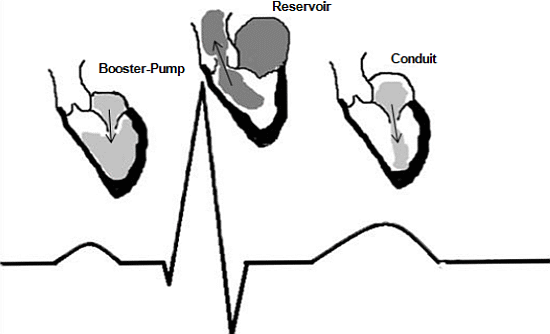
The Three Integrated Phases of Left Atrial Macrophysiology and Their Interactions
Abstract
:1. Introduction—Compelling Physiology

2. Adaptation
3. Left Atrial Appendage: A Sometimes Deadly Attachment
4. Frank-Starling Mechanism
5. Left Atrial Distensibility
6. Pathological Changes
6.1. Left Atrial Function and Neuroendocrine Dependence
6.2. Atrial Fibrillation
6.3. Importance of the Left Atrial Appendage
6.4. Mitral Valve Disease
6.5. Hypertension
6.6. Myocardial Infarction
6.7. Congestive Heart Failure
6.8. Obstructive Sleep Apnea
7. Conclusions
Acknowledgments
Author Contributions
Abbreviations
Conflicts of Interest
References
- Garcia, M.J. Left ventricular filling. Heart Fail. Clin. 2008, 4, 47–56. [Google Scholar] [CrossRef]
- Potter, L.R.; Yoder, A.R.; Flora, D.R.; Antos, L.K.; Dickey, D.M. Natriuretic peptides: Their structures, receptors, physiologic functions and therapeutic applications. Handb. Exp. Pharmacol. 2009, 191, 341–366. [Google Scholar] [CrossRef]
- Serra, J.L.; Bendersky, M. Atrial fibrillation and renin-angiotensin system. Ther. Adv. Cardiovasc. Dis. 2008, 2, 215–223. [Google Scholar] [CrossRef]
- Tsang, T.S.; Abhayaratna, W.P.; Barnes, M.E.; Miyasaka, Y.; Gersh, B.J.; Bailey, K.R.; Cha, S.S.; Seward, J.B. Prediction of cardiovascular outcomes with left atrial size: Is volume superior to area or diameter? J. Am. Coll. Cardiol. 2006, 47, 1018–1023. [Google Scholar] [CrossRef]
- Laukkanen, J.A.; Kurl, S.; Eranen, J.; Huttunen, M.; Salonen, J.T. Left atrium size and the risk of cardiovascular death in middle-aged men. Arch. Intern. Med. 2005, 165, 1788–1793. [Google Scholar] [CrossRef]
- Benjamin, E.J.; D’Agostino, R.B.; Belanger, A.J.; Wolf, P.A.; Levy, D. Left atrial size and the risk of stroke and death. The Framingham Heart Study. Circulation 1995, 92, 835–841. [Google Scholar] [CrossRef]
- Pozzoli, M.; Capomolla, S.; Sanarico, M.; Pinna, G.; Cobelli, F.; Tavazzi, L. Doppler evaluations of left ventricular diastolic filling and pulmonary wedge pressure provide similar prognostic information in patients with systolic dysfunction after myocardial infarction. Am. Heart J. 1995, 129, 716–725. [Google Scholar] [CrossRef]
- Pagel, P.S.; Kehl, F.; Gare, M.; Hettrick, D.A.; Kersten, J.R.; Warltier, D.C. Mechanical function of the left atrium: New insights based on analysis of pressure-volume relations and Doppler echocardiography. Anesthesiology 2003, 98, 975–994. [Google Scholar] [CrossRef]
- Mitchell, J.H.; Shapiro, W. Atrial function and the hemodynamic consequences of atrial fibrillation in man. Am. J. Cardiol. 1969, 23, 556–567. [Google Scholar] [CrossRef]
- Appleton, C.P.; Hatle, L.K.; Popp, R.L. Relation of transmitral flow velocity patterns to left ventricular diastolic function: New insights from a combined hemodynamic and Doppler echocardiographic study. J. Am. Coll. Cardiol. 1988, 12, 426–440. [Google Scholar] [CrossRef]
- Stefanadis, C.; Dernellis, J.; Tsiamis, E.; Toutouzas, P. Effects of pacing-induced and balloon coronary occlusion ischemia on left atrial function in patients with coronary artery disease. J. Am. Coll. Cardiol. 1999, 33, 687–696. [Google Scholar] [CrossRef]
- Kagawa, K.; Arakawa, M.; Miwa, H.; Noda, T.; Nishigaki, K.; Ito, Y.; Hirakawa, S. Left atrial function during left ventricular diastole evaluated by left atrial angiography and left ventriculography. J. Cardiol. 1994, 24, 317–325. [Google Scholar]
- Toutouzas, K.; Trikas, A.; Pitsavos, C.; Barbetseas, J.; Androulakis, A.; Stefanadis, C.; Toutouzas, P. Echocardiographic features of left atrium in elite male athletes. Am. J. Cardiol. 1996, 78, 1314–1317. [Google Scholar] [CrossRef]
- Kihara, Y.; Sasayama, S.; Miyazaki, S.; Onodera, T.; Susawa, T.; Nakamura, Y.; Fujiwara, H.; Kawai, C. Role of the left atrium in adaptation of the heart to chronic mitral regurgitation in conscious dogs. Circ. Res. 1988, 62, 543–553. [Google Scholar] [CrossRef]
- Yoshida, N.; Okamoto, M.; Makita, Y.; Nanba, K.; Yoshizumi, M. Determinants of enhanced left atrial active emptying with aging: Left atrial preload, contractility or both? Intern. Med. 2009, 48, 987–992. [Google Scholar] [CrossRef]
- Nakao, T.; Shimizu, M.; Sugihara, N.; Kita, Y.; Shimizu, K.; Takeda, R. Preload dependency of left atrial pump function in hypertrophic cardiomyopathy. Jpn. Circ. J. 1993, 57, 47–54. [Google Scholar] [CrossRef]
- Stefanadis, C.; Dernellis, J.; Toutouzas, P. A clinical appraisal of left atrial function. Eur. Heart J. 2001, 22, 22–36. [Google Scholar] [CrossRef]
- Williams, J.F., Jr.; Sonnenblick, E.H.; Braunwald, E. Determinants of atrial contractile force in the intact heart. Am. J. Physiol. 1965, 209, 1061–1068. [Google Scholar]
- Triposkiadis, F.; Tentolouris, K.; Androulakis, A.; Trikas, A.; Toutouzas, K.; Kyriakidis, M.; Gialafos, J.; Toutouzas, P. Left atrial mechanical function in the healthy elderly: New insights from a combined assessment of changes in atrial volume and transmitral flow velocity. J. Am. Soc. Echocardiogr. 1995, 8, 801–809. [Google Scholar] [CrossRef]
- Sunagawa, K.; Maughan, W.L.; Burkhoff, D.; Sagawa, K. Left ventricular interaction with arterial load studied in isolated canine ventricle. Am. J. Physiol. 1983, 245, H773–H780. [Google Scholar]
- Stone, H.L. Effect of heart rate on left atrial systolic shortening in the dog. J. Appl. Physiol. 1975, 38, 1110–1116. [Google Scholar]
- Schabelman, S.; Schiller, N.B.; Silverman, N.H.; Ports, T.A. Left atrial volume estimation by two-dimensional echocardiography. Catheter. Cardiovasc. Diagn. 1981, 7, 165–178. [Google Scholar] [CrossRef]
- Prioli, A.; Marino, P.; Lanzoni, L.; Zardini, P. Increasing degrees of left ventricular filling impairment modulate left atrial function in humans. Am. J. Cardiol. 1998, 82, 756–761. [Google Scholar] [CrossRef]
- Payne, R.M.; Stone, H.L.; Engelken, E.J. Atrial function during volume loading. J. Appl. Physiol. 1971, 31, 326–331. [Google Scholar]
- Okamoto, M.; Tsubokura, T.; Morishita, K.; Nakagawa, H.; Yamagata, T.; Kawagoe, T.; Hondo, T.; Tsuchioka, Y.; Matsuura, H.; Kajiyama, G. Effects of volume loading on left atrial systolic time intervals. J. Clin. Ultrasound 1991, 19, 405–411. [Google Scholar] [CrossRef]
- Blondheim, D.S.; Osipov, A.; Meisel, S.R.; Frimerman, A.; Shochat, M.; Shotan, A. Relation of left atrial size to function as determined by transesophageal echocardiography. Am. J. Cardiol. 2005, 96, 457–463. [Google Scholar] [CrossRef]
- Abhayaratna, W.P.; Fatema, K.; Barnes, M.E.; Seward, J.B.; Gersh, B.J.; Bailey, K.R.; Casaclang-Verzosa, G.; Tsang, T.S. Left atrial reservoir function as a potent marker for first atrial fibrillation or flutter in persons > or = 65 years of age. Am. J. Cardiol. 2008, 101, 1626–1629. [Google Scholar] [CrossRef]
- Gutman, J.; Wang, Y.S.; Wahr, D.; Schiller, N.B. Normal left atrial function determined by 2-dimensional echocardiography. Am. J. Cardiol. 1983, 51, 336–340. [Google Scholar] [CrossRef]
- Thomas, L.; Levett, K.; Boyd, A.; Leung, D.Y.; Schiller, N.B.; Ross, D.L. Compensatory changes in atrial volumes with normal aging: Is atrial enlargement inevitable? J. Am. Coll. Cardiol. 2002, 40, 1630–1635. [Google Scholar] [CrossRef]
- Boyd, A.C.; Schiller, N.B.; Leung, D.; Ross, D.L.; Thomas, L. Atrial dilation and altered function are mediated by age and diastolic function but not before the eighth decade. JACC Cardiovasc. Imaging 2011, 4, 234–242. [Google Scholar] [CrossRef]
- D’Ascenzi, F.; Cameli, M.; Lisi, M.; Zacá, V.; Natali, B.; Malandrino, A.; Benincasa, S.; Catanese, S.; Causarano, A.; Mondillo, S. Left atrial remodeling in competitive adolescent soccer players. Int. J. Sports Med. 2012, 33, 795–801. [Google Scholar] [CrossRef]
- Verma, A.; Kilicaslan, F.; Adams, J.R.; Hao, S.; Beheiry, S.; Minor, S.; Ozduran, V.; Claude Elayi, S.; Martin, D.O.; Schweikert, R.A.; et al. Extensive ablation during pulmonary vein antrum isolation has no adverse impact on left atrial function: An echocardiography and cine computed tomography analysis. J. Cardiovasc. Electrophysiol. 2006, 17, 741–746. [Google Scholar] [CrossRef]
- Rodrigues, A.C.; Scannavacca, M.I.; Caldas, M.A.; Hotta, V.T.; Pisani, C.; Sosa, E.A.; Mathias, W., Jr. Left atrial function after ablation for paroxysmal atrial fibrillation. Am. J. Cardiol. 2009, 103, 395–398. [Google Scholar] [CrossRef]
- Tsao, H.M.; Hu, W.C.; Wu, M.H.; Tai, C.T.; Chang, S.L.; Lin, Y.J.; Lo, L.W.; Huang, C.C.; Hu, Y.F.; Sheu, M.H.; et al. The impact of catheter ablation on the dynamic function of the left atrium in patients with atrial fibrillation: Insights from four-dimensional computed tomographic images. J. Cardiovasc. Electrophysiol. 2010, 21, 270–277. [Google Scholar] [CrossRef]
- Hara, H.; Virmani, R.; Holmes, D.R., Jr.; Buchbinder, M.; Lesser, J.R.; van Tassel, R.A.; Mooney, M.R.; Schwartz, R.S. Is the left atrial appendage more than a simple appendage? Catheter. Cardiovasc. Interv. 2009, 74, 234–242. [Google Scholar]
- Johnson, W.D.; Ganjoo, A.K.; Stone, C.D.; Srivyas, R.C.; Howard, M. The left atrial appendage: Our most lethal human attachment—Surgical implications. Eur. J. Cardiothorac. Surg. 2000, 17, 718–722. [Google Scholar] [CrossRef]
- Akosah, K.O.; Funai, J.T.; Porter, T.R.; Jesse, R.L.; Mohanty, P.K. Left atrial appendage contractile function in atrial fibrillation. Influence of heart rate and cardioversion to sinus rhythm. Chest 1995, 107, 690–696. [Google Scholar] [CrossRef]
- Predictors of thromboembolism in atrial fibrillation: II. Echocardiographic features of patients at risk. Ann. Intern. Med. 1992, 116, 6–12. [CrossRef]
- Gage, B.F.; Waterman, A.D.; Shannon, W.; Boechler, M.; Rich, M.W.; Radford, M.J. Validation of clinical classification schemes for predicting stroke: Results from the national registry of atrial fibrillation. JAMA 2001, 285, 2864–2870. [Google Scholar] [CrossRef]
- Boyd, A.C.; McKay, T.; Nasibi, S.; Richards, D.A.; Thomas, L. Left ventricular mass predicts left atrial appendage thrombus in persistent atrial fibrillation. Eur. Heart J. Cardiovasc. Imaging 2013, 14, 269–275. [Google Scholar] [CrossRef]
- Hoit, B.D.; Shao, Y.; Tsai, L.M.; Patel, R.; Gabel, M.; Walsh, R.A. Altered left atrial compliance after atrial appendectomy. Influence on left atrial and ventricular filling. Circ. Res. 1993, 72, 167–175. [Google Scholar] [CrossRef]
- Tabata, T.; Oki, T.; Yamada, H.; Iuchi, A.; Ito, S.; Hori, T.; Kitagawa, T.; Kato, I.; Kitahata, H.; Oshita, S. Role of left atrial appendage in left atrial reservoir function as evaluated by left atrial appendage clamping during cardiac surgery. Am. J. Cardiol. 1998, 81, 327–332. [Google Scholar] [CrossRef]
- Davis, C.A., III; Rembert, J.C.; Greenfield, J.C., Jr. Compliance of left atrium with and without left atrium appendage. Am. J. Physiol. 1990, 259, H1006–H1008. [Google Scholar]
- Chapeau, C.; Gutkowska, J.; Schiller, P.W.; Milne, R.W.; Thibault, G.; Garcia, R.; Genest, J.; Cantin, M. Localization of immunoreactive synthetic atrial natriuretic factor (ANF) in the heart of various animal species. J. Histochem. Cytochem. 1985, 33, 541–550. [Google Scholar] [CrossRef]
- Kappagoda, C.T.; Linden, R.J.; Saunders, D.A. The effect on heart rate of distending the atrial appendages in the dog. J. Physiol. 1972, 225, 705–719. [Google Scholar]
- Henry, J.P.; Gauer, O.H.; Reeves, J.L. Evidence of the atrial location of receptors influencing urine flow. Circ. Res. 1956, 4, 85–90. [Google Scholar] [CrossRef]
- Fatkin, D.; Feneley, M. Stratification of thromboembolic risk of atrial fibrillation by transthoracic echocardiography and transesophageal echocardiography: The relative role of left atrial appendage function, mitral valve disease, and spontaneous echocardiographic contrast. Prog. Cardiovasc. Dis. 1996, 39, 57–68. [Google Scholar] [CrossRef]
- Handke, M.; Harloff, A.; Hetzel, A.; Olschewski, M.; Bode, C.; Geibel, A. Left atrial appendage flow velocity as a quantitative surrogate parameter for thromboembolic risk: Determinants and relationship to spontaneous echocontrast and thrombus formation—A transesophageal echocardiographic study in 500 patients with cerebral ischemia. J. Am. Soc. Echocardiogr. 2005, 18, 1366–1372. [Google Scholar] [CrossRef]
- Panagiotopoulos, K.; Toumanidis, S.; Saridakis, N.; Vemmos, K.; Moulopoulos, S. Left atrial and left atrial appendage functional abnormalities in patients with cardioembolic stroke in sinus rhythm and idiopathic atrial fibrillation. J. Am. Soc. Echocardiogr. 1998, 11, 711–719. [Google Scholar] [CrossRef]
- Alli, O.; Doshi, S.; Kar, S.; Reddy, V.; Sievert, H.; Mullin, C.; Swarup, V.; Whisenant, B.; Holmes, D., Jr. Quality of life assessment in the randomized PROTECT AF (percutaneous closure of the left atrial appendage versus Warfarin therapy for prevention of stroke in patients with atrial fibrillation) trial of patients at risk for stroke with nonvalvular atrial fibrillation. J. Am. Coll. Cardiol. 2013, 61, 1790–1798. [Google Scholar] [CrossRef]
- Tavi, P.; Han, C.; Weckstrom, M. Mechanisms of stretch-induced changes in [Ca2+]i in rat atrial myocytes: Role of increased troponin C affinity and stretch-activated ion channels. Circ. Res. 1998, 83, 1165–1177. [Google Scholar] [CrossRef]
- Henry, W.L.; Gardin, J.M.; Ware, J.H. Echocardiographic measurements in normal subjects from infancy to old age. Circulation 1980, 62, 1054–1061. [Google Scholar] [CrossRef]
- Kircher, B.; Abbott, J.A.; Pau, S.; Gould, R.G.; Himelman, R.B.; Higgins, C.B.; Lipton, M.J.; Schiller, N.B. Left atrial volume determination by biplane two-dimensional echocardiography: Validation by cine computed tomography. Am. Heart J. 1991, 121, 864–871. [Google Scholar] [CrossRef]
- Hsiao, S.H.; Huang, W.C.; Lin, K.L.; Chiou, K.R.; Kuo, F.Y.; Lin, S.K.; Cheng, C.C. Left atrial distensibility and left ventricular filling pressure in acute versus chronic severe mitral regurgitation. Am. J. Cardiol. 2010, 105, 709–715. [Google Scholar] [CrossRef]
- Oki, T.; Tabata, T.; Yamada, H.; Fukuda, K.; Abe, M.; Onose, Y.; Wakatsuki, T.; Iuchi, A.; Ito, S. Assessment of abnormal left atrial relaxation by transesophageal pulsed Doppler echocardiography of pulmonary venous flow velocity. Clin. Cardiol. 1998, 21, 753–758. [Google Scholar] [CrossRef]
- Hoit, B.D.; Shao, Y.; Gabel, M.; Walsh, R.A. Influence of pericardium on left atrial compliance and pulmonary venous flow. Am. J. Physiol. 1993, 264, H1781–H1787. [Google Scholar]
- Parmley, W.W. Neuroendocrine changes in heart failure and their clinical relevance. Clin. Cardiol. 1995, 18, 440–445. [Google Scholar] [CrossRef]
- Brilla, C.G.; Reams, G.P.; Maisch, B.; Weber, K.T. Renin-angiotensin system and myocardial fibrosis in hypertension: Regulation of the myocardial collagen matrix. Eur. Heart J. 1993, 14, 57–61. [Google Scholar] [CrossRef]
- Brilla, C.G.; Matsubara, L.S.; Weber, K.T. Anti-aldosterone treatment and the prevention of myocardial fibrosis in primary and secondary hyperaldosteronism. J. Mol. Cell. Cardiol. 1993, 25, 563–575. [Google Scholar] [CrossRef]
- Casaclang-Verzosa, G.; Gersh, B.J.; Tsang, T.S. Structural and functional remodeling of the left atrium: Clinical and therapeutic implications for atrial fibrillation. J. Am. Coll. Cardiol. 2008, 51, 1–11. [Google Scholar] [CrossRef]
- Dietz, J.R. Mechanisms of atrial natriuretic peptide secretion from the atrium. Cardiovasc. Res. 2005, 68, 8–17. [Google Scholar] [CrossRef]
- Francis, G.S. Neuroendocrine activity in congestive heart failure. Am. J. Cardiol. 1990, 66, 33–38. [Google Scholar] [CrossRef]
- Tsioufis, C.; Stougiannos, P.; Taxiarchou, E.; Skiadas, I.; Chatzis, D.; Thomopoulos, C.; Lalos, S.; Stefanadis, C.; Kallikazaros, I. The interplay between haemodynamic load, brain natriuretic peptide and left atrial size in the early stages of essential hypertension. J. Hypertens. 2006, 24, 965–972. [Google Scholar] [CrossRef]
- Weber, K.T.; Brilla, C.G.; Campbell, S.E.; Guarda, E.; Zhou, G.; Sriram, K. Myocardial fibrosis: Role of angiotensin II and aldosterone. Basic Res. Cardiol. 1993, 88, 107–124. [Google Scholar]
- Leistad, E.; Christensen, G.; Ilebekk, A. Significance of increased atrial pressure on stroke volume during atrial fibrillation in anaesthetized pigs. Acta Physiol. Scand. 1993, 149, 157–161. [Google Scholar] [CrossRef]
- Leistad, E.; Christensen, G.; Ilebekk, A. Effects of atrial fibrillation on left and right atrial dimensions, pressures, and compliances. Am. J. Physiol. 1993, 264, H1093–H1097. [Google Scholar]
- Henry, W.L.; Morganroth, J.; Pearlman, A.S.; Clark, C.E.; Redwood, D.R.; Itscoitz, S.B.; Epstein, S.E. Relation between echocardiographically determined left atrial size and atrial fibrillation. Circulation 1976, 53, 273–279. [Google Scholar] [CrossRef]
- Takahashi, N.; Imataka, K.; Seki, A.; Fujii, J. Left atrial enlargement in patients with paroxysmal atrial fibrillation. Jpn. Heart J. 1982, 23, 677–683. [Google Scholar] [CrossRef]
- Suarez, G.S.; Lampert, S.; Ravid, S.; Lown, B. Changes in left atrial size in patients with lone atrial fibrillation. Clin. Cardiol. 1991, 14, 652–656. [Google Scholar] [CrossRef]
- Caplan, L.R.; D’Cruz, I.; Hier, D.B.; Reddy, H.; Shah, S. Atrial size, atrial fibrillation, and stroke. Ann. Neurol. 1986, 19, 158–161. [Google Scholar] [CrossRef]
- Sanfilippo, A.J.; Abascal, V.M.; Sheehan, M.; Oertel, L.B.; Harrigan, P.; Hughes, R.A.; Weyman, A.E. Atrial enlargement as a consequence of atrial fibrillation: A prospective echocardiographic study. Circulation 1990, 82, 792–797. [Google Scholar] [CrossRef]
- Klein, A.L.; Grimm, R.A.; Murray, R.D.; Apperson-Hansen, C.; Asinger, R.W.; Black, I.W.; Davidoff, R.; Erbel, R.; Halperin, J.L.; Orsinelli, D.A.; et al. Use of transesophageal echocardiography to guide cardioversion in patients with atrial fibrillation. N. Engl. J. Med. 2001, 344, 1411–1420. [Google Scholar] [CrossRef]
- Yu, C.M.; Khattab, A.A.; Bertog, S.C.; Lee, A.P.; Kwong, J.S.; Sievert, H.; Meier, B. Mechanical antithrombotic intervention by LAA occlusion in atrial fibrillation. Nat. Rev. Cardiol. 2013, 10, 707–722. [Google Scholar] [CrossRef]
- Stollberger, C.; Schneider, B.; Finsterer, J. Elimination of the left atrial appendage to prevent stroke or embolism? Anatomic, physiologic, and pathophysiologic considerations. Chest 2003, 124, 2356–2362. [Google Scholar] [CrossRef]
- Keren, G.; Etzion, T.; Sherez, J.; Zelcer, A.A.; Megidish, R.; Miller, H.I.; Laniado, S. Atrial fibrillation and atrial enlargement in patients with mitral stenosis. Am. Heart J. 1987, 114, 1146–1155. [Google Scholar] [CrossRef]
- Boudoulas, H.; Boudoulas, D.; Sparks, E.A.; Pearson, A.C.; Nagaraja, H.N.; Wooley, C.F. Left atrial performance indices in chronic mitral valve disease. J. Heart Valve Dis. 1995, 4, S242–S247. [Google Scholar]
- Sanada, H.; Shimizu, M.; Shimizu, K.; Kita, Y.; Sugihara, N.; Takeda, R. Left atrial afterload mismatch in hypertrophic cardiomyopathy. Am. J. Cardiol. 1991, 68, 1049–1054. [Google Scholar] [CrossRef]
- Cioffi, G.; Mureddu, G.F.; Stefenelli, C.; de Simone, G. Relationship between left ventricular geometry and left atrial size and function in patients with systemic hypertension. J. Hypertens. 2004, 22, 1589–1596. [Google Scholar] [CrossRef]
- Lakatta, E.G. Similar myocardial effects of aging and hypertension. Eur. Heart J. 1990, 11, 29–38. [Google Scholar] [CrossRef]
- Vaziri, S.M.; Larson, M.G.; Lauer, M.S.; Benjamin, E.J.; Levy, D. Influence of blood pressure on left atrial size: The framingham heart study. Hypertension 1995, 25, 1155–1160. [Google Scholar] [CrossRef]
- Dreslinski, G.R.; Frohlich, E.D.; Dunn, F.G.; Messerli, F.H.; Suarez, D.H.; Reisin, E. Echocardiographic diastolic ventricular abnormality in hypertensive heart disease: Atrial emptying index. Am. J. Cardiol. 1981, 47, 1087–1090. [Google Scholar] [CrossRef]
- Healey, J.S.; Connolly, S.J. Atrial fibrillation: Hypertension as a causative agent, risk factor for complications, and potential therapeutic target. Am. J. Cardiol. 2003, 91, 9G–14G. [Google Scholar] [CrossRef]
- Tsang, T.S.; Barnes, M.E.; Gersh, B.J.; Bailey, K.R.; Seward, J.B. Left atrial volume as a morphophysiologic expression of left ventricular diastolic dysfunction and relation to cardiovascular risk burden. Am. J. Cardiol. 2002, 90, 1284–1289. [Google Scholar] [CrossRef]
- Matsuda, M.; Matsuda, Y. Mechanism of left atrial enlargement related to ventricular diastolic impairment in hypertension. Clin. Cardiol. 1996, 19, 954–959. [Google Scholar] [CrossRef]
- Gerdts, E.; Oikarinen, L.; Palmieri, V.; Otterstad, J.E.; Wachtell, K.; Boman, K.; Dahlöf, B.; Devereux, R.B.; Losartan Intervention For Endpoint Reduction in Hypertension (LIFE) Study. Correlates of left atrial size in hypertensive patients with left ventricular hypertrophy: The losartan intervention for endpoint reduction in hypertension (LIFE) study. Hypertension 2002, 39, 739–743. [Google Scholar] [CrossRef]
- Boyd, A.C.; Eshoo, S.; Richards, D.A.; Thomas, L. Hypertension accelerates the “normal” aging process with a premature increase in left atrial volume. J. Am. Soc. Hypertens. 2013, 7, 149–156. [Google Scholar] [CrossRef]
- Kokubu, N.; Yuda, S.; Tsuchihashi, K.; Hashimoto, A.; Nakata, T.; Miura, T.; Ura, N.; Nagao, K.; Tsuzuki, M.; Wakabayashi, C.; et al. Noninvasive assessment of left atrial function by strain rate imaging in patients with hypertension: A possible beneficial effect of renin-angiotensin system inhibition on left atrial function. Hypertens. Res. 2007, 30, 13–21. [Google Scholar] [CrossRef]
- Sigwart, U.; Grbic, M.; Goy, J.J.; Kappenberger, L. Left atrial function in acute transient left ventricular ischemia produced during percutaneous transluminal coronary angioplasty of the left anterior descending coronary artery. Am. J. Cardiol. 1990, 65, 282–286. [Google Scholar] [CrossRef]
- Rahimtoola, S.H.; Ehsani, A.; Sinno, M.Z.; Loeb, H.S.; Rosen, K.M.; Gunnar, R.M. Left atrial transport function in myocardial infarction: Importance of its booster-pump function. Am. J. Med. 1975, 59, 686–694. [Google Scholar] [CrossRef]
- Keren, A.; DeAnda, A.; Komeda, M.; Tye, T.; Handen, C.R.; Daughters, G.T.; Ingels, N.B.; Miller, C.; Popp, R.L.; Nikolic, S.D. Pitfalls in creation of left atrial pressure-area relationships with automated border detection. J. Am. Soc. Echocardiogr. 1995, 8, 669–678. [Google Scholar] [CrossRef]
- Gibson, D.G.; Francis, D.P. Clinical assessment of left ventricular diastolic function. Heart 2003, 89, 231–238. [Google Scholar] [CrossRef]
- Wierzbowska-Drabik, K.; Krzeminska-Pakula, M.; Drozdz, J.; Plewka, M.; Trzos, E.; Kurpesa, M.; Rechciński, T.; Rózga, A.; Plońska-Gościniak, E.; Kasprzak, J.D. Enlarged left atrium is a simple and strong predictor of poor prognosis in patients after myocardial infarction. Echocardiography 2008, 25, 27–35. [Google Scholar]
- Hoit, B.D.; Shao, Y.; Gabel, M.; Walsh, R.A. Left atrial mechanical and biochemical adaptation to pacing induced heart failure. Cardiovasc. Res. 1995, 29, 469–474. [Google Scholar] [CrossRef]
- D’Andrea, A.; Caso, P.; Romano, S.; Scarafile, R.; Riegler, L.; Salerno, G.; Limongelli, G.; di Salvo, G.; Calabrò, P.; del Viscovo, L.; et al. Different effects of cardiac resynchronization therapy on left atrial function in patients with either idiopathic or ischaemic dilated cardiomyopathy: A two-dimensional speckle strain study. Eur. Heart J. 2007, 28, 2738–2748. [Google Scholar] [CrossRef]
- Paraskevaidis, I.A.; Panou, F.; Papadopoulos, C.; Farmakis, D.; Parissis, J.; Ikonomidis, I.; Rigopoulos, A.; Iliodromitis, E.K.; Th Kremastinos, D. Evaluation of left atrial longitudinal function in patients with hypertrophic cardiomyopathy: A tissue Doppler imaging and two-dimensional strain study. Heart 2009, 95, 483–489. [Google Scholar] [CrossRef]
- Rosca, M.; Popescu, B.A.; Beladan, C.C.; Calin, A.; Muraru, D.; Popa, E.C.; Lancellotti, P.; Enache, R.; Coman, I.M.; Jurcuţ, R.; et al. Left atrial dysfunction as a correlate of heart failure symptoms in hypertrophic cardiomyopathy. J. Am. Soc. Echocardiogr. 2010, 23, 1090–1098. [Google Scholar] [CrossRef]
- Shahar, E.; Whitney, C.W.; Redline, S.; Lee, E.T.; Newman, A.B.; Nieto, F.J.; O’Connor, G.T.; Boland, L.L.; Schwartz, J.E.; Samet, J.M. Sleep-disordered breathing and cardiovascular disease. Am. J. Respir. Crit. Care Med. 2001, 163, 19–25. [Google Scholar] [CrossRef]
- Oliveira, W.; Campos, O.; Bezerra Lira-Filho, E.; Cintra, F.D.; Vieira, M.; Ponchirolli, A.; de Paola, A.; Tufik, S.; Poyares, D. Left atrial volume and function in patients with obstructive sleep apnea assessed by real-time three-dimensional echocardiography. J. Am. Soc. Echocardiogr. 2008, 21, 1355–1361. [Google Scholar] [CrossRef]
© 2014 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Mehrzad, R.; Rajab, M.; Spodick, D.H. The Three Integrated Phases of Left Atrial Macrophysiology and Their Interactions. Int. J. Mol. Sci. 2014, 15, 15146-15160. https://doi.org/10.3390/ijms150915146
Mehrzad R, Rajab M, Spodick DH. The Three Integrated Phases of Left Atrial Macrophysiology and Their Interactions. International Journal of Molecular Sciences. 2014; 15(9):15146-15160. https://doi.org/10.3390/ijms150915146
Chicago/Turabian StyleMehrzad, Raman, Mohammad Rajab, and David H. Spodick. 2014. "The Three Integrated Phases of Left Atrial Macrophysiology and Their Interactions" International Journal of Molecular Sciences 15, no. 9: 15146-15160. https://doi.org/10.3390/ijms150915146
APA StyleMehrzad, R., Rajab, M., & Spodick, D. H. (2014). The Three Integrated Phases of Left Atrial Macrophysiology and Their Interactions. International Journal of Molecular Sciences, 15(9), 15146-15160. https://doi.org/10.3390/ijms150915146