Single and Binge Methamphetamine Administrations Have Different Effects on the Levels of Dopamine D2 Autoreceptor and Dopamine Transporter in Rat Striatum
Abstract
:1. Introduction
2. Results and Discussion
2.1. Dopamine D2 Receptor and DAT Species in Rat Striatal Synaptosomes
2.2. Single High Dose of Methamphetamine Causes Transient Hyperthermia but Does not Result in Neurotoxicity to Dopaminergic Terminals
2.3. Methamphetamine Differentially Affects Dopamine D2S Receptor and DAT Immunoreactivity in Rat Striatal Synaptosomes
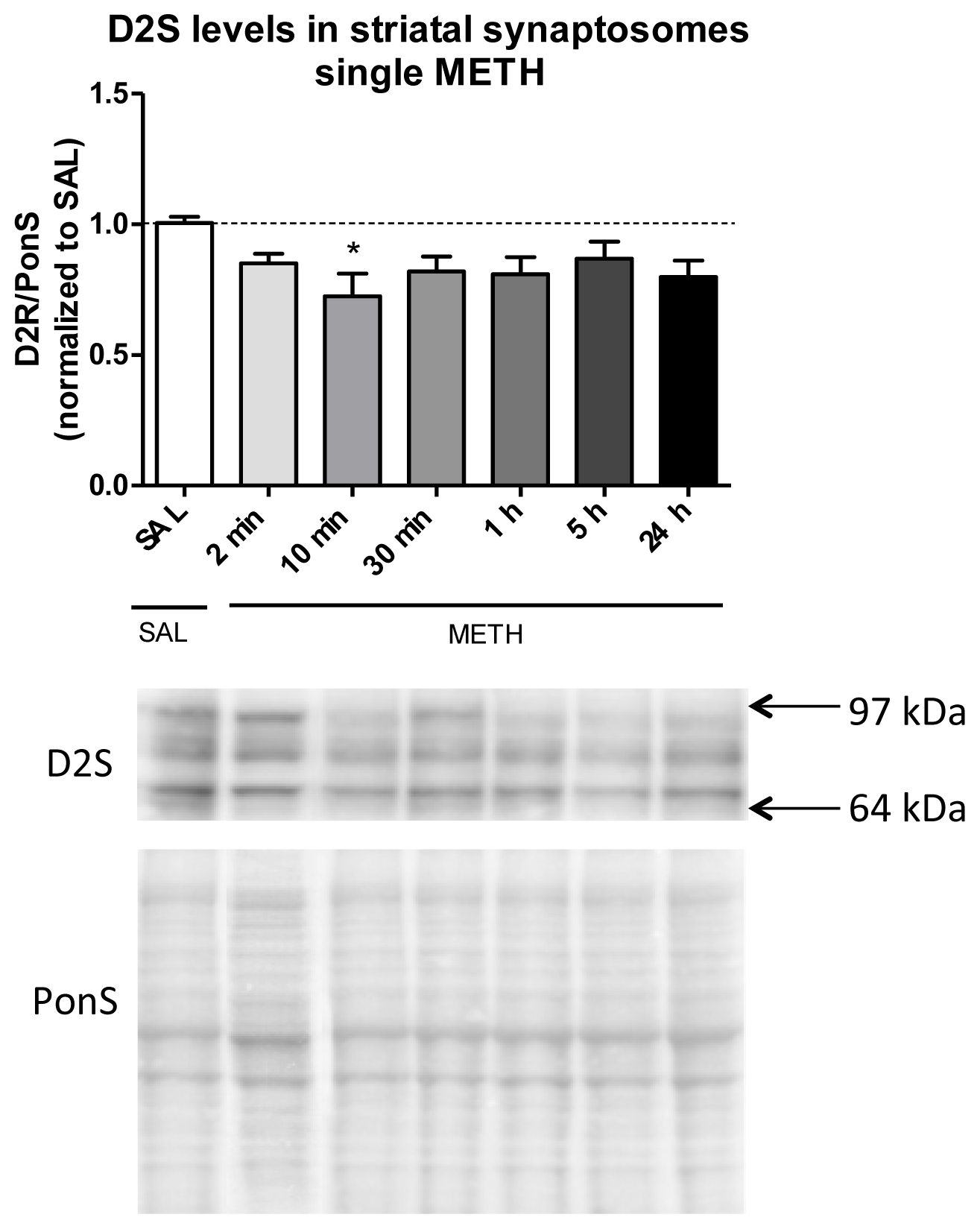
2.3.1. Single Dose of Methamphetamine Decreases the Immunoreactivity of D2S Receptor in Rat Striatal Synaptosomes
2.3.2. Single Dose of Methamphetamine Increases the Immunoreactivity of DAT in Rat Striatal Synaptosomes
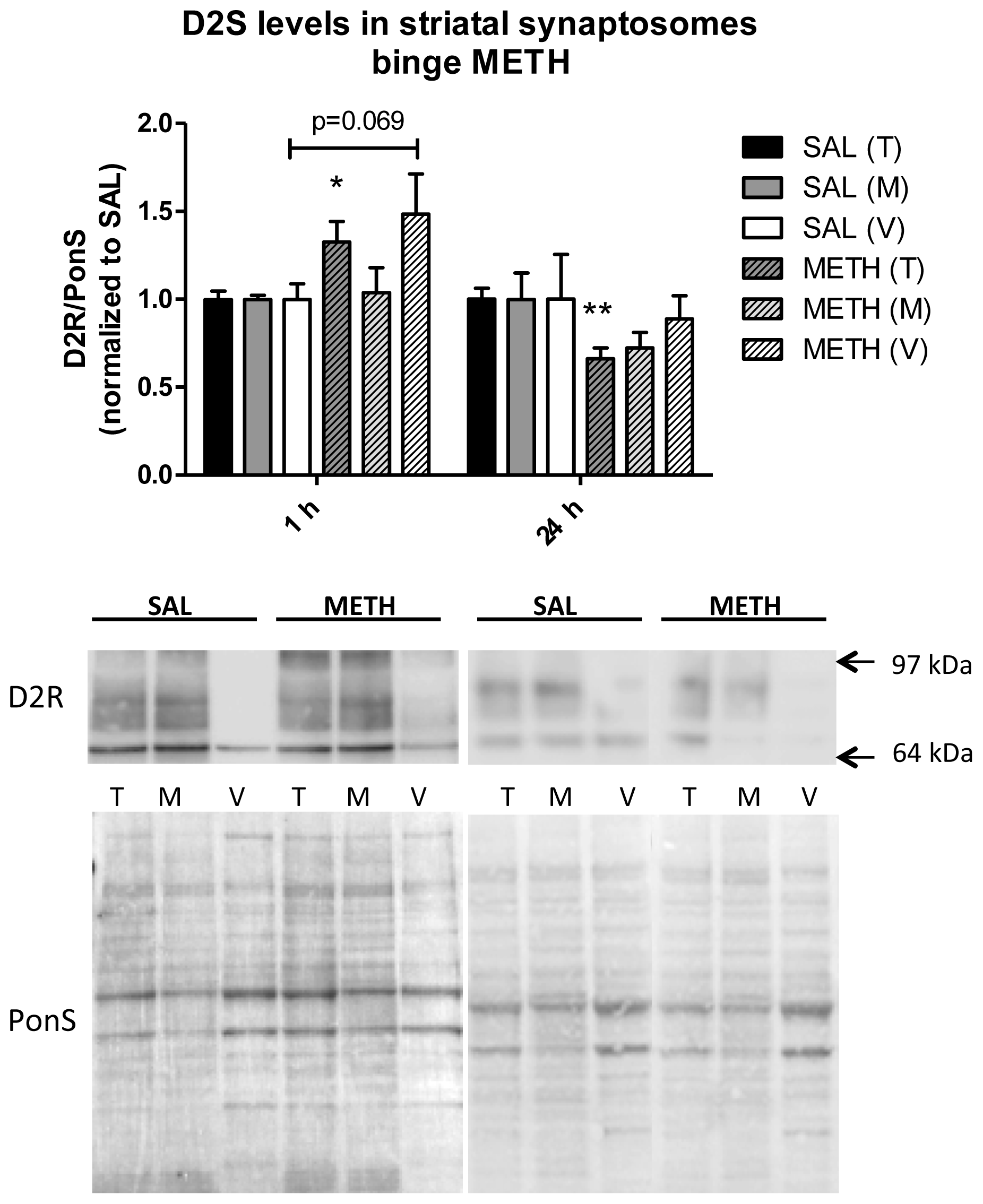
2.3.3. Binge Methamphetamine Increases Immunoreactivity of D2S Receptor in Rat Striatal Synaptosomes Shortly after the Last Injection of the Drug
2.3.4. Binge Methamphetamine Has no Effect on the Immunoreactivity of DAT in Striatal Synaptosomes Shortly after the Last Injection of the Drug
2.4. Binge Methamphetamine Increases D2S Receptor-DAT Protein-Protein Interaction
3. Experimental Section
3.1. Animals
3.2. Administration of Methamphetamine
3.3. Tissue Content of Dopamine
3.4. Preparation of Striatal Synaptosomal Fractions
3.5. SDS-PAGE and Western Blotting
3.6. Co-Immunoprecipitation
3.7. Statistical Analysis
4. Conclusions
Acknowledgments
Conflicts of Interest
- Author ContributionsAnna Moszczynska conceived and designed the study as well as wrote the manuscript; Heli Chauhan carried out most of binge dose and single dose METH experiments; Bryan A. Killinger carried out part of single dose METH experiments; Cheryl V. Miller carried out part of binge dose METH experiments.
References
- Hall, D.A.; Strange, P.G. Comparison of the ability of dopamine receptor agonists to inhibit forskolin-stimulated adenosine 3′5′-cyclic monophosphate (cAMP) accumulation via D2L (long isoform) and D3 receptors expressed in Chinese hamster ovary (CHO) cells. Biochem. Pharmacol 1999, 58, 285–289. [Google Scholar]
- Dal Toso, R.; Sommer, B.; Ewert, M.; Herb, A.; Pritchett, D.B.; Bach, A.; Shivers, B.D.; Seeburg, P.H. The dopamine D2 receptor: Two molecular forms generated by alternative splicing. EMBO J 1989, 8, 4025–4034. [Google Scholar]
- Seeman, P.; Nam, D.; Ulpian, C.; Liu, I.S.; Tallerico, T. New dopamine receptor, D2Longer, with unique TG splice site, in human brain. Mol. Brain Res 2000, 76, 132–141. [Google Scholar]
- Fishburn, C.S.; Elazar, Z.; Fuchs, S. Differential glycosylation and intracellular trafficking for the long and short isoforms of the D2 dopamine receptor. J. Biol. Chem 1995, 270, 29819–29824. [Google Scholar]
- Herdon, H.; Strupish, J.; Nahorski, S.R. Endogenous dopamine release from rat striatal slices and its regulation by D-2 autoreceptors: Effects of uptake inhibitors and synthesis inhibition. Eur. J. Pharmacol 1987, 138, 69–76. [Google Scholar]
- L’Hirondel, M.; Cheramy, A.; Godeheu, G.; Artaud, F.; Saiardi, A.; Borrelli, E.; Glowinski, J. Lack of autoreceptor-mediated inhibitory control of dopamine release in striatal synaptosomes of D2 receptor-deficient mice. Brain Res 1998, 792, 253–262. [Google Scholar]
- Lindgren, N.; Xu, Z.Q.; Herrera-Marschitz, M.; Haycock, J.; Hokfelt, T.; Fisone, G. Dopamine D(2) receptors regulate tyrosine hydroxylase activity and phosphorylation at Ser40 in rat striatum. Eur. J. Neurosci 2001, 13, 773–780. [Google Scholar]
- Mayfield, R.D.; Zahniser, N.R. Dopamine D2 receptor regulation of the dopamine transporter expressed in Xenopus laevis oocytes is voltage-independent. Mol. Pharmacol 2001, 59, 113–121. [Google Scholar]
- Boudanova, E.; Navaroli, D.M.; Melikian, H.E. Amphetamine-induced decreases in dopamine transporter surface expression are protein kinase C-independent. Neuropharmacology 2008, 54, 605–612. [Google Scholar]
- Lee, F.J.; Pei, L.; Moszczynska, A.; Vukusic, B.; Fletcher, P.J.; Liu, F. Dopamine transporter cell surface localization facilitated by a direct interaction with the dopamine D2 receptor. EMBO J 2007, 26, 2127–2136. [Google Scholar]
- Yamamoto, B.K.; Moszczynska, A.; Gudelsky, G.A. Amphetamine toxicities: Classical and emerging mechanisms. Ann. N. Y. Acad. Sci 2010, 1187, 101–121. [Google Scholar]
- Ares-Santos, S.; Granado, N.; Moratalla, R. The role of dopamine receptors in the neurotoxicity of methamphetamine. J. Intern. Med 2013, 273, 437–453. [Google Scholar]
- Marek, G.J.; Vosmer, G.; Seiden, L.S. Dopamine uptake inhibitors block long-term neurotoxic effects of methamphetamine upon dopaminergic neurons. Brain Res 1990, 513, 274–279. [Google Scholar]
- Pu, C.; Fisher, J.E.; Cappon, G.D.; Vorhees, C.V. The effects of amfonelic acid, a dopamine uptake inhibitor, on methamphetamine-induced dopaminergic terminal degeneration and astrocytic response in rat striatum. Brain Res 1994, 649, 217–224. [Google Scholar]
- Sulzer, D.; Rayport, S. Amphetamine and other psychostimulants reduce pH gradients in midbrain dopaminergic neurons and chromaffin granules: A mechanism of action. Neuron 1990, 5, 797–808. [Google Scholar]
- Parker, E.M.; Cubeddu, L.X. Comparative effects of amphetamine, phenylethylamine and related drugs on dopamine efflux, dopamine uptake and mazindol binding. J. Pharmacol. Exp. Ther 1988, 245, 199–210. [Google Scholar]
- Riddle, E.L.; Fleckenstein, A.E.; Hanson, G.R. Role of monoamine transporters in mediating psychostimulant effects. AAPS J 2005, 7, 847–851. [Google Scholar]
- Sun, W.; Ginovart, N.; Ko, F.; Seeman, P.; Kapur, S. In vivo evidence for dopamine-mediated internalization of D2-receptors after amphetamine: Differential findings with [3H]raclopride vs. [3H]spiperone. Mol. Pharmacol 2003, 63, 456–462. [Google Scholar]
- Laruelle, M.; Iyer, R.N.; al-Tikriti, M.S.; Zea-Ponce, Y.; Malison, R.; Zoghbi, S.S.; Baldwin, R.M.; Kung, H.F.; Charney, D.S.; Hoffer, P.B.; et al. Microdialysis and SPECT measurements of amphetamine-induced dopamine release in nonhuman primates. Synapse 1997, 25, 1–14. [Google Scholar]
- Tirotta, E.; Fontaine, V.; Picetti, R.; Lombardi, M.; Samad, T.A.; Oulad-Abdelghani, M.; Edwards, R.; Borrelli, E. Signaling by dopamine regulates D2 receptors trafficking at the membrane. Cell Cycle 2008, 7, 2241–2248. [Google Scholar]
- Gulley, J.M.; Zahniser, N.R. Rapid regulation of dopamine transporter function by substrates, blockers and presynaptic receptor ligands. Eur. J. Pharmacol 2003, 479, 139–152. [Google Scholar]
- Richards, T.L.; Zahniser, N.R. Rapid substrate-induced down-regulation in function and surface localization of dopamine transporters: Rat dorsal striatum versus nucleus accumbens. J. Neurochem 2009, 108, 1575–1584. [Google Scholar]
- German, C.L.; Hanson, G.R.; Fleckenstein, A.E. Amphetamine and methamphetamine reduce striatal dopamine transporter function without concurrent dopamine transporter relocalization. J. Neurochem 2012, 123, 288–297. [Google Scholar]
- Thrash, B.; Thiruchelvan, K.; Ahuja, M.; Suppiramaniam, V.; Dhanasekaran, M. Methamphetamine-induced neurotoxicity: The road to Parkinson’s disease. Pharmacol. Rep 2009, 61, 966–977. [Google Scholar]
- Chu, Y.; Morfini, G.A.; Langhamer, L.B.; He, Y.; Brady, S.T.; Kordower, J.H. Alterations in axonal transport motor proteins in sporadic and experimental Parkinson’s disease. Brain J. Neurol 2012, 135, 2058–2073. [Google Scholar]
- Chen, R.; Daining, C.P.; Sun, H.; Fraser, R.; Stokes, S.L.; Leitges, M.; Gnegy, M.E. Protein kinase Cbeta is a modulator of the dopamine D2 autoreceptor-activated trafficking of the dopamine transporter. J. Neurochem 2013, 125, 663–672. [Google Scholar]
- Bertolino, A.; Fazio, L.; Caforio, G.; Blasi, G.; Rampino, A.; Romano, R.; di Giorgio, A.; Taurisano, P.; Papp, A.; Pinsonneault, J.; et al. Functional variants of the dopamine receptor D2 gene modulate prefronto-striatal phenotypes in schizophrenia. Brain J. Neurol 2009, 132, 417–425. [Google Scholar]
- Wang, M.; Pei, L.; Fletcher, P.J.; Kapur, S.; Seeman, P.; Liu, F. Schizophrenia, amphetamine-induced sensitized state and acute amphetamine exposure all show a common alteration: Increased dopamine D2 receptor dimerization. Mol. Brain 2010, 3. [Google Scholar] [CrossRef]
- David, C.; Fishburn, C.S.; Monsma, F.J., Jr.; Sibley, D.R.; Fuchs, S. Synthesis and processing of D2 dopamine receptors. Biochemistry 1993, 32, 8179–8183. [Google Scholar]
- Zawarynski, P.; Tallerico, T.; Seeman, P.; Lee, S.P.; O’Dowd, B.F.; George, S.R. Dopamine D2 receptor dimers in human and rat brain. FEBS Lett 1998, 441, 383–386. [Google Scholar]
- Torres, G.E.; Carneiro, A.; Seamans, K.; Fiorentini, C.; Sweeney, A.; Yao, W.D.; Caron, M.G. Oligomerization and trafficking of the human dopamine transporter. Mutational analysis identifies critical domains important for the functional expression of the transporter. J. Biol. Chem 2003, 278, 2731–2739. [Google Scholar]
- Torvinen, M.; Torri, C.; Tombesi, A.; Marcellino, D.; Watson, S.; Lluis, C.; Franco, R.; Fuxe, K.; Agnati, L.F. Trafficking of adenosine A2A and dopamine D2 receptors. J. Mol. Neurosci 2005, 25, 191–200. [Google Scholar]
- Lee, S.P.; O’Dowd, B.F.; Rajaram, R.D.; Nguyen, T.; George, S.R. D2 dopamine receptor homodimerization is mediated by multiple sites of interaction, including an intermolecular interaction involving transmembrane domain 4. Biochemistry 2003, 42, 11023–11031. [Google Scholar]
- David, C.; Ewert, M.; Seeburg, P.H.; Fuchs, S. Antipeptide antibodies differentiate between long and short isoforms of the D2 dopamine receptor. Biochem. Biophys. Res. Commun 1991, 179, 824–829. [Google Scholar]
- Prou, D.; Gu, W.-J.; le Crom, S.; Vincent, J.-D.; Salamero, J.; Vernier, P. Intracellular retention of the two isoforms of the D2 dopamine receptor promotes endoplasmic reticulum disruption. J. Cell Sci 2001, 114, 3517–3527. [Google Scholar]
- Cadet, J.L.; Brannock, C.; Krasnova, I.N.; Ladenheim, B.; McCoy, M.T.; Chou, J.; Lehrmann, E.; Wood, W.H.; Becker, K.G.; Wang, Y. Methamphetamine-induced dopamine-independent alterations in striatal gene expression in the 6-hydroxydopamine hemiparkinsonian rats. PLoS One 2010, 5, e15643. [Google Scholar]
- Ali, S.F.; Newport, G.D.; Holson, R.R.; Slikker, W., Jr.; Bowyer, J.F. Low environmental temperatures or pharmacologic agents that produce hypothermia decrease methamphetamine neurotoxicity in mice. Brain Res 1994, 658, 33–38. [Google Scholar]
- Ali, S.F.; Newport, G.D.; Slikker, W., Jr. Methamphetamine-induced dopaminergic toxicity in mice. Role of environmental temperature and pharmacological agents. Ann. N. Y. Acad. Sci 1996, 801, 187–198. [Google Scholar]
- Moszczynska, A.; Yamamoto, B.K. Methamphetamine oxidatively damages parkin and decreases the activity of 26S proteasome in vivo. J. Neurochem. 2011, 116, 1005–1017. [Google Scholar]
- Cappon, G.D.; Pu, C.; Vorhees, C.V. Time-course of methamphetamine-induced neurotoxicity in rat caudate-putamen after single-dose treatment. Brain Res 2000, 863, 106–111. [Google Scholar]
- Beauvais, G.; Atwell, K.; Jayanthi, S.; Ladenheim, B.; Cadet, J.L. Involvement of dopamine receptors in binge methamphetamine-induced activation of endoplasmic reticulum and mitochondrial stress pathways. PLoS One 2011, 6, e28946. [Google Scholar]
- Ng, G.Y.; Varghese, G.; Chung, H.T.; Trogadis, J.; Seeman, P.; O’Dowd, B.F.; George, S.R. Resistance of the dopamine D2L receptor to desensitization accompanies the up-regulation of receptors on to the surface of Sf9 cells. Endocrinology 1997, 138, 4199–4206. [Google Scholar]
- Johnson, L.A.; Furman, C.A.; Zhang, M.; Guptaroy, B.; Gnegy, M.E. Rapid delivery of the dopamine transporter to the plasmalemmal membrane upon amphetamine stimulation. Neuropharmacology 2005, 49, 750–758. [Google Scholar]
- Guo, N.; Guo, W.; Kralikova, M.; Jiang, M.; Schieren, I.; Narendran, R.; Slifstein, M.; Abi-Dargham, A.; Laruelle, M.; Javitch, J.A.; et al. Impact of D2 receptor internalization on binding affinity of neuroimaging radiotracers. Neuropsychopharmacology 2010, 35, 806–817. [Google Scholar]
- Namkung, Y.; Dipace, C.; Javitch, J.A.; Sibley, D.R. G protein-coupled receptor kinase-mediated phosphorylation regulates post-endocytic trafficking of the D2 dopamine receptor. J. Biol. Chem 2009, 284, 15038–15051. [Google Scholar]
- Cho, A.K.; Melega, W.P.; Kuczenski, R.; Segal, D.S. Relevance of pharmacokinetic parameters in animal models of methamphetamine abuse. Synapse 2001, 39, 161–166. [Google Scholar]
- Zombeck, J.A.; Gupta, T.; Rhodes, J.S. Evaluation of a pharmacokinetic hypothesis for reduced locomotor stimulation from methamphetamine and cocaine in adolescent versus adult male C57BL/6J mice. Psychopharmacology 2009, 201, 589–599. [Google Scholar]
- Riviere, G.J.; Byrnes, K.A.; Gentry, W.B.; Owens, S.M. Spontaneous locomotor activity and pharmacokinetics of intravenous methamphetamine and its metabolite amphetamine in the rat. J. Pharmacol. Exp. Ther 1999, 291, 1220–1226. [Google Scholar]
- Gentry, W.B.; Ghafoor, A.U.; Wessinger, W.D.; Laurenzana, E.M.; Hendrickson, H.P.; Owens, S.M. (+)-Methamphetamine-induced spontaneous behavior in rats depends on route of (+)METH administration. Pharmacol. Biochem. Behav 2004, 79, 751–760. [Google Scholar]
- Bartlett, S.E.; Enquist, J.; Hopf, F.W.; Lee, J.H.; Gladher, F.; Kharazia, V.; Waldhoer, M.; Mailliard, W.S.; Armstrong, R.; Bonci, A.; et al. Dopamine responsiveness is regulated by targeted sorting of D2 receptors. Proc. Natl. Acad. Sci. USA 2005, 102, 11521–11526. [Google Scholar]
- Banday, A.A.; Fazili, F.R.; Lokhandwala, M.F. Oxidative stress causes renal dopamine D1 receptor dysfunction and hypertension via mechanisms that involve nuclear factor-κB and protein kinase C. J. Am. Soc. Nephrol 2007, 18, 1446–1457. [Google Scholar]
- Cho, D.I.; Min, C.; Jung, K.S.; Cheong, S.Y.; Zheng, M.; Cheong, S.J.; Oak, M.H.; Cheong, J.H.; Lee, B.K.; Kim, K.M. The N-terminal region of the dopamine D2 receptor, a rhodopsin-like GPCR, regulates correct integration into the plasma membrane and endocytic routes. Br. J. Pharmacol 2012, 166, 659–675. [Google Scholar]
- Schiffmann, S.N.; Fisone, G.; Moresco, R.; Cunha, R.A.; Ferre, S. Adenosine A2A receptors and basal ganglia physiology. Prog. Neurobiol 2007, 83, 277–292. [Google Scholar]
- Ichikawa, J.; Meltzer, H.Y. The effect of chronic atypical antipsychotic drugs and haloperidol on amphetamine-induced dopamine release in vivo. Brain Res. 1992, 574, 98–104. [Google Scholar]
- Saunders, C.; Ferrer, J.V.; Shi, L.; Chen, J.; Merrill, G.; Lamb, M.E.; Leeb-Lundberg, L.M.; Carvelli, L.; Javitch, J.A.; Galli, A. Amphetamine-induced loss of human dopamine transporter activity: an internalization-dependent and cocaine-sensitive mechanism. Proc. Natl. Acad. Sci. USA 2000, 97, 6850–6855. [Google Scholar]
- Chen, R.; Furman, C.A.; Zhang, M.; Kim, M.N.; Gereau, R.W., IV; Leitges, M.; Gnegy, M.E. Protein kinase Cbeta is a critical regulator of dopamine transporter trafficking and regulates the behavioral response to amphetamine in mice. J. Pharmacol. Exp. Ther 2009, 328, 912–920. [Google Scholar]
- Furman, C.A.; Chen, R.; Guptaroy, B.; Zhang, M.; Holz, R.W.; Gnegy, M. Dopamine and amphetamine rapidly increase dopamine transporter trafficking to the surface: Live-cell imaging using total internal reflection fluorescence microscopy. J. Neurosci 2009, 29, 3328–3336. [Google Scholar]
- Gulley, J.M.; Doolen, S.; Zahniser, N.R. Brief, repeated exposure to substrates down-regulates dopamine transporter function in Xenopus oocytes in vitro and rat dorsal striatum in vivo. J. Neurochem. 2002, 83, 400–411. [Google Scholar]
- Fang, C.; Bourdette, D.; Banker, G. Oxidative stress inhibits axonal transport: Implications for neurodegenerative diseases. Mol. Neurodegener 2012, 7. [Google Scholar] [CrossRef]
- Fuxe, K.; Ferre, S.; Canals, M.; Torvinen, M.; Terasmaa, A.; Marcellino, D.; Goldberg, S.R.; Staines, W.; Jacobsen, K.X.; Lluis, C.; et al. Adenosine A2A and dopamine D2 heteromeric receptor complexes and their function. J. Mol. Neurosci 2005, 26, 209–220. [Google Scholar]
- Jones, S.R.; Gainetdinov, R.R.; Hu, X.T.; Cooper, D.C.; Wightman, R.M.; White, F.J.; Caron, M.G. Loss of autoreceptor functions in mice lacking the dopamine transporter. Nat. Neurosci 1999, 2, 649–655. [Google Scholar]
- Schmitz, Y.; Lee, C.J.; Schmauss, C.; Gonon, F.; Sulzer, D. Amphetamine distorts stimulation-dependent dopamine overflow: Effects on D2 autoreceptors, transporters, and synaptic vesicle stores. J. Neurosci 2001, 21, 5916–5924. [Google Scholar]
- Cubells, J.F.; Rayport, S.; Rajendran, G.; Sulzer, D. Methamphetamine neurotoxicity involves vacuolation of endocytic organelles and dopamine-dependent intracellular oxidative stress. J. Neurosci 1994, 14, 2260–2271. [Google Scholar]
- Innis, R.B.; Malison, R.T.; al-Tikriti, M.; Hoffer, P.B.; Sybirska, E.H.; Seibyl, J.P.; Zoghbi, S.S.; Baldwin, R.M.; Laruelle, M.; Smith, E.O.; et al. Amphetamine-stimulated dopamine release competes in vivo for [123I]IBZM binding to the D2 receptor in nonhuman primates. Synapse 1992, 10, 177–184. [Google Scholar]
- Genedani, S.; Carone, C.; Guidolin, D.; Filaferro, M.; Marcellino, D.; Fuxe, K.; Agnati, L.F. Differential sensitivity of A2A and especially D2 receptor trafficking to cocaine compared with lipid rafts in cotransfected CHO cell lines. Novel actions of cocaine independent of the DA transporter. J. Mol. Neurosci 2010, 41, 347–357. [Google Scholar]
- Fleckenstein, A.E.; Metzger, R.R.; Beyeler, M.L.; Gibb, J.W.; Hanson, G.R. Oxygen radicals diminish dopamine transporter function in rat striatum. Eur. J. Pharmacol 1997, 334, 111–114. [Google Scholar]
- Berman, S.B.; Zigmond, M.J.; Hastings, T.G. Modification of dopamine transporter function: Effect of reactive oxygen species and dopamine. J. Neurochem 1996, 67, 593–600. [Google Scholar]
- Kimmel, H.L.; Joyce, A.R.; Carroll, F.I.; Kuhar, M.J. Dopamine D1 and D2 receptors influence dopamine transporter synthesis and degradation in the rat. J. Pharmacol. Exp. Ther 2001, 298, 129–140. [Google Scholar]
- Pristupa, Z.B.; McConkey, F.; Liu, F.; Man, H.Y.; Lee, F.J.; Wang, Y.T.; Niznik, H.B. Protein kinase-mediated bidirectional trafficking and functional regulation of the human dopamine transporter. Synapse 1998, 30, 79–87. [Google Scholar]
- Bertolino, A.; Fazio, L.; di Giorgio, A.; Blasi, G.; Romano, R.; Taurisano, P.; Caforio, G.; Sinibaldi, L.; Ursini, G.; Popolizio, T.W.; et al. Genetically determined interaction between the dopamine transporter and the D2 receptor on prefronto-striatal activity and volume in humans. J. Neurosci 2009, 29, 1224–1234. [Google Scholar]
- Baucum, A.J., II; Rau, K.S.; Riddle, E.L.; Hanson, G.R.; Fleckenstein, A.E. Methamphetamine increases dopamine transporter higher molecular weight complex formation via a dopamine- and hyperthermia-associated mechanism. J. Neurosci 2004, 24, 3436–3443. [Google Scholar]
- Kokoshka, J.M.; Vaughan, R.A.; Hanson, G.R.; Fleckenstein, A.E. Nature of methamphetamine-induced rapid and reversible changes in dopamine transporters. Eur. J. Pharmacol 1998, 361, 269–275. [Google Scholar]





© 2014 by the authors; licensee MDPI, Basel, Switzerland This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Chauhan, H.; Killinger, B.A.; Miller, C.V.; Moszczynska, A. Single and Binge Methamphetamine Administrations Have Different Effects on the Levels of Dopamine D2 Autoreceptor and Dopamine Transporter in Rat Striatum. Int. J. Mol. Sci. 2014, 15, 5884-5906. https://doi.org/10.3390/ijms15045884
Chauhan H, Killinger BA, Miller CV, Moszczynska A. Single and Binge Methamphetamine Administrations Have Different Effects on the Levels of Dopamine D2 Autoreceptor and Dopamine Transporter in Rat Striatum. International Journal of Molecular Sciences. 2014; 15(4):5884-5906. https://doi.org/10.3390/ijms15045884
Chicago/Turabian StyleChauhan, Heli, Bryan A. Killinger, Cheryl V. Miller, and Anna Moszczynska. 2014. "Single and Binge Methamphetamine Administrations Have Different Effects on the Levels of Dopamine D2 Autoreceptor and Dopamine Transporter in Rat Striatum" International Journal of Molecular Sciences 15, no. 4: 5884-5906. https://doi.org/10.3390/ijms15045884
APA StyleChauhan, H., Killinger, B. A., Miller, C. V., & Moszczynska, A. (2014). Single and Binge Methamphetamine Administrations Have Different Effects on the Levels of Dopamine D2 Autoreceptor and Dopamine Transporter in Rat Striatum. International Journal of Molecular Sciences, 15(4), 5884-5906. https://doi.org/10.3390/ijms15045884





