Mesenchymal Stem Cell-Derived Microparticles: A Promising Therapeutic Strategy
Abstract
:1. Introduction
2. Characterization of Mesenchymal Stem Cells (MSCs)
3. Therapeutic Potential of MSCs
3.1. Therapeutic Applications of MSCs
3.1.1. MSCs in Tissue Regeneration
3.1.2. MSCs in Tumor Therapy
3.2. Limitations of MSC Therapy
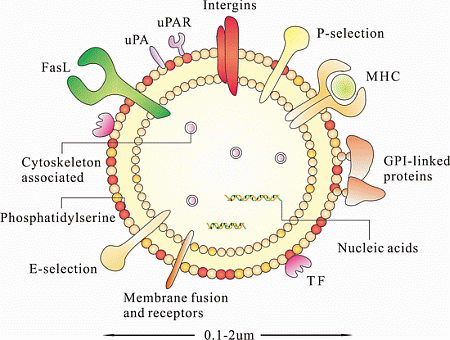
4. Characterization of MSC-MPs

4.1. Nucleic Acids
4.2. Proteins
4.3. Lipids
5. Therapeutic Potential of MSC-MPs
5.1. Intercellular Communication and Phenotypic Change

5.2. Therapeutic Applications of MSC-MPs
5.2.1. Cardiovascular Disease
5.2.2. Renal Disease
5.2.3. Cancer
6. Engineered MSC-MPs for Therapy
7. Prospects and Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Freyssinet, J.-M.; Toti, F.; Hugel, B.; Gidon-Jeangirard, C.; Kunzelmann, C.; Martínez, M.C.; Meyer, D. Apoptosis in vascular disease. Thromb. Haemost. 1999, 82, 727–735. [Google Scholar]
- Pilzer, D.; Gasser, O.; Moskovich, O.; Schifferli, J.A.; Fishelson, Z. Emission of Membrane Vesicles: Roles in Complement Resistance, Immunity and Cancer; Springer Berlin Heidelberg: Belin, Germany, 2005; pp. 375–387. [Google Scholar]
- Al-Nedawi, K.; Meehan, B.; Rak, J. Messengers and mediators of tumor progression. Cell Cycle 2009, 8, 2014–2018. [Google Scholar] [CrossRef]
- Johnstone, R.M. Exosomes biological significance: A concise review. Blood Cells Mol. Dis. 2006, 36, 315–321. [Google Scholar] [CrossRef]
- Cocucci, E.; Racchetti, G.; Meldolesi, J. Shedding microvesicles: Artefacts no more. Trends Cell Biol. 2009, 19, 43–51. [Google Scholar] [CrossRef]
- Takamori, S.; Holt, M.; Stenius, K.; Lemke, E.A.; Grønborg, M.; Riedel, D.; Urlaub, H.; Schenck, S.; Brügger, B.; Ringler, P. Molecular anatomy of a trafficking organelle. Cell 2006, 127, 831–846. [Google Scholar] [CrossRef]
- Al-Nedawi, K.; Meehan, B.; Kerbel, R.S.; Allison, A.C.; Rak, J. Endothelial expression of autocrine VEGF upon the uptake of tumor-derived microvesicles containing oncogenic EGFR. Proc. Natl. Acad. Sci. USA 2009, 106, 3794–3799. [Google Scholar]
- Al-Nedawi, K.; Meehan, B.; Micallef, J.; Lhotak, V.; May, L.; Guha, A.; Rak, J. Intercellular transfer of the oncogenic receptor EGFRvIII by microvesicles derived from tumour cells. Nat. Cell Biol. 2008, 10, 619–624. [Google Scholar]
- Yu, J.; Rak, J. Shedding of tissue factor (TF)-containing microparticles rather than alternatively spliced TF is the main source of TF activity released from human cancer cells. J. Thromb. Haemost. 2004, 2, 2065–2067. [Google Scholar] [CrossRef]
- Zwicker, J.I.; Furie, B.C.; Furie, B. Cancer-associated thrombosis. Crit. Rev. Oncol. Hematol. 2007, 62, 126–136. [Google Scholar] [CrossRef]
- Aharon, A.; Brenner, B. Microparticles, thrombosis and cancer. Best Pract. Res. Clin. Haematol. 2009, 22, 61–69. [Google Scholar] [CrossRef]
- Caplan, A.I.; Dennis, J.E. Mesenchymal stem cells as trophic mediators. J. Cell. Biochem. 2006, 98, 1076–1084. [Google Scholar] [CrossRef]
- Yagi, H.; Soto-Gutierrez, A.; Parekkadan, B.; Kitagawa, Y.; Tompkins, R.G.; Kobayashi, N.; Yarmush, M.L. Mesenchymal stem cells: Mechanisms of immunomodulation and homing. Cell Transplant. 2010, 19, 667. [Google Scholar] [CrossRef]
- Friedenstein, A.J.; Chailakhyan, R.K.; Latsinik, N.V.; Panasyuk, A.F.; Keiliss-Borok, I.V. Stromal cells responsible for transferring the microenvironment of the hemopoietic tissues: Cloning in vitro and retransplantation in vivo. Transplantation 1974, 17, 331–340. [Google Scholar] [CrossRef]
- Friedenstein, A.; Piatetzky-Shapiro, I.; Petrakova, K. Osteogenesis in transplants of bone marrow cells. J. Embryol. Exp. Morphol. 1966, 16, 381–390. [Google Scholar]
- Friedenstein, A.; Chailakhjan, R.; Lalykina, K. The development of fibroblast colonies in monolayer cultures of guinea-pig bone marrow and spleen cells. Cell Prolif. 1970, 3, 393–403. [Google Scholar] [CrossRef]
- Giuliani, A.; Manescu, A.; Langer, M.; Rustichelli, F.; Desiderio, V.; Paino, F.; de Rosa, A.; Laino, L.; d’Aquino, R.; Tirino, V. Three years after transplants in human mandibles, histological and in-line holotomography revealed that stem cells regenerated a compact rather than a spongy bone: Biological and clinical implications. Stem Cells Transl. Med. 2013, 2, 316–324. [Google Scholar] [CrossRef]
- Gangji, V.; Hauzeur, J.-P. Treatment of osteonecrosis of the femoral head with implantation of autologous bone-marrow cells. J. Bone Jt. Surg. 2005, 87, 106–112. [Google Scholar]
- Le Blanc, K.; Götherström, C.; Ringdén, O.; Hassan, M.; McMahon, R.; Horwitz, E.; Anneren, G.; Axelsson, O.; Nunn, J.; Ewald, U. Fetal mesenchymal stem-cell engraftment in bone after in utero transplantation in a patient with severe osteogenesis imperfecta. Transplantation 2005, 79, 1607–1614. [Google Scholar] [CrossRef]
- Wakitani, S.; Nawata, M.; Tensho, K.; Okabe, T.; Machida, H.; Ohgushi, H. Repair of articular cartilage defects in the patello-femoral joint with autologous bone marrow mesenchymal cell transplantation: Three case reports involving nine defects in five knees. J. Tissue Eng. Regen. Med. 2007, 1, 74–79. [Google Scholar] [CrossRef]
- Ichim, T.E.; Solano, F.; Lara, F.; Paris, E.; Ugalde, F.; Rodriguez, J.P.; Minev, B.; Bogin, V.; Ramos, F.; Woods, E.J. Feasibility of combination allogeneic stem cell therapy for spinal cord injury: A case report. Int. Arch. Med. 2010, 3, 30. [Google Scholar] [CrossRef]
- Liu, J.; Han, D.; Wang, Z.; Xue, M.; Zhu, L.; Yan, H.; Zheng, X.; Guo, Z.; Wang, H. Clinical analysis of the treatment of spinal cord injury with umbilical cord mesenchymal stem cells. Cytotherapy 2013, 15, 185–191. [Google Scholar] [CrossRef]
- Bhansali, A.; Upreti, V.; Khandelwal, N.; Marwaha, N.; Gupta, V.; Sachdeva, N.; Sharma, R.; Saluja, K.; Dutta, P.; Walia, R. Efficacy of autologous bone marrow-derived stem cell transplantation in patients with type 2 diabetes mellitus. Stem Cells Dev. 2009, 18, 1407–1416. [Google Scholar] [CrossRef]
- Estrada, E.J.; Valacchi, F.; Nicora, E.; Brieva, S.; Esteve, C.; Echevarria, L.; Froud, T.; Bernetti, K.; Cayetano, S.M.; Velazquez, O. Combined treatment of intrapancreatic autologous bone marrow stem cells and hyperbaric oxygen in type 2 diabetes mellitus. Cell Transplant. 2008, 17, 1295–1304. [Google Scholar] [CrossRef]
- Duijvestein, M.; Vos, A.C.W.; Roelofs, H.; Wildenberg, M.E.; Wendrich, B.B.; Verspaget, H.W.; Kooy-Winkelaar, E.M.; Koning, F.; Zwaginga, J.J.; Fidder, H.H. Autologous bone marrow-derived mesenchymal stromal cell treatment for refractory luminal Crohn’s disease: Results of a phase I study. Gut 2010, 59, 1662–1669. [Google Scholar] [CrossRef]
- Liang, J.; Zhang, H.; Wang, D.; Feng, X.; Wang, H.; Hua, B.; Liu, B.; Sun, L. Allogeneic mesenchymal stem cell transplantation in seven patients with refractory inflammatory bowel disease. Gut 2011. [Google Scholar] [CrossRef]
- Chen, S.-L.; Fang, W.-W.; Ye, F.; Liu, Y.-H.; Qian, J.; Shan, S.-J.; Zhang, J.-J.; Chunhua, R.Z.; Liao, L.-M.; Lin, S. Effect on left ventricular function of intracoronary transplantation of autologous bone marrow mesenchymal stem cell in patients with acute myocardial infarction. Am. J. Cardiol. 2004, 94, 92–95. [Google Scholar] [CrossRef]
- Hare, J.M.; Traverse, J.H.; Henry, T.D.; Dib, N.; Strumpf, R.K.; Schulman, S.P.; Gerstenblith, G.; DeMaria, A.N.; Denktas, A.E.; Gammon, R.S. A randomized, double-blind, placebo-controlled, dose-escalation study of intravenous adult human mesenchymal stem cells (prochymal) after acute myocardial infarction. J. Am. Coll. Cardiol. 2009, 54, 2277–2286. [Google Scholar] [CrossRef]
- Stagg, J. Mesenchymal stem cells in cancer. Stem Cell Rev. 2008, 4, 119–124. [Google Scholar] [CrossRef]
- Ritter, E.; Perry, A.; Yu, J.; Wang, T.; Tang, L.; Bieberich, E. Breast cancer cell-derived fibroblast growth factor 2 and vascular endothelial growth factor are chemoattractants for bone marrow stromal stem cells. Ann. Surg. 2008, 247, 310–314. [Google Scholar] [CrossRef]
- Dwyer, R.; Potter-Beirne, S.; Harrington, K.; Lowery, A.; Hennessy, E.; Murphy, J.; Barry, F.; OʼBrien, T.; Kerin, M. Monocyte chemotactic protein-1 secreted by primary breast tumors stimulates migration of mesenchymal stem cells. Clin. Cancer Res. 2007, 13, 5020–5027. [Google Scholar] [CrossRef]
- Xu, W.-T.; Bian, Z.-Y.; Fan, Q.-M.; Li, G.; Tang, T.-T. Human mesenchymal stem cells (hMSCs) target osteosarcoma and promote its growth and pulmonary metastasis. Cancer Lett. 2009, 281, 32–41. [Google Scholar] [CrossRef]
- Stoff-Khalili, M.A.; Rivera, A.A.; Mathis, J.M.; Banerjee, N.S.; Moon, A.S.; Hess, A.; Rocconi, R.P.; Numnum, T.M.; Everts, M.; Chow, L.T. Mesenchymal stem cells as a vehicle for targeted delivery of CRAds to lung metastases of breast carcinoma. Breast Cancer Res. Treat. 2007, 105, 157–167. [Google Scholar] [CrossRef]
- Sonabend, A.M.; Ulasov, I.V.; Tyler, M.A.; Rivera, A.A.; Mathis, J.M.; Lesniak, M.S. Mesenchymal stem cells effectively deliver an oncolytic adenovirus to intracranial glioma. Stem Cells 2008, 26, 831–841. [Google Scholar] [CrossRef]
- Yong, R.L.; Shinojima, N.; Fueyo, J.; Gumin, J.; Vecil, G.G.; Marini, F.C.; Bogler, O.; Andreeff, M.; Lang, F.F. Human bone marrow-derived mesenchymal stem cells for intravascular delivery of oncolytic adenovirus Δ24-RGD to human gliomas. Cancer Res. 2009, 69, 8932–8940. [Google Scholar] [CrossRef]
- Komarova, S.; Kawakami, Y.; Stoff-Khalili, M.A.; Curiel, D.T.; Pereboeva, L. Mesenchymal progenitor cells as cellular vehicles for delivery of oncolytic adenoviruses. Mol. Cancer Ther. 2006, 5, 755–766. [Google Scholar] [CrossRef]
- Garcia-Castro, J.; Alemany, R.; Cascallo, M.; Martinez-Quintanilla, J.; del Mar Arriero, M.; Lassaletta, A.; Madero, L.; Ramírez, M. Treatment of metastatic neuroblastoma with systemic oncolytic virotherapy delivered by autologous mesenchymal stem cells: An exploratory study. Cancer Gene Ther. 2010, 17, 476–483. [Google Scholar] [CrossRef]
- Pannuti, A.; Foreman, K.; Rizzo, P.; Osipo, C.; Golde, T.; Osborne, B.; Miele, L. Targeting Notch to target cancer stem cells. Clin. Cancer Res. 2010, 16, 3141–3152. [Google Scholar] [CrossRef]
- Frank, N.Y.; Schatton, T.; Frank, M.H. The therapeutic promise of the cancer stem cell concept. J. Clin. Investig. 2010, 120, 41–50. [Google Scholar] [CrossRef]
- Matushansky, I.; Hernando, E.; Socci, N.D.; Mills, J.E.; Matos, T.A.; Edgar, M.A.; Singer, S.; Maki, R.G.; Cordon-Cardo, C. Derivation of sarcomas from mesenchymal stem cells via inactivation of the Wnt pathway. J. Clin. Investig. 2007, 117, 3248–3257. [Google Scholar] [CrossRef]
- Riggi, N.; Suvà, M.-L.; Suvà, D.; Cironi, L.; Provero, P.; Tercier, S.; Joseph, J.-M.; Stehle, J.-C.; Baumer, K.; Kindler, V. EWS-FLI-1 expression triggers a Ewing’s sarcoma initiation program in primary human mesenchymal stem cells. Cancer Res. 2008, 68, 2176–2185. [Google Scholar] [CrossRef]
- Suvà, M.-L.; Riggi, N.; Stehle, J.-C.; Baumer, K.; Tercier, S.; Joseph, J.-M.; Suvà, D.; Clément, V.; Provero, P.; Cironi, L. Identification of cancer stem cells in Ewing’s sarcoma. Cancer Res. 2009, 69, 1776–1781. [Google Scholar] [CrossRef]
- Lim, P.; Patel, S.A.; Rameshwar, P. Effective tissue repair and immunomodulation by mesenchymal stem cells within a milieu of cytokines. Stem Cell-Based Tissue Repair 2011, 346–365. [Google Scholar]
- Rubio, D.; Garcia, S.; Paz, M.F.; de la Cueva, T.; Lopez-Fernandez, L.A.; Lloyd, A.C.; Garcia-Castro, J.; Bernad, A. Molecular characterization of spontaneous mesenchymal stem cell transformation. PLoS One 2008, 3, e1398. [Google Scholar]
- Xu, X.; Zhang, X.; Wang, S.; Qian, H.; Zhu, W.; Cao, H.; Wang, M.; Chen, Y.; Xu, W. Isolation and comparison of mesenchymal stem-like cells from human gastric cancer and adjacent non-cancerous tissues. J. Cancer Res. Clin. Oncol. 2011, 137, 495–504. [Google Scholar]
- Lin, T.-M.; Chang, H.-W.; Wang, K.-H.; Kao, A.-P.; Chang, C.-C.; Wen, C.-H.; Lai, C.-S.; Lin, S.-D. Isolation and identification of mesenchymal stem cells from human lipoma tissue. Biochem. Biophys. Res. Commun. 2007, 361, 883–889. [Google Scholar] [CrossRef]
- Brune, J.C.; Tormin, A.; Johansson, M.C.; Rissler, P.; Brosjö, O.; Löfvenberg, R.; von Steyern, F.V.; Mertens, F.; Rydholm, A.; Scheding, S. Mesenchymal stromal cells from primary osteosarcoma are non-malignant and strikingly similar to their bone marrow counterparts. Int. J. Cancer 2011, 129, 319–330. [Google Scholar]
- Kidd, S.; Spaeth, E.; Watson, K.; Burks, J.; Lu, H.; Klopp, A.; Andreeff, M.; Marini, F.C. Origins of the tumor microenvironment: Quantitative assessment of adipose-derived and bone marrow-derived stroma. PLoS One 2012, 7, e30563. [Google Scholar]
- Lai, R.C.; Arslan, F.; Lee, M.M.; Sze, N.S.K.; Choo, A.; Chen, T.S.; Salto-Tellez, M.; Timmers, L.; Lee, C.N.; El Oakley, R.M. Exosome secreted by MSC reduces myocardial ischemia/reperfusion injury. Stem Cell Res. 2010, 4, 214–222. [Google Scholar] [CrossRef]
- Farsad, K. Exosomes: Novel organelles implicated in immunomodulation and apoptosis. Yale J. Biol. Med. 2002, 75, 95. [Google Scholar]
- Uccelli, A.; Moretta, L.; Pistoia, V. Mesenchymal stem cells in health and disease. Nat. Rev. Immunol. 2008, 8, 726–736. [Google Scholar]
- Buyanovskaya, O.; Kuleshov, N.; Nikitina, V.; Voronina, E.; Katosova, L.; Bochkov, N. Spontaneous aneuploidy and clone formation in adipose tissue stem cells during different periods of culturing. Bull. Exp. Biol. Med. 2009, 148, 109–112. [Google Scholar]
- Zhou, Y.F.; Bosch-Marce, M.; Okuyama, H.; Krishnamachary, B.; Kimura, H.; Zhang, L.; Huso, D.L.; Semenza, G.L. Spontaneous transformation of cultured mouse bone marrow-derived stromal cells. Cancer Res. 2006, 66, 10849–10854. [Google Scholar] [CrossRef]
- Bruno, S.; Grange, C.; Deregibus, M.C.; Calogero, R.A.; Saviozzi, S.; Collino, F.; Morando, L.; Falda, M.; Bussolati, B.; Tetta, C. Mesenchymal stem cell-derived microvesicles protect against acute tubular injury. J. Am. Soc. Nephrol. 2009, 20, 1053–1067. [Google Scholar] [CrossRef]
- VanWijk, M.J.; VanBavel, E.; Sturk, A.; Nieuwland, R. Microparticles in cardiovascular diseases. Cardiovasc. Res. 2003, 59, 277–287. [Google Scholar] [CrossRef]
- Diamant, M.; Tushuizen, M.E.; Sturk, A.; Nieuwland, R. Cellular microparticles: New players in the field of vascular disease? Eur. J. Clin. Investig. 2004, 34, 392–401. [Google Scholar]
- Bian, S.; Cui, H.; Zhang, X.; Qi, L.; Li, D. Mesenchymal stem cells release membrane microparticles in the process of apoptosis. Zhongguo Shi Yan Xue Ye Xue Za Zhi 2012, 20, 453–457. (In Chinese) [Google Scholar]
- Herring, J.; McMichael, M.; Smith, S. Microparticles in health and disease. J. Vet. Intern. Med. 2013, 27, 1020–1033. [Google Scholar] [CrossRef]
- Schroit, A.; Tanaka, Y.; Madsen, J.; Fidler, I. The recognition of red blood cells by macrophages: Role of phosphatidylserine and possible implications of membrane phospholipid asymmetry. Biol. Cell 1984, 51, 227–238. [Google Scholar] [CrossRef]
- Connor, J.; Pak, C.H.; Zwaal, R.; Schroit, A. Bidirectional transbilayer movement of phospholipid analogs in human red blood cells. Evidence for an ATP-dependent and protein-mediated process. J. Biol. Chem. 1992, 267, 19412–19417. [Google Scholar]
- Zwaal, R.F.; Comfurius, P.; Bevers, E.M. Mechanism and function of changes in membrane-phospholipid asymmetry in platelets and erythrocytes. Biochem. Soc. Trans. 1993, 21, 248–253. [Google Scholar]
- Freyssinet, J.-M.; Toti, F. Formation of procoagulant microparticles and properties. Thromb. Res. 2010, 125, S46–S48. [Google Scholar]
- Baron, M.; Boulanger, C.M.; Staels, B.; Tailleux, A. Cell-derived microparticles in atherosclerosis: Biomarkers and targets for pharmacological modulation? J. Cell. Mol. Med. 2012, 16, 1365–1376. [Google Scholar]
- Demarchi, F.; Schneider, C. The calpain system as a modulator of stress/damage response. Cell Cycle 2007, 6, 136. [Google Scholar] [CrossRef]
- Cauwenberghs, S.; Feijge, M.A.; Harper, A.G.; Sage, S.O.; Curvers, J.; Heemskerk, J.W. Shedding of procoagulant microparticles from unstimulated platelets by integrin-mediated destabilization of actin cytoskeleton. FEBS Lett. 2006, 580, 5313–5320. [Google Scholar] [CrossRef]
- Curtis, A.; Wilkinson, P.; Gui, M.; Gales, T.; Hu, E.; Edelberg, J. p38 mitogen-activated protein kinase targets the production of proinflammatory endothelial microparticles. J. Thromb. Haemost. 2009, 7, 701–709. [Google Scholar] [CrossRef]
- Crespin, M.; Vidal, C.; Picard, F.; Lacombe, C.; Fontenay, M. Activation of PAK1/2 during the shedding of platelet microvesicles. Blood Coagul. Fibrinolysis 2009, 20, 63–70. [Google Scholar] [CrossRef]
- Abramovici, H.; Mojtabaie, P.; Parks, R.J.; Zhong, X.-P.; Koretzky, G.A.; Topham, M.K.; Gee, S.H. Diacylglycerol kinase ζ regulates actin cytoskeleton reorganization through dissociation of Rac1 from RhoGDI. Mol. Biol. Cell 2009, 20, 2049–2059. [Google Scholar] [CrossRef]
- Lai, R.C.; Chen, T.S.; Lim, S.K. Mesenchymal stem cell exosome: A novel stem cell-based therapy for cardiovascular disease. Regen. Med. 2011, 6, 481–492. [Google Scholar]
- Wang, X.Q.; Zhu, X.J.; Zou, P. Research progress of mesenchymal stem cell-derived microvesicle. Zhongguo Shi Yan Xue Ye Xue Za Zhi 2013, 21, 227–230. (In Chinese) [Google Scholar]
- Chen, T.S.; Lai, R.C.; Lee, M.M.; Choo, A.B.H.; Lee, C.N.; Lim, S.K. Mesenchymal stem cell secretes microparticles enriched in pre-microRNAs. Nucleic Acids Res. 2010, 38, 215–224. [Google Scholar] [CrossRef] [Green Version]
- Collino, F.; Deregibus, M.C.; Bruno, S.; Sterpone, L.; Aghemo, G.; Viltono, L.; Tetta, C.; Camussi, G. Microvesicles derived from adult human bone marrow and tissue specific mesenchymal stem cells shuttle selected pattern of miRNAs. PLoS One 2010, 5, e11803. [Google Scholar] [CrossRef]
- Tomasoni, S.; Longaretti, L.; Rota, C.; Morigi, M.; Conti, S.; Gotti, E.; Capelli, C.; Introna, M.; Remuzzi, G.; Benigni, A. Transfer of growth factor receptor mRNA via exosomes unravels the regenerative effect of mesenchymal stem cells. Stem Cells Dev. 2012, 22, 772–780. [Google Scholar]
- Ohtani, K.; Dimmeler, S. Control of cardiovascular differentiation by microRNAs. Basic Res. Cardiol. 2011, 106, 5–11. [Google Scholar] [CrossRef]
- Ryan, B.M.; Robles, A.I.; Harris, C.C. Genetic variation in microRNA networks: The implications for cancer research. Nat. Rev. Cancer 2010, 10, 389–402. [Google Scholar]
- Kim, H.-S.; Choi, D.-Y.; Yun, S.J.; Choi, S.-M.; Kang, J.W.; Jung, J.W.; Hwang, D.; Kim, K.P.; Kim, D.-W. Proteomic analysis of microvesicles derived from human mesenchymal stem cells. J. Proteome Res. 2011, 11, 839–849. [Google Scholar]
- Van der Pol, E.; Böing, A.N.; Harrison, P.; Sturk, A.; Nieuwland, R. Classification, functions, and clinical relevance of extracellular vesicles. Pharmacol. Rev. 2012, 64, 676–705. [Google Scholar]
- Weerheim, A.; Kolb, A.; Sturk, A.; Nieuwland, R. Phospholipid composition of cell-derived microparticles determined by one-dimensional high-performance thin-layer chromatography. Anal. Biochem. 2002, 302, 191–198. [Google Scholar] [CrossRef]
- Tan, K.T.; Lip, G.Y. The potential role of platelet microparticles in atherosclerosis. Thromb. Haemost. 2005, 94, 488. [Google Scholar]
- Rustom, A.; Saffrich, R.; Markovic, I.; Walther, P.; Gerdes, H.-H. Nanotubular highways for intercellular organelle transport. Science 2004, 303, 1007–1010. [Google Scholar] [CrossRef]
- Ratajczak, J.; Wysoczynski, M.; Hayek, F.; Janowska-Wieczorek, A.; Ratajczak, M. Membrane-derivedmicrovesicles: Important and underappreciated mediators of cell-to-cell communication. Leukemia 2006, 20, 1487–1495. [Google Scholar]
- Camussi, G.; Deregibus, M.C.; Bruno, S.; Cantaluppi, V.; Biancone, L. Exosomes/microvesicles as a mechanism of cell-to-cell communication. Kidney Int. 2010, 78, 838–848. [Google Scholar]
- Ratajczak, J.; Miekus, K.; Kucia, M.; Zhang, J.; Reca, R.; Dvorak, P.; Ratajczak, M. Embryonic stem cell-derived microvesicles reprogram hematopoietic progenitors: Evidence for horizontal transfer of mRNA and protein delivery. Leukemia 2006, 20, 847–856. [Google Scholar] [CrossRef]
- Valadi, H.; Ekström, K.; Bossios, A.; Sjöstrand, M.; Lee, J.J.; Lötvall, J.O. Exosome-mediated transfer of mRNAs and microRNAs is a novel mechanism of genetic exchange between cells. Nat. Cell Biol. 2007, 9, 654–659. [Google Scholar]
- Skog, J.; Würdinger, T.; van Rijn, S.; Meijer, D.H.; Gainche, L.; Curry, W.T.; Carter, B.S.; Krichevsky, A.M.; Breakefield, X.O. Glioblastoma microvesicles transport RNA and proteins that promote tumour growth and provide diagnostic biomarkers. Nat. Cell Biol. 2008, 10, 1470–1476. [Google Scholar]
- Pegtel, D.M.; Cosmopoulos, K.; Thorley-Lawson, D.A.; van Eijndhoven, M.A.; Hopmans, E.S.; Lindenberg, J.L.; de Gruijl, T.D.; Würdinger, T.; Middeldorp, J.M. Functional delivery of viral miRNAs via exosomes. Proc. Natl. Acad. Sci. USA 2010, 107, 6328–6333. [Google Scholar] [CrossRef]
- Chen, T.S.; Arslan, F.; Yin, Y.; Tan, S.S.; Lai, R.C.; Choo, A.; Padmanabhan, J.; Lee, C.N.; de Kleijn, D.; Lim, S.K. Enabling a robust scalable manufacturing process for therapeutic exosomes through oncogenic immortalization of human ESC-derived MSCs. J. Transl. Med. 2011, 9, 1471–1482. [Google Scholar]
- Alexandre, C.S.; Volpini, R.A.; Shimizu, M.H.; Sanches, T.R.; Semedo, P.; di Jura, V.L.; Camara, N.O.; Seguro, A.C.; Andrade, L. Lineage-negative bone marrow cells protect against chronic renal failure. Stem Cells 2009, 27, 682–692. [Google Scholar] [CrossRef]
- Zhen-Qiang, F.; Bing-Wei, Y.; Yong-Liang, L.; Xiang-Wei, W.; Shan-Hong, Y.; Yuan-Ning, Z.; Wei-Sheng, J.; Wei, C.; Ye, G. Localized expression of human BMP-7 by BM-MSCs enhances renal repair in an in vivo model of ischemia-reperfusion injury. Genes Cells 2012, 17, 53–64. [Google Scholar] [CrossRef]
- Morigi, M.; Imberti, B.; Zoja, C.; Corna, D.; Tomasoni, S.; Abbate, M.; Rottoli, D.; Angioletti, S.; Benigni, A.; Perico, N. Mesenchymal stem cells are renotropic, helping to repair the kidney and improve function in acute renal failure. J. Am. Soc. Nephrol. 2004, 15, 1794–1804. [Google Scholar] [CrossRef]
- Choi, H.Y.; Moon, S.J.; Ratliff, B.B.; Ahn, S.H.; Jung, A.; Lee, M.; Lee, S.; Lim, B.J.; Kim, B.S.; Plotkin, M.D. Microparticles from kidney-derived mesenchymal stem cells act as carriers of proangiogenic signals and contribute to recovery from acute kidney injury. PLoS One 2014, 9, e87853. [Google Scholar]
- Gatti, S.; Bruno, S.; Deregibus, M.C.; Sordi, A.; Cantaluppi, V.; Tetta, C.; Camussi, G. Microvesicles derived from human adult mesenchymal stem cells protect against ischaemia–reperfusion-induced acute and chronic kidney injury. Nephrol. Dial. Transplant. 2011, 26, 1474–1483. [Google Scholar]
- Bruno, S.; Collino, F.; Deregibus, M.C.; Grange, C.; Tetta, C.; Camussi, G. Microvesicles derived from human bone marrow mesenchymal stem cells inhibit tumor growth. Stem Cells Dev. 2012, 22, 758–771. [Google Scholar]
- Roorda, B.D.; ter Elst, A.; Kamps, W.A.; de Bont, E.S. Bone marrow-derived cells and tumor growth: Contribution of bone marrow-derived cells to tumor micro-environments with special focus on mesenchymal stem cells. Crit. Rev. Oncol. Hematol. 2009, 69, 187–198. [Google Scholar] [CrossRef]
- Spaeth, E.L.; Dembinski, J.L.; Sasser, A.K.; Watson, K.; Klopp, A.; Hall, B.; Andreeff, M.; Marini, F. Mesenchymal stem cell transition to tumor-associated fibroblasts contributes to fibrovascular network expansion and tumor progression. PLoS One 2009, 4, e4992. [Google Scholar] [CrossRef]
- Zhu, W.; Huang, L.; Li, Y.; Zhang, X.; Gu, J.; Yan, Y.; Xu, X.; Wang, M.; Qian, H.; Xu, W. Exosomes derived from human bone marrow mesenchymal stem cells promote tumor growth in vivo. Cancer Lett. 2012, 315, 28–37. [Google Scholar] [CrossRef]
© 2014 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Tan, X.; Gong, Y.-Z.; Wu, P.; Liao, D.-F.; Zheng, X.-L. Mesenchymal Stem Cell-Derived Microparticles: A Promising Therapeutic Strategy. Int. J. Mol. Sci. 2014, 15, 14348-14363. https://doi.org/10.3390/ijms150814348
Tan X, Gong Y-Z, Wu P, Liao D-F, Zheng X-L. Mesenchymal Stem Cell-Derived Microparticles: A Promising Therapeutic Strategy. International Journal of Molecular Sciences. 2014; 15(8):14348-14363. https://doi.org/10.3390/ijms150814348
Chicago/Turabian StyleTan, Xi, Yong-Zhen Gong, Ping Wu, Duan-Fang Liao, and Xi-Long Zheng. 2014. "Mesenchymal Stem Cell-Derived Microparticles: A Promising Therapeutic Strategy" International Journal of Molecular Sciences 15, no. 8: 14348-14363. https://doi.org/10.3390/ijms150814348
APA StyleTan, X., Gong, Y.-Z., Wu, P., Liao, D.-F., & Zheng, X.-L. (2014). Mesenchymal Stem Cell-Derived Microparticles: A Promising Therapeutic Strategy. International Journal of Molecular Sciences, 15(8), 14348-14363. https://doi.org/10.3390/ijms150814348