HSPC117 Is Regulated by Epigenetic Modification and Is Involved in the Migration of JEG-3 Cells
Abstract
:1. Introduction
2. Results and Discussion
2.1. Results
2.1.1. Effect of Histone Deacetylation and Methylation on HSPC117 Expression

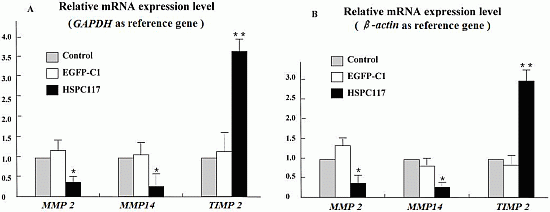
2.1.2. Differential Regulation of HSPC117 on MMP 2, MMP 14, and TIMP 2 Expressions in JEG-3 Cells Line

2.1.3. Effect of HSPC117 on JEG-3 Cell Migration and Wound Closure in Vitro


2.2. Discussion
2.2.1. Relationship of HSPC117 with Epigenetic Inheritance
2.2.2. The Relationship among HSPC117, MMPs, TIMPs, and Cell Migration
3. Materials and Methods
3.1. Gene Cloning
3.2. Construction and Transfection of EGFP-HSPC117 Expression Plasmid
3.3. Cell Culture
3.4. RNA Extraction and Quality Control
3.5. Reverse Transcription Real-Time Quantitative PCR (PT-qPCR)
| Gene | qPCR Primers | |
|---|---|---|
| HSPC117 | F: | 5' ATGACCCTGAAGCAGTAGTATCCC 3' |
| R: | 5' TACTCCTCGCAGTGCTCCTTGTC 3' | |
| MMP 2 | F: | 5' GTGGATGATGCCTTTGCTCG 3' |
| R: | 5' CCATCGGCGTTCCCATACTT 3' | |
| MMP 14 | F: | 5' CCCAACATCTGTGACGGGAACT 3' |
| R: | 5' GAGCAGCATCAATCTTGTCGGTAG 3' | |
| TIMP 2 | F: | 5' CCAAAGCGGTCAGTGAGAAGG 3' |
| R: | 5' TGGGTGGTGCTCAGGGTGTC 3' | |
| GAPDH | F: | 5' ACGGATTTGGTCGTATTGGG 3' |
| R: | 5' CGCTCCTGGAAGATGGTGAT 3' | |
| β-actin | F: | 5' TCCCTGGAGAAGAGCTACGA 3' |
| R: | 5' AGCACTGTGTTGGCGTACAG 3' | |
3.6. Western Blot Analysis
3.7. Scratch Wound Assay
3.8. Statistical Analysis
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Ding, N.Z.; He, M.; He, C.Q.; Hu, J.S.; Teng, J.L.; Chen, J. Yin yang-1 regulates the characterized murine focal adhesion-associated protein promoter. DNA Cell Biol. 2012, 31, 496–503. [Google Scholar] [CrossRef]
- Genschik, P.; Drabikowski, K.; Filipowicz, W. Characterization of the Escherichia coli RNA 3'-terminal phosphate cyclase and its sigma54-regulated operon. J. Biol. Chem. 1998, 273, 25516–2526. [Google Scholar]
- Tanaka, N.; Chakravarty, A.K.; Maughan, B.; Shuman, S. Novel mechanism of RNA repair by RtcB via sequential 2',3'-cyclic phosphodiesterase and 3'-Phosphate/5'-hydroxyl ligation reactions. J. Biol. Chem. 2011, 286, 43134–43143. [Google Scholar]
- Popow, J.; Schleiffer, A.; Martinez, J. Diversity and roles of (t)RNA ligases. Cell. Mol. Life Sci. 2012, 69, 2657–2670. [Google Scholar] [CrossRef]
- Hu, J.; Teng, J.; Ding, N.; He, M.; Sun, Y.; Yu, A.C.; Chen, J. FAAP, a novel murine protein, is involved in cell adhesion through regulating vinculin-paxillin association. Front. Biosci. 2008, 13, 7123–7131. [Google Scholar]
- Sweeney, M.J.; Ko, B.C.; Chung, S.S.; Turck, C.W. Protein identification by automated nanospray mass spectrometry-“zoomscan walking”. J. Biomol. Tech. 2002, 13, 43–48. [Google Scholar]
- De Hoog, C.L.; Foster, L.J.; Mann, M. RNA and RNA binding proteins participate in early stages of cell spreading through spreading initiation centers. Cell 2004, 117, 649–662. [Google Scholar] [CrossRef]
- Wang, Y.; Hai, T.; Liu, Z.; Zhou, S.; Lv, Z.; Ding, C.; Liu, L.; Niu, Y.; Zhao, X.; Tong, M.; et al. HSPC117 deficiency in cloned embryos causes placental abnormality and fetal death. Biochem. Biophys. Res. Commun. 2010, 397, 407–412. [Google Scholar] [CrossRef]
- Wells, D.N. Cloning in livestock agriculture. Reprod. Suppl. 2003, 61, 131–150. [Google Scholar]
- Gourvas, V.; Dalpa, E.; Konstantinidou, A.; Vrachnis, N.; Spandidos, D.A.; Sifakis, S. Angiogenic factors in placentas from pregnancies complicated by fetal growth restriction (review). Mol. Med. Rep. 2012, 6, 23–27. [Google Scholar]
- Ling, C.; Groop, L. Epigenetics: A molecular link between environmental factors and type 2 diabetes. Diabetes 2009, 58, 2718–2725. [Google Scholar]
- Mossman, D.; Kim, K.T.; Scott, R.J. Demethylation by 5-aza-2'-deoxycytidine in colorectal cancer cells targets Genomic DNA whilst Promoter CpGisland methylation persists. BMC Cancer 2010, 10, 366. [Google Scholar] [CrossRef]
- Demuth, T.; Berens, M.E. Molecular mechanisms of glioma cell migration and invasion. J. Neurooncol. 2004, 70, 217–228. [Google Scholar] [CrossRef]
- Zhao, J.; Ju, X.; Zhang, L.; An, Y.; Zhang, Z.; Sun, Z.; Ding, N.; Teng, C.B. Spatiotemporal expression of D10Wsu52e in the Developing mouse pancreas. Front. Biosci. 2011, 3, 355–363. [Google Scholar] [CrossRef]
- Desai, K.K.; Bingman, C.A.; Phillips, G.N., Jr.; Raines, R.T. Structures of the noncanonical RNA ligase RtcB reveal the mechanism of histidine guanylylation. Biochemistry 2013, 52, 2518–2525. [Google Scholar]
- Chakravarty, A.K.; Subbotin, R.; Chait, B.T.; Shuman, S. RNA ligase RtcB splices 3'-phosphate and 5'-OH ends via covalent RtcB-(histidinyl)-GMP and polynucleotide-(3')pp(5')G intermediates. Proc. Natl. Acad. Sci. USA 2012, 109, 6072–6072. [Google Scholar]
- Popow, J.; Englert, M.; Weitzer, S.; Schleiffer, A.; Mierzwa, B.; Mechtler, K.; Trowitzsch, S.; Will, C.L.; Lührmann, R.; Söll, D. HSPC117 is the essential subunit of a human tRNA splicing ligase complex. Science 2011, 331, 760–764. [Google Scholar] [CrossRef]
- Desai, K.K.; Raines, R.T. tRNA ligase catalyzes the GTP-dependent ligation of RNA with 3'-phosphate and 5'-hydroxyl termini. Biochemistry 2012, 51, 1333–1335. [Google Scholar] [CrossRef]
- Ygberg, S.E.; Clements, M.O.; Rytkönen, A.; Thompson, A.; Holden, D.W.; Hinton, J.C.; Rhen, M. Polynucleotide phosphorylase negatively controls spv virulence gene expression in Salmonella enterica. Infect. Immun. 2006, 74, 1243–1254. [Google Scholar] [CrossRef]
- Hamamichi, S.; Rivas, R.N.; Knight, A.L.; Cao, S.; Caldwell, K.A.; Caldwell, G.A. Hypothesis-based RNAi screening identifies neuroprotective genes in a Parkinson’s disease model. Proc. Natl. Acad. Sci. USA 2008, 10, 728–733. [Google Scholar]
- Cross, S.H.; Clark, V.H.; Simmen, M.W.; Bickmore, W.A.; Maroon, H.; Langford, C.F.; Carter, N.P.; Bird, A.P. CpG island libraries from human Chromosomes 18 and 22: Landmarks for novel genes. Mamm. Genome 2000, 11, 373–383. [Google Scholar] [CrossRef]
- Pinnick, K.E.; Karpe, F. Symposium 1: Nutrition and epigenetics DNA methylation of genes in adipose tissue. Proc. Nutr. Soc. 2011, 70, 57–63. [Google Scholar] [CrossRef]
- Schmelz, K.; Sattler, N.; Wagner, M.; Lübbert, M.; Dörken, B.; Tamm, I. Induction of gene expression by 5-Aza-29-deoxycytidine in acute myeloid leukemia (AML) and myelodysplastic syndrome (MDS) but not epithelial cells by DNAmethylation-dependent and independent mechanisms. Leukemia 2005, 19, 103–111. [Google Scholar]
- Mai, A.; Altucci, L. Epi-drugs to fight cancer: From chemistry to cancer treatment, the road ahead. Int. J. Biochem. Cell Biol. 2009, 41, 199–213. [Google Scholar] [CrossRef]
- Zhang, Y.W.; Staal, B.; Dykema, K.J.; Furge, K.A.; vande Woude, G.F. Cancer-type regulation of mig-6 expression by inhibitors of methylation and histone deacetylation. PLoS One 2012, 7, e38955. [Google Scholar]
- Yoo, C.B.; Jones, P.A. Epigenetic therapy of cancer: Past, present and future. Nat. Rev. Drug Discov. 2006, 5, 37–50. [Google Scholar]
- Alcazar, O.; Cousins, S.W.; Marin-Castaño, M.E. MMP-14 and TIMP-2 overexpression protects against hydroquinone-induced oxidant injury in RPE: Implications for extracellular matrix turnover. Investig. Ophthalmol. Vis. Sci. 2007, 48, 5662–5670. [Google Scholar] [CrossRef]
- Segarra, M.; García-Martínez, A.; Sánchez, M.; Hernández-Rodríguez, J.; Lozano, E.; Grau, J.M.; Cid, M.C. Gelatinase expression and proteolytic activity in giant-cell Arteritis. Ann. Rheum. Dis. 2007, 66, 1429–1435. [Google Scholar] [CrossRef]
- Itoh, Y.; Seiki, M. MT1-MMP: A potent modifier of pericellular microenvironment. J. Cell. Physiol. 2006, 206, 1–8. [Google Scholar] [CrossRef]
- Schenk, S.; Hintermann, E.; Bilban, M.; Koshikawa, N.; Hojilla, C.; Khokha, R.; Quaranta, V. Binding to EGF receptor of a laminin-5 EGF-like fragment liberated during MMP-dependent mammary gland involution. J. Cell Biol. 2003, 161, 197–209. [Google Scholar] [CrossRef]
- Woessner, J.F., Jr. MMPs and TIMPs—An historical perspective. Mol. Biotechnol. 2002, 22, 33–49. [Google Scholar] [CrossRef]
- Strongin, A.Y.; Collier, I.; Bannikov, G.; Marmer, B.L.; Grant, G.A.; Goldberg, G.I. Mechanism of cell surface activation of 72-kDa type IV collagenase. Isolation of the activated form of the membrane metalloprotease. J. Biol. Chem. 1995, 270, 5331–5338. [Google Scholar]
- Butler, G.S.; Butler, M.J.; Atkinson, S.J.; Will, H.; Tamura, T.; van Westrum, S.S.; Crabbe, T.; Clements, J.; d’Ortho, M.P.; Murphy, G. The TIMP2 membrane type 1 metalloproteinase “receptor” regulates the concentration and efficient activation of progelatinase A: A kinetic study. J. Biol. Chem. 1998, 273, 871–880. [Google Scholar]
© 2014 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Ma, H.; Qi, M.-Y.; Zhang, X.; Zhang, Y.-L.; Wang, L.; Li, Z.-Q.; Fu, B.; Wang, W.-T.; Liu, D. HSPC117 Is Regulated by Epigenetic Modification and Is Involved in the Migration of JEG-3 Cells. Int. J. Mol. Sci. 2014, 15, 10936-10949. https://doi.org/10.3390/ijms150610936
Ma H, Qi M-Y, Zhang X, Zhang Y-L, Wang L, Li Z-Q, Fu B, Wang W-T, Liu D. HSPC117 Is Regulated by Epigenetic Modification and Is Involved in the Migration of JEG-3 Cells. International Journal of Molecular Sciences. 2014; 15(6):10936-10949. https://doi.org/10.3390/ijms150610936
Chicago/Turabian StyleMa, Hong, Mei-Yu Qi, Xu Zhang, Yue-Ling Zhang, Liang Wang, Zhong-Qiu Li, Bo Fu, Wen-Tao Wang, and Di Liu. 2014. "HSPC117 Is Regulated by Epigenetic Modification and Is Involved in the Migration of JEG-3 Cells" International Journal of Molecular Sciences 15, no. 6: 10936-10949. https://doi.org/10.3390/ijms150610936
APA StyleMa, H., Qi, M.-Y., Zhang, X., Zhang, Y.-L., Wang, L., Li, Z.-Q., Fu, B., Wang, W.-T., & Liu, D. (2014). HSPC117 Is Regulated by Epigenetic Modification and Is Involved in the Migration of JEG-3 Cells. International Journal of Molecular Sciences, 15(6), 10936-10949. https://doi.org/10.3390/ijms150610936