Systemic Injection of Low-Dose Lipopolysaccharide Fails to Break down the Blood–Brain Barrier or Activate the TLR4-MyD88 Pathway in Neonatal Rat Brain
Abstract
:1. Introduction
2. Results and Discussion
2.1. Unchanged Blood–Brain Barrier (BBB) after Systemic Lipopolysaccharide (LPS)
2.1.1. Evans Blue (EB) Extravasation
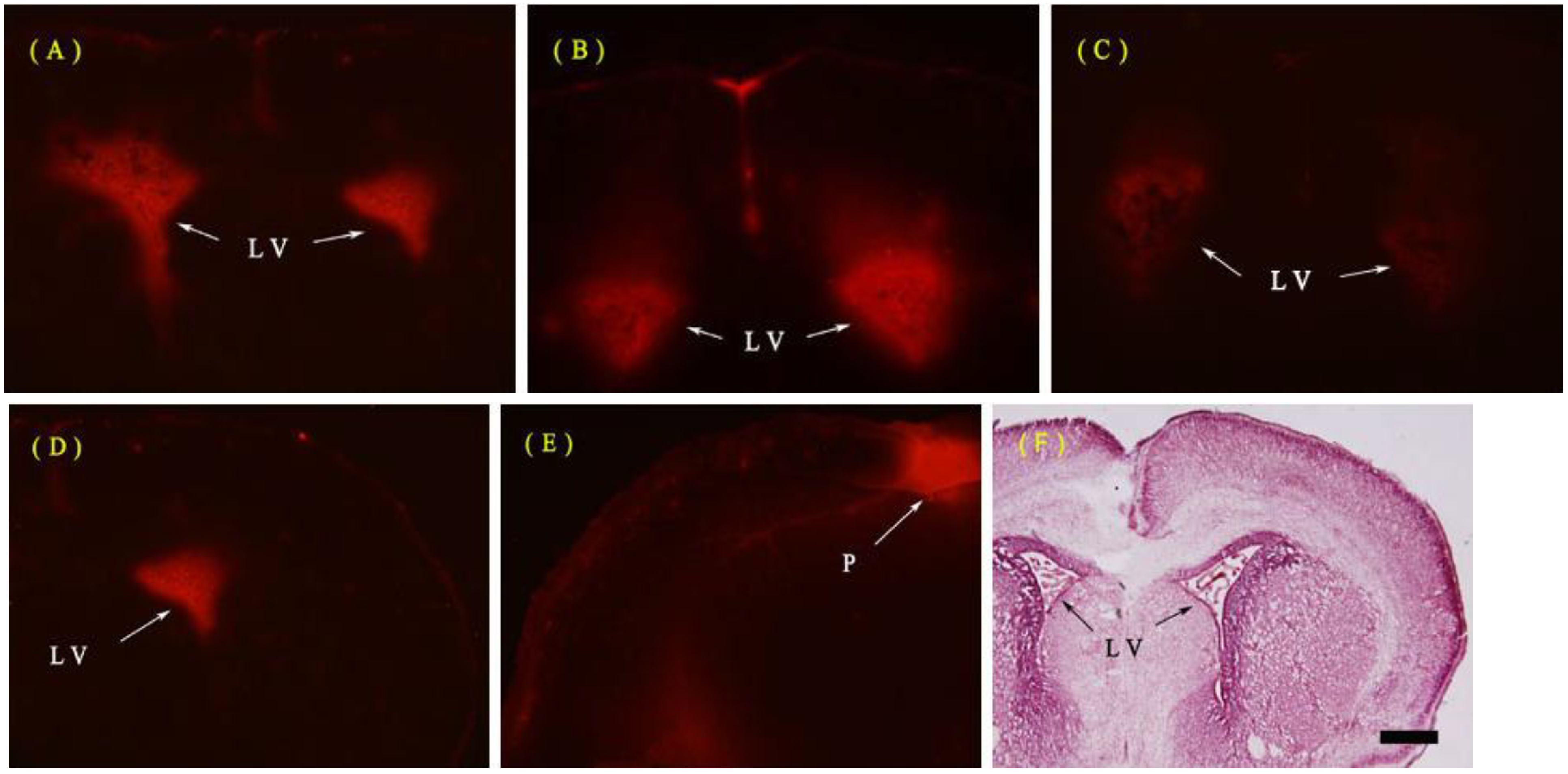
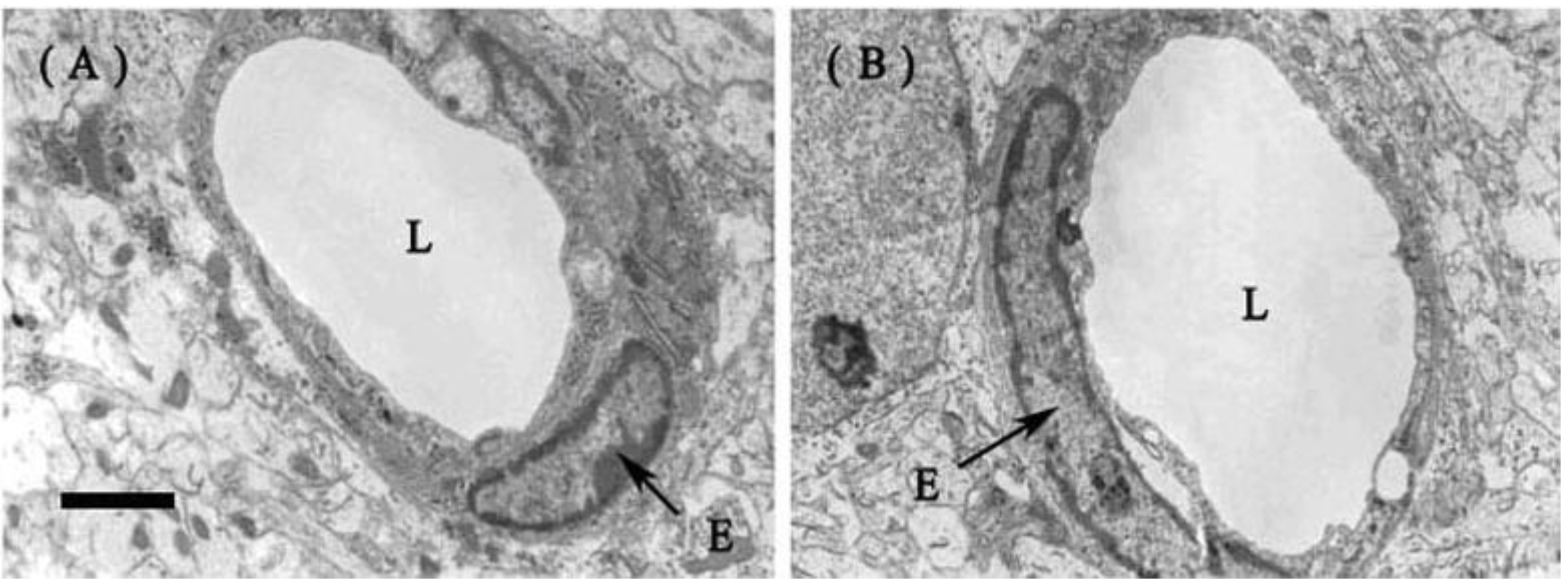
2.1.2. Fine Structure of the BBB
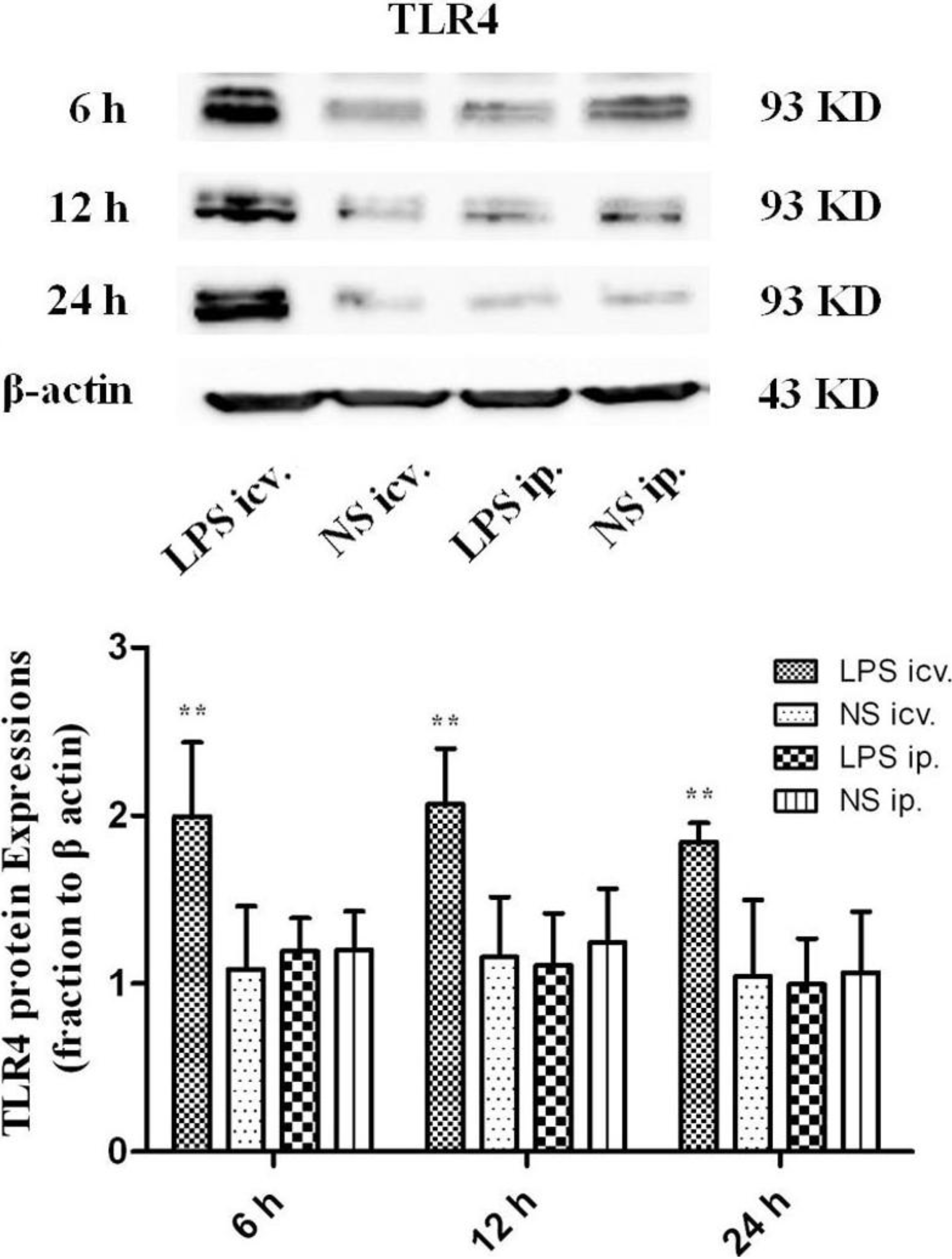
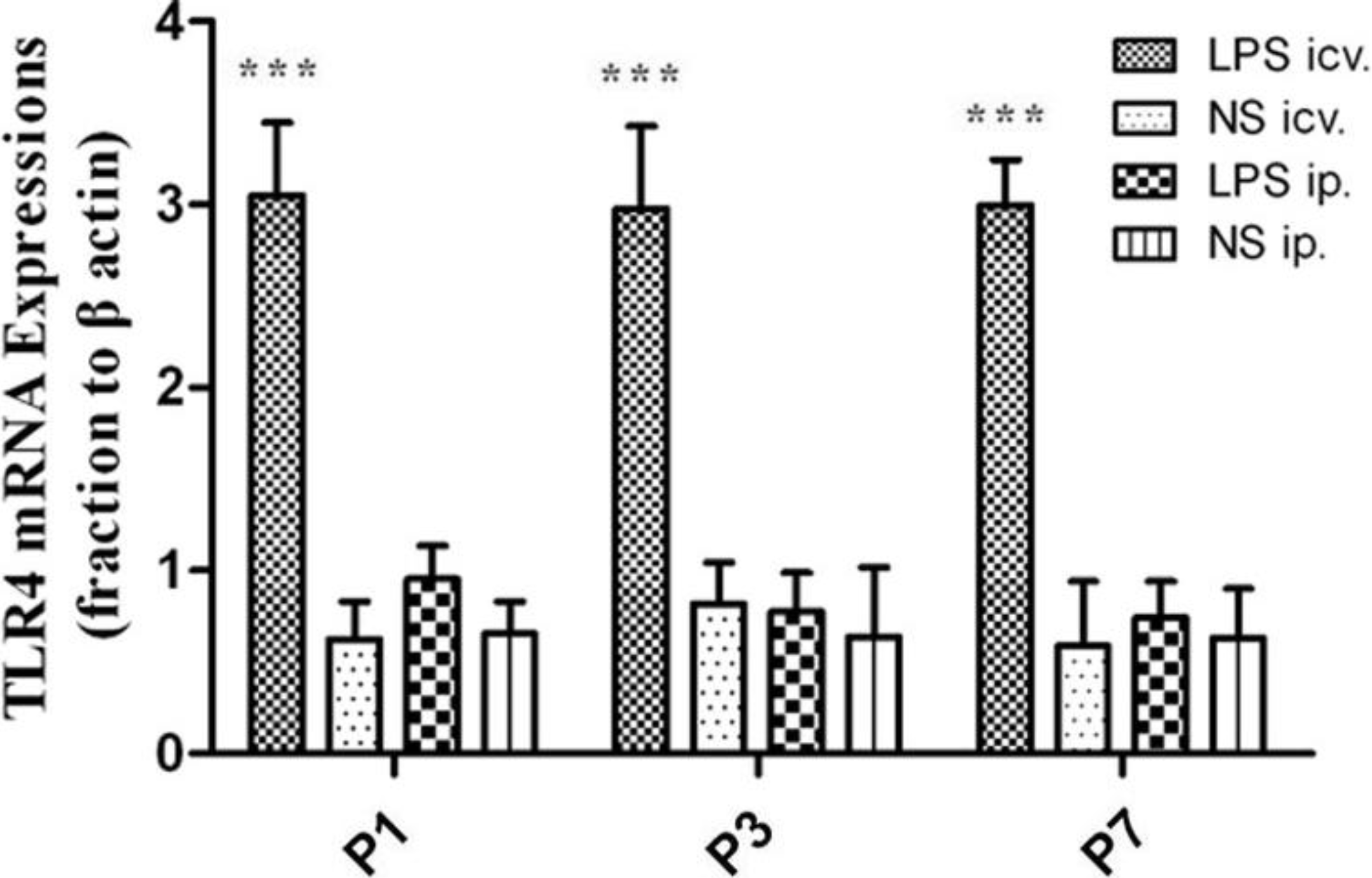
2.2. No Toll-Like Receptor 4 (TLR4) Protein and mRNA Responses to Peripheral LPS
2.3. Myeloid Differentiation Factor 88 (MyD88)–Nuclear Transcription Factor Kappa-B (MyD88–NF-κB) Pathway Is not Activated by Systemic LPS
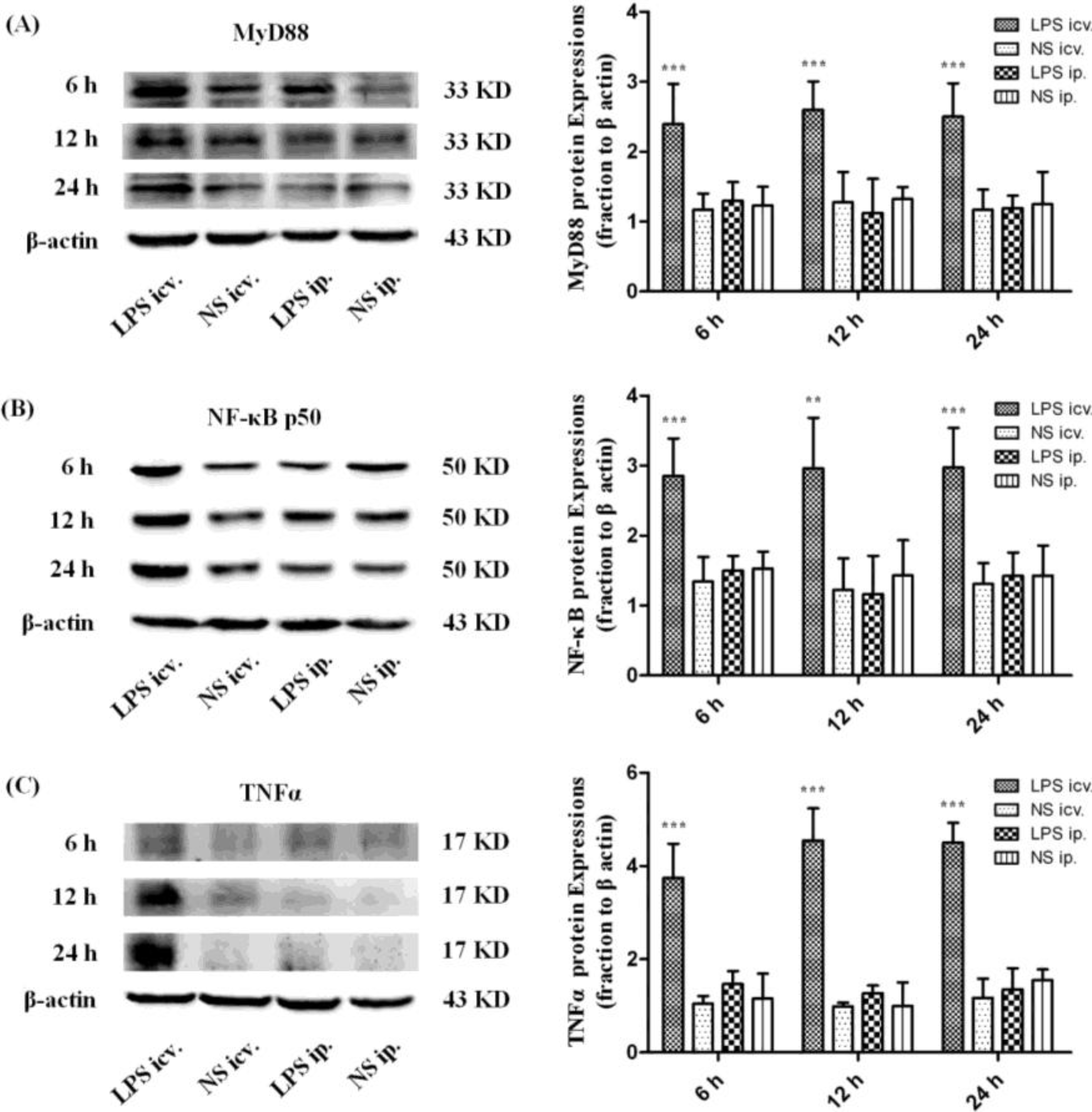
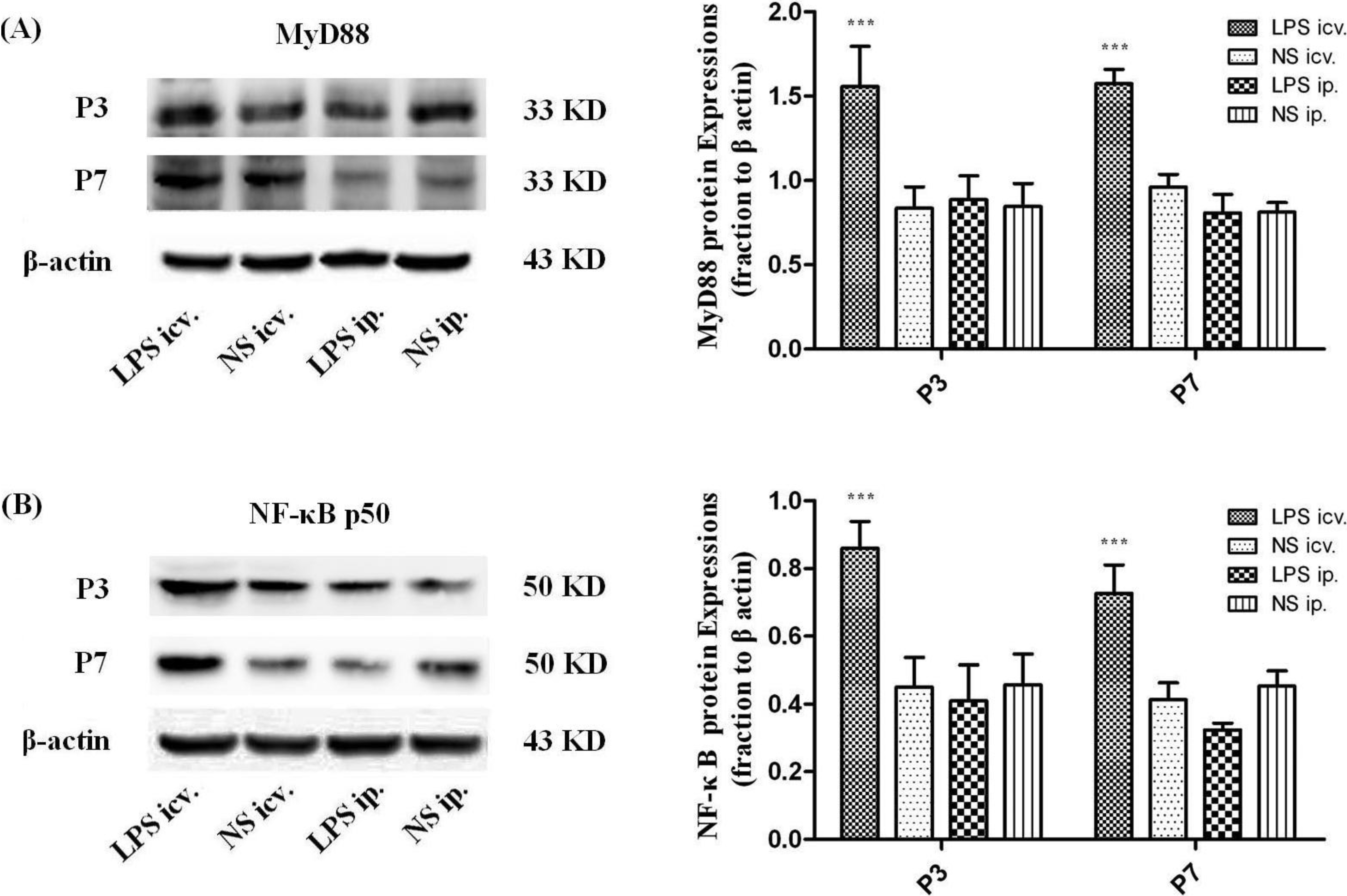
2.4. Microglia Is not Activated after Systemic LPS in Neonatal Rat Brain
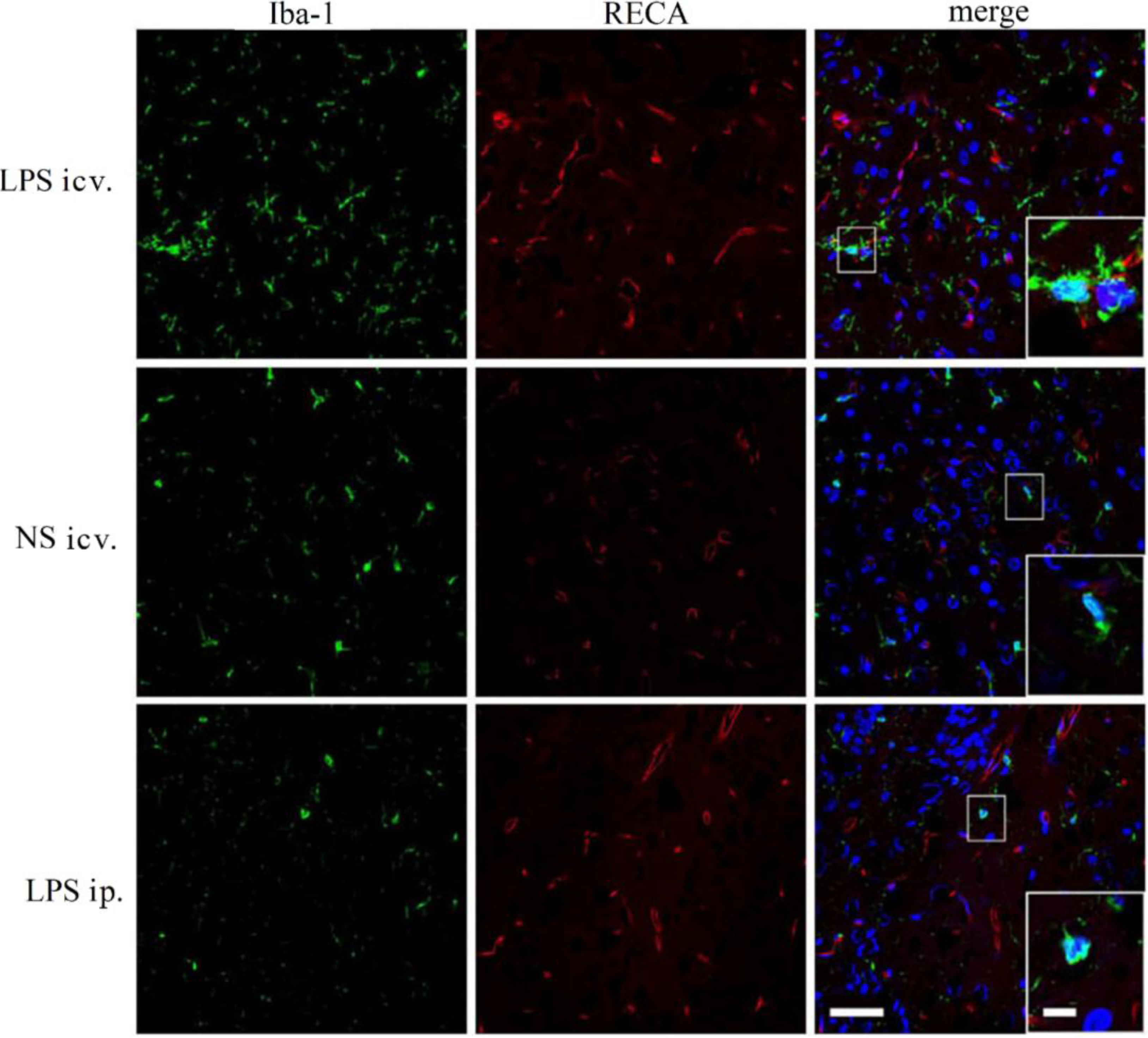
2.5. Discussion
3. Experimental Section
3.1. Animals and Experiment Procedure
3.2. Evaluation of the BBB Permeability by EB
3.3. Electron Microscopy
3.4. Western Blot Assay
3.5. Quantitative Real-Time Reverse-Transcriptase (PCR) Analysis
3.6. Immunohistochemical Analysis
3.7. Statistical Analysis
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Park, B.S.; Lee, J.O. Recognition of lipopolysaccharide pattern by TLR4 complexes. Exp. Mol. Med. 2013, 45, e66. [Google Scholar] [CrossRef]
- Lehnardt, S.; Massillon, L.; Follett, P.; Jensen, F.E.; Ratan, R.; Rosenberg, P.A.; Volpe, J.J.; Vartanian, T. Activation of innate immunity in the CNS triggers neurodegeneration through a Toll-like receptor 4-dependent pathway. Proc. Natl. Acad. Sci. USA 2003, 100, 8514–8519. [Google Scholar] [CrossRef]
- Eichenberger, E.; Schmidhauser-Kopp, M.; Hurni, H.; Fricsay, M.; Westphal, O. Biological effects of a highly purified pyrogen (lipopolysaccaride) from Salmonella abortus equi. Schweiz. Med. Wochenschr. 1955, 85, 1213–1218. (In German) [Google Scholar]
- Goehler, L.E.; Gaykema, R.P.; Nguyen, K.T.; Lee, J.E.; Tilders, F.J.; Maier, S.F.; Watkins, L.R. Interleukin-1β in immune cells of the abdominal vagus nerve: A link between the immune and nervous systems? J. Neurosci. 1999, 19, 2799–2806. [Google Scholar]
- Romeo, H.E.; Tio, D.L.; Rahman, S.U.; Chiappelli, F.; Taylor, A.N. The glossopharyngeal nerve as a novel pathway in immune-to-brain communication: Relevance to neuroimmune surveillance of the oral cavity. J. Neuroimmunol. 2001, 115, 91–100. [Google Scholar] [CrossRef]
- Qin, L.; Wu, X.; Block, M.L.; Liu, Y.; Breese, G.R.; Hong, J.S.; Knapp, D.J.; Crews, F.T. Systemic LPS causes chronic neuroinflammation and progressive neurodegeneration. Glia 2007, 55, 453–462. [Google Scholar] [CrossRef]
- Quan, N.; He, L.; Lai, W. Endothelial activation is an intermediate step for peripheral lipopolysaccharide induced activation of paraventricular nucleus. Brain Res. Bull. 2003, 59, 447–452. [Google Scholar] [CrossRef]
- Xaio, H.; Banks, W.A.; Niehoff, M.L.; Morley, J.E. Effect of LPS on the permeability of the blood–brain barrier to insulin. Brain Res. 2001, 896, 36–42. [Google Scholar] [CrossRef]
- Calabria, A.R.; Shusta, E.V. Blood–brain barrier genomics and proteomics: Elucidating phenotype, identifying disease targets and enabling brain drug delivery. Drug Discov. Today 2006, 11, 792–799. [Google Scholar] [CrossRef]
- Wolburg, H.; Noell, S.; Mack, A.; Wolburg-Buchholz, K.; Fallier-Becker, P. Brain endothelial cells and the glio-vascular complex. Cell Tissue Res. 2009, 335, 75–96. [Google Scholar] [CrossRef]
- Deli, M.A.; Abraham, C.S.; Kataoka, Y.; Niwa, M. Permeability studies on in vitro blood–brain barrier models: Physiology, pathology, and pharmacology. Cell Mol. Neurobiol. 2005, 25, 59–127. [Google Scholar] [CrossRef]
- Banks, W.A.; Robinson, S.M. Minimal penetration of lipopolysaccharide across the murine blood–brain barrier. Brain Behav. Immun. 2010, 24, 102–109. [Google Scholar] [CrossRef]
- Lu, P.M.; Gonzales, C.; Chen, Y.; Adedoyin, A.; Hummel, M.; Kennedy, J.D.; Whiteside, G.T. CNS penetration of small molecules following local inflammation, widespread systemic inflammation or direct injury to the nervous system. Life Sci. 2009, 85, 450–456. [Google Scholar] [CrossRef]
- Brochu, M.E.; Girard, S.; Lavoie, K.; Sebire, G. Developmental regulation of the neuroinflammatory responses to LPS and/or hypoxia–ischemia between preterm and term neonates: An experimental study. J. Neuroinflamm. 2011, 8, 2094–2098. [Google Scholar]
- Lin, L.; Knowlton, A.A. Innate immunity and cardiomyocytes in ischemic heart disease. Life Sci. 2014, 100, 1–8. [Google Scholar] [CrossRef]
- Peri, F.; Calabrese, V. Toll-like receptor 4 (TLR4) modulation by synthetic and natural compounds: An update. J. Med. Chem. 2014, 57, 3612–3622. [Google Scholar] [CrossRef]
- Liu, S.; Kielian, T. Microglial activation by Citrobacter koseri is mediated by TLR4- and MyD88-dependent pathways. J. Immunol. 2009, 183, 5537–5547. [Google Scholar]
- Eklind, S.; Mallard, C.; Leverin, A.L.; Gilland, E.; Blomgren, K.; Mattsby-Baltzer, I.; Hagberg, H. Bacterial endotoxin sensitizes the immature brain to hypoxic–ischaemic injury. Eur. J. Neurosci. 2001, 13, 1101–1106. [Google Scholar]
- Wang, L.W.; Chang, Y.C.; Lin, C.Y.; Hong, J.S.; Huang, C.C. Low-dose lipopolysaccharide selectively sensitizes hypoxic ischemia-induced white matter injury in the immature brain. Pediatr. Res. 2010, 68, 41–47. [Google Scholar]
- Hickey, E.; Shi, H.; van Arsdell, G.; Askalan, R. Lipopolysaccharide-induced preconditioning against ischemic injury is associated with changes in toll-like receptor 4 expression in the rat developing brain. Pediatr. Res. 2011, 70, 10–14. [Google Scholar]
- Bordet, R.; Deplanque, D.; Maboudou, P.; Puisieux, F.; Pu, Q.; Robin, E.; Martin, A.; Bastide, M.; Leys, D.; Lhermitte, M.; et al. Increase in endogenous brain superoxide dismutase as a potential mechanism of lipopolysaccharide-induced brain ischemic tolerance. J. Cereb. Blood Flow Metab. 2000, 20, 1190–1196. [Google Scholar]
- Ikeda, T.; Yang, L.; Ikenoue, T.; Mallard, C.; Hagberg, H. Endotoxin-induced hypoxic–ischemic tolerance is mediated by up-regulation of corticosterone in neonatal rat. Pediatr. Res. 2006, 59, 56–60. [Google Scholar]
- Breder, C.D.; Hazuka, C.; Ghayur, T.; Klug, C.; Huginin, M.; Yasuda, K.; Teng, M.; Saper, C.B. Regional induction of tumor necrosis factor alpha expression in the mouse brain after systemic lipopolysaccharide administration. Proc. Natl. Acad. Sci. USA 1994, 91, 11393–11397. [Google Scholar] [CrossRef]
- Tasaki, K.; Ruetzler, C.A.; Ohtsuki, T.; Martin, D.; Nawashiro, H.; Hallenbeck, J.M. Lipopolysaccharide pre-treatment induces resistance against subsequent focal cerebral ischemic damage in spontaneously hypertensive rats. Brain Res. 1997, 748, 267–270. [Google Scholar] [CrossRef]
- Laye, S.; Parnet, P.; Goujon, E.; Dantzer, R. Peripheral administration of lipopolysaccharide induces the expression of cytokine transcripts in the brain and pituitary of mice. Mol. Brain Res. 1994, 27, 157–162. [Google Scholar] [CrossRef]
- Zimmermann, C.; Ginis, I.; Furuya, K.; Klimanis, D.; Ruetzler, C.; Spatz, M.; Hallenbeck, J.M. Lipopolysaccharide-induced ischemic tolerance is associated with increased levels of ceramide in brain and in plasma. Brain Res. 2001, 895, 59–65. [Google Scholar]
- Banks, W.A.; Erickson, M.A. The blood–brain barrier and immune function and dysfunction. Neurobiol. Dis. 2010, 37, 26–32. [Google Scholar] [CrossRef]
- Murakami, K.; Kondo, T.; Yang, G.; Chen, S.F.; Morita-Fujimura, Y.; Chan, P.H. Cold injury in mice: A model to study mechanisms of brain edema and neuronal apoptosis. Prog. Neurobiol. 1999, 57, 289–299. [Google Scholar] [CrossRef]
- Scaini, G.; Morais, M.O.; Galant, L.S.; Vuolo, F.; Dalligna, D.M.; Pasquali, M.A.; Ramos, V.M.; Gelain, D.P.; Moreira, J.C.; Schuck, P.F.; et al. Coadministration of branched-chain amino acids and lipopolysaccharide causes matrix metalloproteinase activation and blood–brain barrier breakdown. Mol. Neurobiol. 2014. [Google Scholar] [CrossRef]
- Le, V.H.; Fishman, W.H. Combination of Evans blue with plasma protein; its significance in capillary permeability studies, blood dye disappearance curves, and its use as a protein tag. Am. J. Physiol. 1947, 151, 26–33. [Google Scholar]
- Marsh, B.J.; Williams-Karnesky, R.L.; Stenzel-Poore, M.P. Toll-like receptor signaling in endogenous neuroprotection and stroke. Neuroscience 2009, 158, 1007–1020. [Google Scholar] [CrossRef]
- Wieland, C.W.; Florquin, S.; Maris, N.A.; Hoebe, K.; Beutler, B.; Takeda, K.; Akira, S.; van der Poll, T. The MyD88-dependent, but not the MyD88-independent, pathway of TLR4 signaling is important in clearing nontypeable haemophilus influenzae from the mouse lung. J. Immunol. 2005, 175, 6042–6049. [Google Scholar] [CrossRef]
© 2014 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Wang, P.; You, S.-W.; Yang, Y.-J.; Wei, X.-Y.; Wang, Y.-Z.; Wang, X.; Hao, D.-J.; Kuang, F.; Shang, L.-X. Systemic Injection of Low-Dose Lipopolysaccharide Fails to Break down the Blood–Brain Barrier or Activate the TLR4-MyD88 Pathway in Neonatal Rat Brain. Int. J. Mol. Sci. 2014, 15, 10101-10115. https://doi.org/10.3390/ijms150610101
Wang P, You S-W, Yang Y-J, Wei X-Y, Wang Y-Z, Wang X, Hao D-J, Kuang F, Shang L-X. Systemic Injection of Low-Dose Lipopolysaccharide Fails to Break down the Blood–Brain Barrier or Activate the TLR4-MyD88 Pathway in Neonatal Rat Brain. International Journal of Molecular Sciences. 2014; 15(6):10101-10115. https://doi.org/10.3390/ijms150610101
Chicago/Turabian StyleWang, Peng, Si-Wei You, Yin-Jie Yang, Xiao-Yan Wei, Ya-Zhou Wang, Xin Wang, Ding-Jun Hao, Fang Kuang, and Li-Xin Shang. 2014. "Systemic Injection of Low-Dose Lipopolysaccharide Fails to Break down the Blood–Brain Barrier or Activate the TLR4-MyD88 Pathway in Neonatal Rat Brain" International Journal of Molecular Sciences 15, no. 6: 10101-10115. https://doi.org/10.3390/ijms150610101
APA StyleWang, P., You, S. -W., Yang, Y. -J., Wei, X. -Y., Wang, Y. -Z., Wang, X., Hao, D. -J., Kuang, F., & Shang, L. -X. (2014). Systemic Injection of Low-Dose Lipopolysaccharide Fails to Break down the Blood–Brain Barrier or Activate the TLR4-MyD88 Pathway in Neonatal Rat Brain. International Journal of Molecular Sciences, 15(6), 10101-10115. https://doi.org/10.3390/ijms150610101



