Inhibitors of Intracellular Signaling Pathways that Lead to Stimulated Epidermal Pigmentation: Perspective of Anti-Pigmenting Agents
Abstract
:1. Introduction
2. Paracrine Cytokine Mechanisms Underlying the Hyperpigmentation of UVB-Melanosis, Solar Lentigo and Melasma
3. Intracellular Signaling Mechanisms Associated with Endothelin-1 (EDN1) and Stem Cell Factor (SCF)
4. Effects of Specific Signaling Inhibitors on EDN1 or SCF Signaling
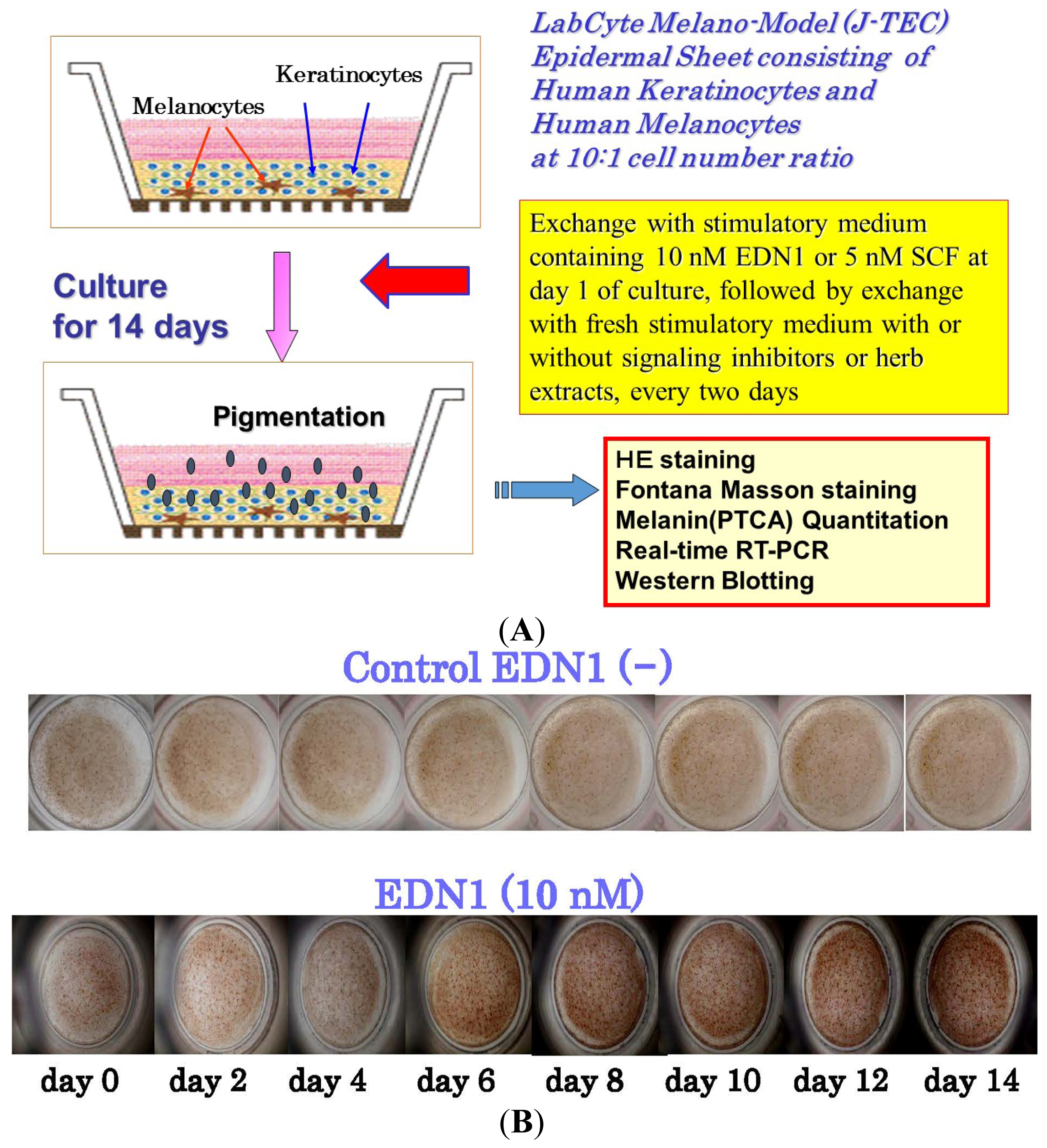
5. Effects of EDN1 or SCF on the Pigmentation of Human Epidermal Equivalents (HEEs)
6. Effects of Signaling Inhibitors on EDN1 or SCF-Stimulated Pigmentation
7. Screening of Herb Extracts or Natural Chemicals Capable of Interrupting the EDN1 or SCF-Triggered Intracellular Signaling Cascades
8. Withania somnifera
9. Effect of the Withania somnifera Extract (WSE) on EDN1 Signaling in Acral Lentigo Malignant (ALM) Melanoma Cells
10. Effect of the WSE on the EDN1-Stimulated Pigmentation of HEEs
11. Effect of the WSE on the Expression of Melanocyte-Specific Genes and Proteins
12. Summary of Substances Identified that Are Capable of Interrupting the SCF- or EDN1-Activated Intracellular Signaling Cascades and Their Abrogating Effects on the SCF- or EDN1-Stimulated Pigmentation in HEEs
13. Conclusions
Conflicts of Interest
Abbreviations
| EDN1 | endothelin-1 |
| EDNRB | endothelin B receptor |
| IL | interleukin |
| MAPK | mitogen activated protein kinase |
| MITF | microphthalmia associated transcription factor |
| NHM | normal human melanocytes |
| PKA | protein kinase A |
| PKC | protein kinase C |
| SCF | stem cell factor |
| TYRP-1 | tyrosinase-related protein-1 |
| DCT | dopachrome tautomerase |
| TYK | tyrosine kinase |
| WSE | Withania somnifera extract |
| MTE | Melia toosendan extract |
| TNF | tumor necrosis factor |
| αMSH | alpha melanocyte stimulating hormone |
| bFGF | basic fibroblast growth factor |
| ACTH | adrenocorticotropic hormone |
| MC1R | melanocortin 1 receptor |
| HEEs | human epidermal equivalents |
| PAR2 | proteinase-activated receptor 2 |
References
- Chung, K.W.; Park, Y.J.; Choi, Y.J.; Park, M.H.; Ha, Y.M.; Uehara, Y.; Yoon, J.H.; Chun, P.; Moon, H.R.; Chung, H.Y. Evaluation of in vitro and in vivo anti-melanogenic activity of a newly synthesized strong tyrosinase inhibitor (E)-3-(2,4-dihydroxybenzylidene) pyrrolidine-2,5-dione (3-DBP). Biochim. Biophys. Acta 2012, 1820, 962–969. [Google Scholar]
- Ando, H.; Funasaka, Y.; Oka, M.; Ohashi, A.; Furumura, M.; Matsunaga, J.; Matsunaga, N.; Hearing, V.J.; Ichihashi, M. Possible involvement of proteolytic degradation of tyrosinase in the regulatory effect of fatty acids on melanogenesis. J. Lipid Res 1999, 40, 1312–1316. [Google Scholar]
- Paine, C.; Sharlow, E.; Liebel, F.; Eisinger, M.; Shapiro, S.; Seiberg, M. An alternative approach to depigmentation by soybean extracts via inhibition of the PAR-2 pathway. J. Investig. Dermatol 2001, 116, 587–595. [Google Scholar]
- Yada, Y.; Higuchi, K.; Imokawa, G. Effects of endothelins on signal transduction and proliferation in human melanocytes. J. Biol. Chem 1991, 266, 18352–18357. [Google Scholar]
- Imokawa, G.; Yada, Y.; Miyagishi, M. Endothelins secreted from human keratinocytes are intrinsic mitogens for human melanocytes. J. Biol. Chem 1992, 267, 24675–24680. [Google Scholar]
- Imokawa, G.; Miyagishi, M.; Yada, Y. Endothelin-1 as a new melanogen: Coordinated expression of its gene and the tyrosinase gene in UVB-exposed human epidermis. J. Investig. Dermatol 1995, 105, 32–37. [Google Scholar]
- Imokawa, G.; Yada, Y.; Kimura, M. Signaling mechanisms of endothelin-induced mitogenesis and melanogenesis in human melanocytes. Biochem. J 1996, 314, 305–312. [Google Scholar]
- Imokawa, G.; Kobayashi, T.; Miyagishi, M.; Higashi, K.; Yada, Y. The role of endothelin-1 in epidermal hyperpigmentation and signaling mechanisms of mitogenesis and melanogenesis. Pigment Cell Res 1997, 10, 218–228. [Google Scholar]
- Hachiya, A.; Kobayashi, A.; Ohuchi, A.; Takema, Y.; Imokawa, G. The paracrine role of stem cell factor/c-kit signaling in the activation of human melanocytes in ultraviolet B-induced pigmentation. J. Investig. Dermatol 2001, 116, 578–586. [Google Scholar]
- Hachiya, A.; Kobayashi, T.; Takema, Y.; Imokawa, G. Biochemical characterization of endothelin-converting enzyme-1α in cultured skin-derived cells and its postulated role in the stimulation of melanogenesis in human epidermis. J. Biol. Chem 2002, 277, 5395–5403. [Google Scholar]
- Hachiya, A.; Kobayashi, A.; Yoshida, Y.; Kitahara, T.; Takema, Y.; Imokawa, G. Biphasic expression of two paracrine melanogenic cytokines, stem cell factor and endothelin-1, in ultraviolet B-induced human melanogenesis. Am. J. Pathol 2004, 65, 2099–2109. [Google Scholar]
- Kadono, S.; Manaka, I.; Kawashima, M.; Kobayashi, T.; Imokawa, G. The role of the epidermal endothelin cascade in the hyperpigmentation mechanism of lentigo senilis. J. Investig. Dermatol 2001, 116, 571–577. [Google Scholar]
- Hattori, H.; Kawashima, M.; Ichikawa, Y.; Imokawa, G. The epidermal stem cell factor is over-expressed in lentigo senilis: Implication for the mechanism of hyperpigmentation. J. Investig. Dermatol 2004, 122, 1256–1265. [Google Scholar]
- Kang, H.Y.; Hwang, J.S.; Lee, J.Y.; Ahn, J.H.; Kim, J.Y.; Lee, E.S.; Kang, W.H. The dermal stem cell factor and c-kit are overexpressed in melasma. Br. J. Dermatol 2006, 154, 1094–1099. [Google Scholar]
- Imokawa, G.; Yada, Y.; Morisaki, N.; Kimura, M. Biological characterization of human fibroblast-derived mitogenic factors for human melanocytes. Biochem. J 1998, 330, 1235–1239. [Google Scholar]
- Chakraborty, A.K.; Funasaka, Y.; Slominski, A.; Ermak, G.; Hwang, J.; Pawelek, J.; Ichihashi, M. Production and release of proopiomelanocortin (POMC) derived peptides by human melanocytes and keratinocytes in culture: Regulation by ultraviolet B. Biochim. Biophys. Acta 1996, 1313, 130–138. [Google Scholar]
- Schauer, E.; Trautinger, F.; Köck, A.; Schwarz, A.; Bhardwaj, R.; Simon, M.; Ansel, J.C.; Schwarz, T.; Luger, T.A. Proopiomelanocortin-derived peptides are synthesized and released by human keratinocytes. J. Clin. Investig 1994, 93, 2258–2262. [Google Scholar]
- Abdel-Malek, Z.; Swope, V.B.; Suzuki, I.; Akcali, C.; Harriger, M.D.; Boyce, S.T.; Urabe, K.; Hearing, V.J. Mitogenic and melanogenic stimulation of normal human melanocytes by melanotropic peptides. Proc. Natl. Acad. Sci. USA 1995, 92, 1789–1793. [Google Scholar]
- Suzuki, I.; Cone, R.D.; Im, S.; Nordlund, J.; Abdel-Malek, Z.A. Binding of melanotropic hormones to the melanocortin receptor MC1R on human melanocytes stimulates proliferation and melanogenesis. Endocrinology 1996, 137, 1627–1633. [Google Scholar]
- Kawashima, M.; Imokawa, G. Hyperpigmentation mechanisms involved in UVB-melanosis and solar lentigo and clinical effects of Chamomira ET extract on the pigmentation. Mon. Book Derma 2005, 98, 43–61. [Google Scholar]
- Kobayashi, T.; Imokawa, G.; Hearing, V.J. Differentiation of epidermal melanocytes by UVB irradiation in tail skin of mouse (C57BL/6J mice-aa/ee, recessive yellow) with mutation of melanocortin receptor. Jpn. J. Dermatol 1996, 106, 716. [Google Scholar]
- Niwano, T.; Nakajima, H.; Imokawa, G. Paracrine interaction between UVB-exposed human keratinocytes and human melanocytes in co-culture system with cell insert leading to an increased expression of tyrosinase and its blockade by Witherferin A. Pigment Cell Melanoma Res 2012, 25, 917. [Google Scholar]
- Kolch, W.; Heidecker, G.; Kochs, G.; Hummel, R.; Vahidi, H.; Mischak, H.; Finkenzeller, G.; Marme, D.; Rapp, U.R. Protein kinase Cα activates RAF-1 by direct phosphorylation. Nature 1993, 364, 249–252. [Google Scholar]
- Schonwasser, D.C.; Marais, R.M.; Marshall, C.J.; Parker, P.J. Activation of the mitogen-activated protein kinase/extracellular signal-regulated kinase pathway by conventional, novel, and atypical protein kinase C isotypes. Mol. Cell. Biol 1998, 18, 790–798. [Google Scholar]
- Mason, C.S.; Springer, C.J.; Cooper, R.G.; Superti-Furga, G.; Marshall, C.J.; Marais, R. Serine and tyrosine phosphorylations cooperate in Raf-1, but not B-Raf activation. EMBO J 1999, 18, 2137–2148. [Google Scholar]
- Marais, R.; Light, Y.; Mason, C.; Paterson, H.; Olson, M.F.; Marshall, C.J. Requirement of Ras-GTP-Raf complexes for activation of Raf-1 by protein kinase C. Science 1998, 280, 109–112. [Google Scholar]
- Imokawa, G.; Kobayashi, T.; Miyagishi, M. Intracellular signaling mechanisms leading to synergistic effects of endothelin-1 and stem cell factor on proliferation of cultured human melanocytes: Cross-talk via trans-activation of the tyrosine kinase c-kit receptor. J. Biol. Chem 2000, 275, 33321–33328. [Google Scholar]
- Sato-Jin, K.; Nishimura, E.K.; Akasaka, E.; Huber, W.; Nakano, H.; Miller, A.; Du, J.; Wu, M.; Hanada, K.; Sawamura, D.; et al. Epistatic connections between MITF and endothelin signaling in Waardenburg syndrome and other pigmentary disorders. FASEB J 2008, 22, 1155–1168. [Google Scholar]
- Blume-Jensen, P.; Claesson-Welsh, L.; Siegbahn, A.; Zsebo, K.M.; Westermark, B.; Heldin, C.H. Activation of the human c-kit product by ligand-induced dimerization mediates circular actin reorganization and chemotaxis. EMBO J 1991, 10, 4121–4128. [Google Scholar]
- Cutler, R.L.; Liu, L.; Damen, J.E.; Krystal, G. Multiple cytokines induce the tyrosine phosphorylation of Shc and its association with Grb2 in hemopoietic cells. J. Biol. Chem 1993, 268, 21463–21465. [Google Scholar]
- Lennartsson, J.; Blume-Jensen, P.; Hermanson, M.; Ponten, E.; Carlberg, M.; Ronnstrand, L. Phosphorylation of Shc by Src family kinases is necessary for stem cell factor receptor/c-kit mediated activation of the Ras/MAP kinase pathway and c-fos induction. Oncogene 1999, 18, 5546–5553. [Google Scholar]
- Liu, L.; Damen, J.E.; Cutler, R.L.; Krystal, G. Multiple cytokines stimulate the binding of a common 145-kilodalton protein to Shc at the Grb2 recognition site of Shc. Mol. Cell. Biol 1994, 14, 6926–6935. [Google Scholar]
- Wan, P.; Hu, Y.; He, L. Regulation of melanocyte pivotal transcription factor MITF by some other transcription factors. Mol. Cell. Biochem 2011, 354, 241–246. [Google Scholar]
- Potterf, S.B.; Furumura, M.; Dunn, K.J.; Arnheiter, H.; Pavan, W.J. Transcription factor hierarchy in Waardenburg syndrome: Regulation of MITF expression by SOX10 and PAX3. Hum. Genet 2000, 107, 1–6. [Google Scholar]
- Bentley, N.J.; Eisen, T.; Goding, C.R. Melanocyte-specific expression of the human tyrosinase promoter: Activation by the microphthalmia gene product and role of the initiator. Mol. Cell. Biol 1994, 14, 7996–8006. [Google Scholar]
- Fang, D.; Setaluri, V. Role of microphthalmia transcription factor in regulation of melanocyte differentiation marker TRP-1. Biochem. Biophys. Res. Commun 1999, 256, 657–663. [Google Scholar]
- Du, J.; Miller, A.J.; Widlund, H.R.; Horstmann, M.A.; Ramaswamy, S.; Fisher, D.E. MLANA/MART1 and SILV/PMEL17/GP100 are transcriptionally regulated by MITF in melanocytes and melanoma. Am. J. Pathol 2003, 163, 333–343. [Google Scholar]
- Mizutani, Y.; Hayashi, N.; Kawashima, M.; Imokawa, G. A single UVB exposure increases the expression of functional KIT in human melanocytes by up-regulating MITF expression through the phosphorylation of p38/CREB. Arch. Dermatol. Res 2010, 302, 283–294. [Google Scholar]
- Nakajima, H.; Wakabayashi, Y.; Wakamats, K.; Imokawa, G. An extract of Melia toosendan attenuates endothelin-1-stimulated pigmentation in human epidermal equivalents through the interruption of PKC activity within melanocytes. Arch. Dermatol. Res 2011, 303, 263–276. [Google Scholar]
- Nakajima, H.; Fukazawa, K.; Wakabayashi, Y.; Wakamatsu, K.; Imokawa, G. WSE attenuates stem cell factor-stimulated pigmentation in human epidermal equivalent through interruption of ERK phosphorylation within melanocytes. J. Nat. Med 2012, 66, 435–446. [Google Scholar]
- Nakajima, H.; Wakabayashi, Y.; Wakamatsu, K.; Imokawa, G. An extract of Withania somnifera attenuates endothelin-1-stimulated pigmentation in human epidermal equivalents through the interruption of PKC activity within melanocytes. Phytother. Res 2011, 25, 1398–1411. [Google Scholar]
- Nakajima, H.; Fukazawa, K.; Wakabayashi, Y.; Wakamatsu, K.; Senda, K.; Imokawa, G. Abrogating effect of a xanthophyll carotenoid astaxanthin on the stem cell factor-induced stimulation of human epidermal pigmentation. Arch. Dermatol. Res 2012, 304, 803–816. [Google Scholar]
- Agarwal, R.; Diwanay, S.; Patki, P.; Patwardhan, B. Studies on immunomodulatory activity of Withania somnifera (Ashwagandha) extract in experimental immune inflammation. J. Ethnopharmacol 1999, 67, 27–35. [Google Scholar]
- Gupta, S.K.; Mohanty, I.; Talwar, K.K.; Dinda, A.; Joshi, S.; Bansal, P.; Saxena, A.; Arya, D.S. Cardioprotection from ischemia and reperfusion injury by Withania somnifera: A hemodynamic, biochemical and histopathological assessment. Mol. Cell. Biochem 2004, 260, 39–47. [Google Scholar]
- Ahmad, M.; Saleem, S.; Ahmad, A.S.; Ansari, M.A.; Yousuf, S.; Hoda, M.N.; Islam, F. Neuroprotective effects of Withania somnifera on 6-hydroxydopamine induced Parkisonism in rats. Hum. Exp. Toxicol 2005, 24, 137–147. [Google Scholar]
- Owais, M.; Sharad, K.S.; Shehbaz, A.; Saleemuddin, M. Antibacterial efficacy of Withania somnifera (Ashwagandha) an indigenous medicinal plant against experimental murine salmonellosis. Phytomedicine 2005, 12, 229–235. [Google Scholar]
- Rasool, M.; Varalakshmi, P. Immunomodulatory role of Withania somnifera root powder on experimental induced inflammation: An in vivo and in vitro study. Vascul. Pharmacol 2006, 44, 406–410. [Google Scholar]
- Singh, D.; Aggarwal, A.; Maurya, R.; Naik, S. Withania somnifera inhibits NF-κB and AP-1 transcription factors in human peripheral blood and synovial fluid mononuclear cells. Phytother. Res 2007, 21, 905–913. [Google Scholar]
- Ichikawa, H.; Takad, Y.; Shishodia, S.; Jayaprakasam, B.; Nair, M.G.; Aggarwal, B.B. Withanolides potentiate apoptosis, inhibit invasion, and abolish osteoclastogenesis through suppression of nuclear factor-κB (NF-κB) activation and NF-κB-regulated gene expression. Mol. Cancer Ther 2006, 5, 1434–1445. [Google Scholar]
- Kaileh, M.; van den Berghe, W.; Heyerick, A.; Horion, J.; Piette, J.; Libert, C.; de Keukeleire, D.; Essawi, T.; Haegeman, G. Withaferin A strongly elicits IκB kinase β hyperphosphorylation concomitant with potent inhibition of its kinase activity. J. Biol. Chem 2007, 282, 4253–4264. [Google Scholar]
- Aalinkeel, R.; Hu, Z.; Nair, B.B.; Sykes, D.E.; Reynolds, J.L.; Mahajan, S.D.; Schwartz, S.A. Genomic analysis highlights the role of the JAK-STAT signaling in the anti-proliferative. Altern. Med 2010, 7, 177–187. [Google Scholar]
- Terazawa, S.; Nakajima, H.; Niwano, T.; Wakabayashi, Y.; Fukazawa, K.; Imokawa, G. Withaferin A attenuates SCF-stimulated pigmentation in human epidermal equivalents by interrupting c-KIT activation within human melanocytes. Pigment Cell Melanoma Res 2013, 26, E5–E6. [Google Scholar]














| Five criteria to determine if a cytokine is an intrinsic factor involved in UVB hyperpigmentation | EDN1 | mSCF | αMSH | bFGF |
|---|---|---|---|---|
| EDNRB | c-KIT | MC1R | c-Met | |
| 1. The cytokine(s) should have the potential to activate melanocytes at physiological concentration in vitro. | ○ (1–10 nM) | ○ (1–10 nM) | Δ (>100 nM) | Δ (>100 nM) |
| 2. The cytokine(s) should exist in supernatants of UVB-exposed keratinocytes at concentrations sufficient to stimulate melanocytes. | ○ | – | ○ | × |
| 3. The stimulatory effect of culture supernatants on melanocytes should be neutralized by an antibody to the cytokine if it is secretable. | ○ | – | ○ | × |
| 4. The cytokine(s) should be highly expressed in UVB-exposed epidermis. | ○ | ○ | ○ | ○ |
| 5. The hyperpigmentation induced should be suppressed by antibodies that inhibit the corresponding receptor or by receptor antagonists in vivo. | ○ | ○ | × | × |
 Withaferin A (WFA) |  Astaxanthin (AX) | |||||||
|---|---|---|---|---|---|---|---|---|
| Herb extract of natural chemical | Tyrosinase activity | Cytotoxicity (MTT assay in NHM) | Inhibitory effects on signaling and targeted signal molecules | 3D human epidermal equivalent | Reference | |||
| Direct inhibition | Abrogating effect | SCF signaling | EDN1 signaling | SCF | EDN1 | |||
| Withania somnfera (WSE) | ○ | × | ○ | Ras/Raf-1 | PKC/Raf-1 | × | × | [40,50] |
| Melia toosendan (MTE) | ○ | × | ○ | ○ | PKC/Raf-1 | ○ | × | [39] |
| Astaxanthin (AX) | ○ | × | ○ | Raf-1/MEK | ○ | × | ○ | [51] |
| Withaferin A (WFA) | ○ | × | ○ | c-KIT | PKC/Raf-1 | × | × | [22,39,52] |
© 2014 by the authors; licensee MDPI, Basel, Switzerland This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Imokawa, G.; Ishida, K. Inhibitors of Intracellular Signaling Pathways that Lead to Stimulated Epidermal Pigmentation: Perspective of Anti-Pigmenting Agents. Int. J. Mol. Sci. 2014, 15, 8293-8315. https://doi.org/10.3390/ijms15058293
Imokawa G, Ishida K. Inhibitors of Intracellular Signaling Pathways that Lead to Stimulated Epidermal Pigmentation: Perspective of Anti-Pigmenting Agents. International Journal of Molecular Sciences. 2014; 15(5):8293-8315. https://doi.org/10.3390/ijms15058293
Chicago/Turabian StyleImokawa, Genji, and Koichi Ishida. 2014. "Inhibitors of Intracellular Signaling Pathways that Lead to Stimulated Epidermal Pigmentation: Perspective of Anti-Pigmenting Agents" International Journal of Molecular Sciences 15, no. 5: 8293-8315. https://doi.org/10.3390/ijms15058293
APA StyleImokawa, G., & Ishida, K. (2014). Inhibitors of Intracellular Signaling Pathways that Lead to Stimulated Epidermal Pigmentation: Perspective of Anti-Pigmenting Agents. International Journal of Molecular Sciences, 15(5), 8293-8315. https://doi.org/10.3390/ijms15058293





