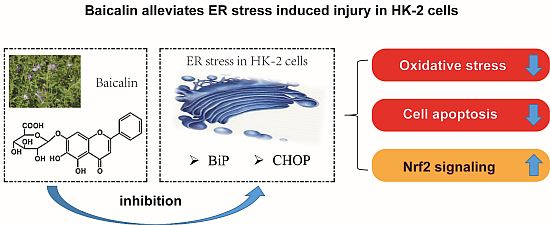
Baicalin Ameliorates H2O2 Induced Cytotoxicity in HK-2 Cells through the Inhibition of ER Stress and the Activation of Nrf2 Signaling
Abstract
:1. Introduction
2. Results
2.1. Baicalin Pretreatment Increased Cell Viability after H2O2 Stimulation

2.2. Baicalin Pretreatment Decreased Oxidative Stress after H2O2 Stimulation

2.3. Baicalin Pretreatment Inhibited Caspase-3 Activation and Cell Apoptosis after H2O2 Stimulation

2.4. Baicalin Pretreatment Reduced Endoplasmic Reticulum (ER) Stress Induced by H2O2 Stimulation

2.5. Baicalin Pretreatment Reduced ER Stress Induced by Tunicamycin Stimulation

2.6. Baicalin Pretreatment Promoted the Expression of Nrf2 Induced by H2O2 Stimulation
2.7. The Influences of ER Stress and Nrf2 Regulation on Baicalin Mediated Protection against H2O2-Induced Injury

3. Discussion

4. Experimental Section
4.1. Materials and Reagents
| Gene | The Sequence of Gene-Specific Primer |
|---|---|
| BiP | AAAGAAGACGGGCAAAGATGTTGCTTGATGCTGAGAAGACAG |
| CHOP | ACCACTCTTGACCCTGCTTCTCTCTGGGAGGTGCTTGTGAC |
| β-actin | GTTGTCGACGACGAGCG GCACAGAGCCTCGCCTT |
4.2. Cell Culture and H2O2 Treatment
4.3. Cell Viability Analysis
4.4. Caspase-3 Activity Assay
4.5. Hoechst Staining
4.6. Transferase-Mediated dUTP-Biotin Nick End Labeling (TUNEL) Staining
4.7. Measurement of Oxidative Stress
4.8. Western Blot Analysis
4.9. Quantitative Real-Time Polymerase Chain Reaction
4.10. Immunocytochemistry
4.11. Statistical Analysis
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Kosieradzki, M.; Rowinski, W. Ischemia/reperfusion injury in kidney transplantation: Mechanisms and prevention. Transplant. Proc. 2008, 40, 3279–88. [Google Scholar] [CrossRef]
- Cooper, J.E.; Wiseman, A.C. Acute kidney injury in kidney transplantation. Curr. Opin. Nephrol. Hypertens. 2013, 22, 698–703. [Google Scholar] [CrossRef]
- Xie, J.; Guo, Q. Apoptosis antagonizing transcription factor protects renal tubule cells against oxidative damage and apoptosis induced by ischemia-reperfusion. J. Am. Soc. Nephrol. 2006, 17, 3336–3346. [Google Scholar] [CrossRef]
- Inagi, R. Endoplasmic reticulum stress in the kidney as a novel mediator of kidney injury. Nephron. Exp. Nephrol. 2009, 112, e1–e9. [Google Scholar] [CrossRef]
- Inagi, R. Endoplasmic reticulum stress as a progression factor for kidney injury. Curr. Opin. Pharmacol. 2010, 10, 156–165. [Google Scholar] [CrossRef]
- Grall, S.; Prunier-Mirebeau, D.; Tamareille, S.; Mateus, V.; Lamon, D.; Furber, A.; Prunier, F. Endoplasmic reticulum stress pathway involvement in local and remote myocardial ischemic conditioning. Shock 2013, 39, 433–439. [Google Scholar] [CrossRef]
- Hetz, C. The unfolded protein response: Controlling cell fate decisions under ER stress and beyond. Nat. Rev. Mol. Cell Biol. 2012, 13, 89–102. [Google Scholar]
- Rasheva, V.I.; Domingos, P.M. Cellular responses to endoplasmic reticulum stress and apoptosis. Apoptosis 2009, 14, 996–1007. [Google Scholar] [CrossRef]
- Shelton, L.M.; Park, B.K.; Copple, I.M. Role of Nrf2 in protection against acute kidney injury. Kidney Int. 2013, 84, 1090–1095. [Google Scholar] [CrossRef]
- Liu, M.; Grigoryev, D.N.; Crow, M.T.; Haas, M.; Yamamoto, M.; Reddy, S.P.; Rabb, H. Transcription factor Nrf2 is protective during ischemic and nephrotoxic acute kidney injury in mice. Kidney Int. 2009, 76, 277–285. [Google Scholar] [CrossRef]
- Digaleh, H.; Kiaei, M.; Khodagholi, F. Nrf2 and Nrf1 signaling and ER stress crosstalk: Implication for proteasomal degradation and autophagy. Cell. Mol. Life Sci. 2013, 70, 4681–4694. [Google Scholar] [CrossRef]
- Cao, Y.; Mao, X.; Sun, C.; Zheng, P.; Gao, J.; Wang, X.; Min, D.; Sun, H.; Xie, N.; Cai, J. Baicalin attenuates global cerebral ischemia/reperfusion injury in gerbils via anti-oxidative and anti-apoptotic pathways. Brain Res. Bull. 2011, 85, 396–402. [Google Scholar] [CrossRef]
- Tang, Y.J.; Zhou, F.W.; Luo, Z.Q.; Li, X.Z.; Yan, H.M.; Wang, M.J.; Huang, F.R.; Yue, S.J. Multiple therapeutic effects of adjunctive baicalin therapy in experimental bacterial meningitis. Inflammation 2010, 33, 180–188. [Google Scholar] [CrossRef]
- Zhu, J.; Wang, J.; Sheng, Y.; Zou, Y.; Bo, L.; Wang, F.; Lou, J.; Fan, X.; Bao, R.; Wu, Y.; et al. Baicalin improves survival in a murine model of polymicrobial sepsis via suppressing inflammatory response and lymphocyte apoptosis. PLoS One 2012, 7, e35523. [Google Scholar]
- Lin, M.; Li, L.; Li, L.; Pokhrel, G.; Qi, G.; Rong, R.; Zhu, T. The protective effect of baicalin against renal ischemia-reperfusion injury through inhibition of inflammation and apoptosis. BMC Complement. Altern. Med. 2014, 14, 19. [Google Scholar] [CrossRef]
- Omura, T.; Asari, M.; Yamamoto, J.; Oka, K.; Hoshina, C.; Maseda, C.; Awaya, T.; Tasaki, Y.; Shiono, H.; Yonezawa, A.; et al. Sodium tauroursodeoxycholate prevents paraquat-induced cell death by suppressing endoplasmic reticulum stress responses in human lung epithelial A549 cells. Biochem. Biophys. Res. Commun. 2013, 432, 689–694. [Google Scholar] [CrossRef]
- Ren, D.; Villeneuve, N.F.; Jiang, T.; Wu, T.; Lau, A.; Toppin, H.A.; Zhang, D.D. Brusatol enhances the efficacy of chemotherapy by inhibiting the Nrf2-mediated defense mechanism. Proc. Nat. Acad. Sci. USA 2011, 108, 1433–1438. [Google Scholar]
- Li, J.; Johnson, D.; Calkins, M.; Wright, L.; Svendsen, C.; Johnson, J. Stabilization of Nrf2 by tBHQ confers protection against oxidative stress-induced cell death in human neural stem cells. Toxic Sci. 2005, 83, 313–328. [Google Scholar]
- Gobe, G.; Willgoss, D.; Hogg, N.; Schoch, E.; Endre, Z. Cell survival or death in renal tubular epithelium after ischemia-reperfusion injury. Kidney Int. 1999, 56, 1299–1304. [Google Scholar] [CrossRef]
- Carlisle, R.E.; Brimble, E.; Werner, K.E.; Cruz, G.L.; Ask, K.; Ingram, A.J.; Dickhout, J.G. 4-Phenylbutyrate inhibits tunicamycin-induced acute kidney injury via CHOP/GADD153 repression. PLoS One 2014, 9, e84663. [Google Scholar]
- Yang, J.R.; Yao, F.H.; Zhang, J.G.; Ji, Z.Y.; Li, K.L.; Zhan, J.; Tong, Y.N.; Lin, L.R.; He, Y.N. Ischemia-reperfusion induces renal tubule pyroptosis via the CHOP-caspase-11 pathway. Am. J. Physiol. Ren. Physiol. 2014, 306, F75–F84. [Google Scholar] [CrossRef]
- Yang, B.; Jain, S.; Ashra, S.Y.; Furness, P.N.; Nicholson, M.L. Apoptosis and caspase-3 in long-term renal ischemia/reperfusion injury in rats and divergent effects of immunosuppressants. Transplantation 2006, 81, 1442–1450. [Google Scholar] [CrossRef]
- Linkermann, A.; de Zen, F.; Weinberg, J.; Kunzendorf, U.; Krautwald, S. Programmed necrosis in acute kidney injury. Nephrol. Dial. Transpl. 2012, 27, 3412–3419. [Google Scholar] [CrossRef]
- Morris, J.A.; Dorner, A.J.; Edwards, C.A.; Hendershot, L.M.; Kaufman, R.J. Immunoglobulin binding protein (BiP) function is required to protect cells from endoplasmic reticulum stress but is not required for the secretion of selective proteins. J. Biol. Chem. 1997, 272, 4327–4334. [Google Scholar] [CrossRef]
- Giordano, E.; Davalos, A.; Nicod, N.; Visioli, F. Hydroxytyrosol attenuates tunicamycin-induced endoplasmic reticulum stress in human hepatocarcinoma cells. Mol. Nutr. Food Res. 2013, 58, 954–962. [Google Scholar]
- Zinszner, H.; Kuroda, M.; Wang, X.; Batchvarova, N.; Lightfoot, R.T.; Remotti, H.; Stevens, J.L.; Ron, D. CHOP is implicated in programmed cell death in response to impaired function of the endoplasmic reticulum. Genes Dev. 1998, 12, 982–995. [Google Scholar] [CrossRef]
- McCullough, K.D.; Martindale, J.L.; Klotz, L.O.; Aw, T.Y.; Holbrook, N.J. Gadd153 sensitizes cells to endoplasmic reticulum stress by down-regulating Bcl2 and perturbing the cellular redox state. Mol. Cell. Biol. 2001, 21, 1249–1259. [Google Scholar] [CrossRef]
- Tabas, I.; Ron, D. Integrating the mechanisms of apoptosis induced by endoplasmic reticulum stress. Nat. Cell Biol. 2011, 13, 184–190. [Google Scholar] [CrossRef]
- Walter, P.; Ron, D. The unfolded protein response: From stress pathway to homeostatic regulation. Science 2011, 334, 1081–1086. [Google Scholar] [CrossRef]
- Hayes, J.D.; Dinkova-Kostova, A.T. The Nrf2 regulatory network provides an interface between redox and intermediary metabolism. Trends Biochem. Sci. 2014, 39, 199–218. [Google Scholar] [CrossRef]
- Okada, K.; Minamino, T.; Tsukamoto, Y.; Liao, Y.; Tsukamoto, O.; Takashima, S.; Hirata, A.; Fujita, M.; Nagamachi, Y.; Nakatani, T.; et al. Prolonged endoplasmic reticulum stress in hypertrophic and failing heart after aortic constriction: Possible contribution of endoplasmic reticulum stress to cardiac myocyte apoptosis. Circulation 2004, 110, 705–712. [Google Scholar] [CrossRef]
- Ma, Y.; Yang, F.; Wang, Y.; Du, Z.; Liu, D.; Guo, H.; Shen, J.; Peng, H. CaMKKβ is involved in AMP-activated protein kinase activation by baicalin in LKB1 deficient cell lines. PLoS One 2012, 7, e47900. [Google Scholar]
- Lim, H.A.; Lee, E.K.; Kim, J.M.; Park, M.H.; Kim, D.H.; Choi, Y.J.; Ha, Y.M.; Yoon, J.H.; Choi, J.S.; Yu, B.P.; et al. PPARγ activation by baicalin suppresses NF-κB-mediated inflammation in aged rat kidney. Biogerontology 2012, 13, 133–145. [Google Scholar] [CrossRef]
- Wu, Y.X.; Sato, E.; Kimura, W.; Miura, N. Baicalin and scutellarin are proteasome inhibitors that specifically target chymotrypsin-like catalytic activity. Phytother. Res. 2013, 27, 1362–1367. [Google Scholar] [CrossRef]
- Liu, L.L.; Gong, L.K.; Wang, H.; Xiao, Y.; Wu, X.F.; Zhang, Y.H.; Xue, X.; Qi, X.M.; Ren, J. Baicalin inhibits macrophage activation by lipopolysaccharide and protects mice from endotoxin shock. Biochem. Pharmacol. 2008, 75, 914–922. [Google Scholar] [CrossRef]
- Wang, A.M.; Ku, H.H.; Liang, Y.C.; Chen, Y.C.; Hwu, Y.M.; Yeh, T.S. The autonomous notch signal pathway is activated by baicalin and baicalein but is suppressed by niclosamide in K562 cells. J. Cell. Biochem. 2009, 106, 682–692. [Google Scholar] [CrossRef]
© 2014 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Lin, M.; Li, L.; Zhang, Y.; Zheng, L.; Xu, M.; Rong, R.; Zhu, T. Baicalin Ameliorates H2O2 Induced Cytotoxicity in HK-2 Cells through the Inhibition of ER Stress and the Activation of Nrf2 Signaling. Int. J. Mol. Sci. 2014, 15, 12507-12522. https://doi.org/10.3390/ijms150712507
Lin M, Li L, Zhang Y, Zheng L, Xu M, Rong R, Zhu T. Baicalin Ameliorates H2O2 Induced Cytotoxicity in HK-2 Cells through the Inhibition of ER Stress and the Activation of Nrf2 Signaling. International Journal of Molecular Sciences. 2014; 15(7):12507-12522. https://doi.org/10.3390/ijms150712507
Chicago/Turabian StyleLin, Miao, Long Li, Yi Zhang, Long Zheng, Ming Xu, Ruiming Rong, and Tongyu Zhu. 2014. "Baicalin Ameliorates H2O2 Induced Cytotoxicity in HK-2 Cells through the Inhibition of ER Stress and the Activation of Nrf2 Signaling" International Journal of Molecular Sciences 15, no. 7: 12507-12522. https://doi.org/10.3390/ijms150712507