioactive Ceramic Scaffolds for Bone Repair and Regeneration. Int. J. Mol. Sci. 2014, 15, 4714-4732. https://doi.org/10.3390/ijms15034714
Gao C, Deng Y, Feng P, Mao Z, Li P, Yang B, Deng J, Cao Y, Shuai C, Peng S. Current Progress in Bioactive Ceramic Scaffolds for Bone Repair and Regeneration. International Journal of Molecular Sciences. 2014; 15(3):4714-4732. https://doi.org/10.3390/ijms15034714
Chicago/Turabian StyleGao, Chengde, Youwen Deng, Pei Feng, Zhongzheng Mao, Pengjian Li, Bo Yang, Junjie Deng, Yiyuan Cao, Cijun Shuai, and Shuping Peng. 2014. "Current Progress in Bioactive Ceramic Scaffolds for Bone Repair and Regeneration" International Journal of Molecular Sciences 15, no. 3: 4714-4732. https://doi.org/10.3390/ijms15034714
APA StyleGao, C., Deng, Y., Feng, P., Mao, Z., Li, P., Yang, B., Deng, J., Cao, Y., Shuai, C., & Peng, S. (2014). Current Progress in Bioactive Ceramic Scaffolds for Bone Repair and Regeneration. International Journal of Molecular Sciences, 15(3), 4714-4732. https://doi.org/10.3390/ijms15034714





