Triethanolamine Stabilization of Methotrexate-β-Cyclodextrin Interactions in Ternary Complexes
Abstract
:1. Introduction
2. Results and Discussion
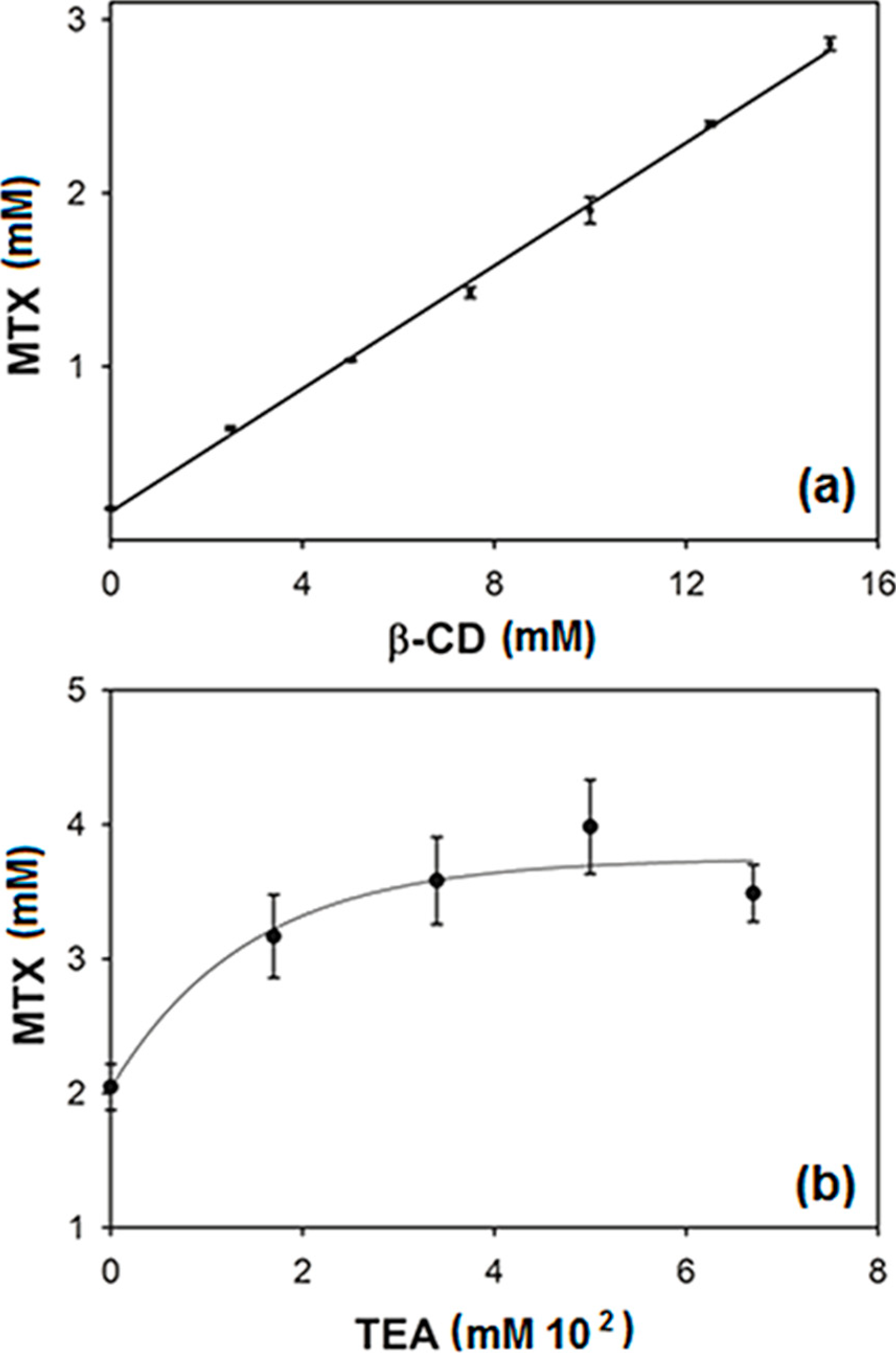
2.1. Effect of Triethanolamine (TEA) on Methotrexate (MTX)–Beta-Cyclodextrin (β-CD) Interactions in Aqueous Medium
2.2. 1H-NMR Spectroscopic Studies
| Studied Protons | Free State (ppm) | Binary Complex (ppm) | Δδ (ppm) | Ternary Complex (ppm) | Δδ (ppm) |
|---|---|---|---|---|---|
| MTX | |||||
| Ha | 8.6892 | 8.3530 | −0.3362 | 8.3677 | −0.3215 |
| Hc | 3.2404 | 3.4216 | 0.1812 | 3.3696 | 0.1292 |
| Hd | 6.9292 | 6.7362 | −0.1930 | 6.7828 | −0.1464 |
| He | 7.7425 | 7.7527 | 0.0102 | 7.7676 | 0.0251 |
| β-CD | |||||
| H1 | 5.0984 | 5.0875 | −0.0109 | 5.0845 | −0.0139 |
| H2 | 3.6778 | 3.6762 | −0.0016 | 3.6670 | −0.0108 |
| H3 | 3.9936 | 3.9065 | −0.0871 | 3.9416 | −0.0520 |
| H4 | 3.6118 | 3.6079 | −0.0039 | 3.6051 | −0.0067 |
| H5 | 3.9063 | 3.8395 | −0.0668 | 3.8103 | −0.0960 |
| H6 | 3.9063 | 3.8782 | −0.0281 | 3.8694 | −0.0369 |

2.3. Molecular Modeling Studies

| Energetic Component | Value (kcal·mol−1) | |
|---|---|---|
| Binary Complex | Ternary Complex | |
| Electrostatic | −30.07 | −220.22 |
| Van der Waals | −33.20 | −30.09 |
| Total Gas Energy | −63.27 | −250.30 |
| Solvation Energy | 43.76 | 217.87 |
| Estimated ∆G | −19.50 | −32.43 |
| Atoms | Occupancy (%) | Average Distance (Å) |
|---|---|---|
| MTX(O3):β-CD (O-H6) | 24.54 | 2.68 (±0.11) |
| MTX(O4):β-CD (O-H6) | 15.80 | 2.65 (±0.15) |
| MTX(O2):β-CD (O-H6) | 5.52 | 2.70 (±0.12) |
| β-CD (O3):MTX (NH28) | 6.35 | 2.88 (±0.08) |
2.4. Drug-Loading Analysis and FTIR Studies
| Sample | Theoretical %N from MTX | Analytical Content (%) | ||
|---|---|---|---|---|
| C | H | N | ||
| MTX | 24.65 | 46.67 | 5.45 | 21.65 |
| β-CD | – | 38.01 | 6.78 | 0.01 |
| β-CD:MTX | 1.75% | 38.96 | 6.71 | 1.55 |
| β-CD:MTX:TEA | 1.80% | 40.65 | 6.77 | 2.74 |

2.5. Thermal and Structural Studies


2.6. In Vitro Drug Release Studies

3. Experimental Section
3.1. Materials
3.2. Phase Solubility Studies

3.4. Molecular Modeling Studies
3.5. Solid Samples Preparation
3.6. Physicochemical Aspects of Freeze-Dried Complexes
3.7. In Vitro Dissolution Studies
3.8. Statistics
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Carretero, G.; Puig, L.; Dehesa, L.; Carrascosa, J.; Ribera, M.; Sánchez-Regaña, M.; Daudén, E.; Vidal, D.; Alsina, M.; Muñoz-Santos, C. Guidelines on the use of methotrexate in psoriasis. Actas Dermo-Sifiliogr. 2010, 101, 13. [Google Scholar]
- Olsen, E.A. The pharmacology of methotrexate. J. Am. Acad. Dermatol. 1991, 25, 306–318. [Google Scholar]
- Singka, G.S.L.; Samah, N.A.; Zulfakar, M.H.; Yurdasiper, A.; Heard, C.M. Enhanced topical delivery and anti-inflammatory activity of methotrexate from an activated nanogel. Eur. J. Pharm. Biopharm. 2010, 76, 275–281. [Google Scholar]
- Orenberg, E.K.; Luck, E.E.; Brown, D.M.; Kitchell, B.E. Implant delivery system: Intralesional delivery of chemotherapeutic agents for treatment of spontaneous skin tumors in veterinary patients. Clin. Dermatol. 1991, 9, 561–568. [Google Scholar]
- Alvarez-Figueroa, M.J.; Blanco-Méndez, J. Transdermal delivery of methotrexate: Iontophoretic delivery from hydrogels and passive delivery from microemulsions. Int. J. Pharm. 2001, 215, 57–65. [Google Scholar]
- Dubey, V.; Mishra, D.; Dutta, T.; Nahar, M.; Saraf, D.K.; Jain, N.K. Dermal and transdermal delivery of an anti-psoriatic agent via ethanolic liposomes. J. Control. Release 2007, 123, 148–154. [Google Scholar]
- Yang, F.; Kamiya, N.; Goto, M. Transdermal delivery of the anti-rheumatic agent methotrexate using a solid-in-oil nanocarrier. Eur. J. Pharm. Biopharm. 2012, 82, 158–163. [Google Scholar]
- Bouhadir, K.H.; Alsberg, E.; Mooney, D.J. Hydrogels for combination delivery of antineoplastic agents. Biomaterials 2001, 22, 2625–2633. [Google Scholar]
- Lindenberg, M.; Kopp, S.; Dressman, J.B. Classification of orally administered drugs on the World Health Organization Model list of essential medicines according to the biopharmaceutics classification system. Eur. J. Pharm. Biopharm. 2004, 58, 265–278. [Google Scholar]
- Connors, K.A. The stability of cyclodextrin complexes in solution. Chem. Rev. 1997, 97, 32. [Google Scholar]
- Granero, G.E.; Maitre, M.M.; Garnero, C.; Longhi, M.R. Synthesis, characterization and in vitro release studies of a new acetazolamide–HP-β-CD–TEA inclusion complex. Eur. J. Med. Chem. 2008, 43, 464–470. [Google Scholar]
- Palma, S.D.; Tartara, L.I.; Quinteros, D.; Allemandi, D.A.; Longhi, M.R.; Granero, G.E. An efficient ternary complex of acetazolamide with HP-β-CD and TEA for topical ocular administration. J. Control. Release 2009, 138, 24–31. [Google Scholar]
- Soica, C.; Danciu, C.; Savoiu-Balint, G.; Borcan, F.; Ambrus, R.; Zupko, I.; Bojin, F.; Coricovac, D.; Ciurlea, S.; Avram, S.; et al. Betulinic acid in complex with a γ-cyclodextrin derivative decreases proliferation and in vivo tumor development of non-metastatic and metastatic B164A5 cells. Int. J. Mol. Sci. 2014, 15, 8235–8255. [Google Scholar]
- Cui, L.; Zhang, Z.-H. Effect of β-cyclodextrin complexation on solubility and enzymatic conversion of naringin. Int. J. Mol. Sci. 2012, 13, 14251–14261. [Google Scholar]
- Szejtli, J. Introduction and general overview of cyclodextrin chemistry. Chem. Rev. 1998, 98, 11. [Google Scholar]
- Onnainty, R.; Schenfeld, E.M.; Quevedo, M.A.; Fernández, M.A.; Longhi, M.R.; Granero, G.E. Characterization of the hydrochlorothiazide: β-Cyclodextrin inclusion complex. Experimental and theoretical methods. J. Phys. Chem. B 2012, 117, 206–217. [Google Scholar]
- Jansook, P.; Loftsson, T. CDs as solubilizers: Effects of excipients and competing drugs. Int. J. Pharm. 2009, 379, 32–40. [Google Scholar]
- Loftsson, T.; Fri∂riksdóttir, H.; Gu∂mundsdóttir, T.K. The effect of water-soluble polymers on aqueous solubility of drugs. Int. J. Pharm. 1996, 127, 293–296. [Google Scholar]
- Messner, M.; Kurkov, S.V.; Jansook, P.; Loftsson, T. Self-assembled cyclodextrin aggregates and nanoparticles. Int. J. Pharm. 2010, 387, 199–208. [Google Scholar]
- Higuchi, T.; Connors, K.A. Phase solubility techniques. Adv. Anal. Chem. Instrum. 1965, 4, 95. [Google Scholar]
- Granero, G.; Granero, C.; Longhi, M. The effect of pH and triethanolamine on sulfisoxazole complexation with hydroxypropyl-β-cyclodextrin. Eur. J. Pharm. Sci. 2003, 20, 285–293. [Google Scholar]
- Mora, M.J.; Longhi, M.R.; Granero, G.E. Synthesis and characterization of binary and ternary complexes of diclofenac with a methyl-β-CD and monoethanolamine and in vitro transdermal evaluation. Eur. J. Med. Chem. 2010, 45, 4079–4088. [Google Scholar]
- Swiech, O.; Mieczkowska, A.; Chmurski, K.; Bilewicz, R. Intermolecular interactions between doxorubicin and β-cyclodextrin 4-methoxyphenol conjugates. J. Phys. Chem. B 2012, 116, 1765–1771. [Google Scholar]
- Karoyo, A.H.; Sidhu, P.S.; Wilson, L.; Hazendonk, P. Characterization and dynamic properties for the solid inclusion complexes of β-cyclodextrin and perfluorooctanoic acid. J. Phys. Chem. B 2013, 117, 8269–8282. [Google Scholar]
- Danciu, C.; Soica, C.; Oltean, M.; Avram, S.; Borcan, F.; Csanyi, E.; Ambrus, R.; Zupko, I.; Muntean, D.; Dehelean, C.A.; et al. Genistein in 1:1 inclusion complexes with ramified cyclodextrins: Theoretical, physicochemical and biological evaluation. Int. J. Mol. Sci. 2014, 15, 1962–1982. [Google Scholar]
- He, P.; Davis, S.S.; Illum, L. Chitosan microspheres prepared by spray drying. Int. J. Pharm. 1999, 187, 53–65. [Google Scholar]
- Sambasevam, K.P.; Mohamad, S.; Sarih, N.M.; Ismail, N.A. Synthesis and characterization of the inclusion complex of β-cyclodextrin and azomethine. Int. J. Mol. Sci. 2013, 14, 3671–3682. [Google Scholar]
- Lis-Cieplak, A.; Sitkowski, J.; Kolodziejski, W. Comparative proton nuclear magnetic resonancestudies of amantadine complexes formed in aqueous solutions with three major cyclodextrins. J. Pharm. Sci. 2014, 103, 274–282. [Google Scholar]
- Domańska, U.; Pelczarska, A.; Pobudkowska, A. Effect of 2-hydroxypropyl-β-cyclodextrin on solubility of sparingly soluble drug derivatives of anthranilic acid. Int. J. Mol. Sci. 2011, 12, 2383–2394. [Google Scholar]
- Melo, P.N.; Barbosa, E.G.; Caland, L.B.; Carpegianni, H.; Garnero, C.; Longhi, M.; Freitas, M.F.P.; Silva-Júnior, A.A. Host–guest interactions between benznidazole and β-cyclodextrin in multicomponent complex systems involving hydrophilic polymers and triethanolamine in aqueous solution. J. Mol. Liq. 2013, 186, 147–156. [Google Scholar]
- Mohamad, S.; Surikumaran, H.; Raoov, M.; Marimuthu, T.; Chandrasekaram, K.; Subramaniam, P. Conventional study on novel dicationic ionic liquid inclusion with β-cyclodextrin. Int. J. Mol. Sci. 2011, 12, 6329–6345. [Google Scholar]
- Iacovino, R.; Rapuano, F.; Caso, J.V.; Russo, A.; Lavorgna, M.; Russo, C.; Isidori, M.; Russo, L.; Malgieri, G.; Isernia, C. β-Cyclodextrin inclusion complex to improve physicochemical properties of pipemidic acid: Characterization and bioactivity evaluation. Int. J. Mol. Sci. 2013, 14, 13022–13041. [Google Scholar]
- Giordano, F.; Novak, C.; Moyano, J.R. Thermal analysis of cyclodextrins and their inclusion compounds. Thermochim. Acta 2001, 380, 123–151. [Google Scholar]
- Hak-Kim, C.; Gonda, I. Methotrexate: Existence of different types of solid. Int. J. Pharm. 1991, 68, 179–190. [Google Scholar]
- Hambley, T.; Chan, H.; Gonda, I. Crystal and molecular structure of methotrexate. J. Am. Chem. Soc. 1986, 108, 2103–2105. [Google Scholar]
- Sutton, P.A.; Cody, V.; Smith, G.D. Crystal structure of methotrexate tetrahydrate. J. Am. Chem. Soc. 1986, 108, 4155–4158. [Google Scholar]
- Oliveira, A.R.; Molina, E.F.; Castro, P.M.; Fonseca, J.L.C.; Rossanezi, G.; Freitas, M.F.P.; Oliveira, A.G.; Silva-Júnior, A.A. Structural and thermal properties of spray-dried methotrexate-loaded biodegradable microparticles. J. Therm. Anal. Calorim. 2013, 112, 555–565. [Google Scholar]
- Li, N.; Zhang, Y.H.; Wu, Y.N.; Xiong, X.L.; Zhang, Y.H. Inclusion complex of trimethoprim with β-cyclodextrin. J. Pharm. Biomed. Anal. 2005, 39, 824–829. [Google Scholar]
- Ribeiro, L.; Loftsson, T.; Ferreira, D.; Veiga, F. Investigation and physicochemical characterizationof vinpocetine–sulfobutyl ether & β-cyclodextrin binary and ternary complexes. Chem. Pharm. Bull. 2003, 51, 914–922. [Google Scholar]
- Okumura, H.; Kawaguchi, Y.; Harada, A. Preparation and characterization of inclusion complexes of poly (dimethylsiloxane) s with cyclodextrins. Macromolecules 2001, 34, 6338–6343. [Google Scholar]
- Giron, D. Thermal analysis and calorimetric methods in the characterisation of polymorphs and solvates. Thermochim. Acta 1995, 248, 1–59. [Google Scholar]
- Figueiras, A.; Sarraguça, J.M.G.; Carvalho, R.; Pais, A.A.C.C.; Veiga, F.B. Interaction of omeprazole with a methylated derivative of β-cyclodextrin: Phase solubility, NMR spectroscopy and molecular simulation. Pharm. Res. 2007, 24, 377–389. [Google Scholar]
- Ikuta, N.; Sugiyama, H.; Shimosegawa, H.; Nakane, R.; Ishida, Y.; Uekaji, Y.; Nakata, D.; Pallauf, K.; Rimbach, G.; Terao, K.; et al. Analysis of the enhanced Sstability of R(+)-α lipoic acid by the complex formation with cyclodextrins. Int. J. Mol. Sci. 2013, 14, 3639–3655. [Google Scholar]
- Zhang, X.; Zhang, Y.; Zhong, D.; Chen, Y.; Lis, S. Investigation and physicochemical characterization of claritromycin–citric acid–cyclodextrins ternary complex. Drug Dev. Ind. Pharm. 2007, 33, 8. [Google Scholar]
- Allouche, A.R. Gabedita—A graphical user interface for computational chemistry softwares. J. Comput. Chem. 2011, 32, 174–182. [Google Scholar]
- OpenEye.Scientific.Software. Available online: http://www.eyesopen.com/ (accessed in May 2013).
- McGann, M. FRED and HYBRID docking performance on standardized datasets. J. Comput. Aided Mol. Des. 2012, 26, 897–906. [Google Scholar]
- McGann, M. FRED pose prediction and virtual screening accuracy. J. Chem. Inf. Model. 2011, 51, 18. [Google Scholar]
- VIDA.4.2.1. OpenEye Scientific Software, Santa Fe, NM. Available online: http://www.eyesopen.com (accessed on May 2013).
- Case, D.A.; Cheatham, T.E.; Darden, T.; Gohlke, H.; Luo, R.; Merz, K.M.; Onufriev, A.; Simmerling, C.; Wang, B.; Woods, R.J. The Amber biomolecular simulation programs. J. Comput. Chem. 2005, 26, 1668–1688. [Google Scholar]
- Wang, J.; Wolf, R.M.; Caldwell, J.W.; Kollman, P.A.; Case, D.A. Development and testing of a general amber force field. J. Comput. Chem. 2004, 25, 1157–1174. [Google Scholar]
- Kirschner, K.N.; Yongye, A.B.; Tschampel, S.M.; González-Outeiriño, J.; Daniels, C.R.; Foley, B.L.; Woods, R.J. GLYCAM06: A generalizable biomolecular force field. Carbohydr. J. Comput. Chem. 2008, 29, 622–655. [Google Scholar]
- Kuhn, B.; Gerber, P.; Schulz-Gasch, T.; Stahl, M. Validation and use of the MM-PBSA approach for drug discovery. J. Med. Chem. 2005, 48, 8. [Google Scholar]
- Humphrey, W.; Dalke, A.; Schulten, K. VMD: Visual molecular dynamics. J. Mol. Graph. 1996, 14, 5. [Google Scholar]
- GPGPU Computing Group. Available online: http://www.famaf.unc.edu.ar/grupos/GPGPU/ (accessed on May 2013).
- Khan, K.A.; Rhodes, C.T. Effect of compaction pressure on the dissolution efficiency of some direct compression systems. Pharm. Acta Helv. 1972, 47, 14. [Google Scholar]
© 2014 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Barbosa, J.A.A.; Zoppi, A.; Quevedo, M.A.; De Melo, P.N.; De Medeiros, A.S.A.; Streck, L.; De Oliveira, A.R.; Fernandes-Pedrosa, M.F.; Longhi, M.R.; Da Silva-Júnior, A.A. Triethanolamine Stabilization of Methotrexate-β-Cyclodextrin Interactions in Ternary Complexes. Int. J. Mol. Sci. 2014, 15, 17077-17099. https://doi.org/10.3390/ijms150917077
Barbosa JAA, Zoppi A, Quevedo MA, De Melo PN, De Medeiros ASA, Streck L, De Oliveira AR, Fernandes-Pedrosa MF, Longhi MR, Da Silva-Júnior AA. Triethanolamine Stabilization of Methotrexate-β-Cyclodextrin Interactions in Ternary Complexes. International Journal of Molecular Sciences. 2014; 15(9):17077-17099. https://doi.org/10.3390/ijms150917077
Chicago/Turabian StyleBarbosa, Jahamunna A. A., Ariana Zoppi, Mario A. Quevedo, Polyanne N. De Melo, Arthur S. A. De Medeiros, Letícia Streck, Alice R. De Oliveira, Matheus F. Fernandes-Pedrosa, Marcela R. Longhi, and Arnóbio A. Da Silva-Júnior. 2014. "Triethanolamine Stabilization of Methotrexate-β-Cyclodextrin Interactions in Ternary Complexes" International Journal of Molecular Sciences 15, no. 9: 17077-17099. https://doi.org/10.3390/ijms150917077
APA StyleBarbosa, J. A. A., Zoppi, A., Quevedo, M. A., De Melo, P. N., De Medeiros, A. S. A., Streck, L., De Oliveira, A. R., Fernandes-Pedrosa, M. F., Longhi, M. R., & Da Silva-Júnior, A. A. (2014). Triethanolamine Stabilization of Methotrexate-β-Cyclodextrin Interactions in Ternary Complexes. International Journal of Molecular Sciences, 15(9), 17077-17099. https://doi.org/10.3390/ijms150917077




