Abstract
Oxidative stress is recognized as one of the primary processes underlying the initiation and progression of atherosclerotic vascular disease. Under physiological conditions, the balance between reactive oxygen species (ROS) generation and ROS scavenging is tightly controlled. As part of normal cellular metabolism, regulated oxidative stress is responsible for a variety of cellular responses. Excess generation of ROS that could not be compensated by antioxidant system has been suggested to be responsible for a number of pathological conditions. Due to their short biological half-lives, direct measurement of ROS is not available and surrogate measures are commonly used. Plasma lipoproteins, by virtue of their close interactions with endothelial cells in the vasculature and the susceptibility of their surface lipids to oxidative modification, are perfect biological sensors of oxidative stress in the arterial wall. In particular, with each consumed meal, triglyceride-rich lipoproteins, secreted by the intestine into the circulation, are responsible for the delivery of 20–40 grams of fat to the peripheral tissues. This flux of dietary lipids is accompanied by concomitant increases in glucose, insulin and other meal-associated metabolites. The contribution of postprandial lipemia to the pathogenesis of atherosclerosis has been previously suggested by several lines of investigation. We have extended this hypothesis by demonstrating the acute generation of oxidative epitopes on plasma lipoproteins as well as transient changes in the oxidative susceptibility of plasma lipoproteins.
1. Oxidative Stress and Reactive Oxygen Species (ROS)
Reactive oxygen species (ROS) are produced from molecular oxygen as a result of normal cellular function and can be classified as either free radicals or nonradicals. Some of the common free radicals are superoxide anion (O2−·), hydroxyl radical (·OH), and hydrogen peroxide (H2O2) as a nonradical ROS. Superoxide anions could be generated in vivo by a number of processes involving molecules such as (NAD(P)H) oxidase, xanthine oxidase, or by the electron transport system of mitochondria. Superoxide anions can also be converted to hydrogen peroxide (an example of a nonradical species) by superoxide dismutase (SOD). The hydroxyl radical is the most reactive form of ROS and can initiate lipid peroxidation by attacking polyunsaturated fatty acids (PUFA). Other oxygen-derived free radicals such as peroxyl radicals (ROO··), in particular hydroxyperoxyl radical (HOO··), could also contribute to fatty acid peroxidation.
Heavy metal ions can also interact with superoxide anions to form hydrogen peroxide (Haber–Weiss reaction), which can subsequently be converted to OH radicals (Fenton reaction). In the presence of polyunsaturated fatty acids, a lipid radical could be formed (seeding process). The lipid radical can subsequently react with oxygen to produce peroxyl radicals, which can initiate a chain reaction resulting in the formation of lipid hydroperoxides. If these radicals are not quenched by antioxidants, this process will propagate until all of the PUFA molecules are modified. Lipid hydroperoxides, being very unstable, are rapidly converted to secondary products such as malondialdehydes (MDA) and 4-hydroxy-2,3-nonenals (HNE).
While most of the enzymes responsible for the generation of ROS are intracellular [1], excess ROS can find its way into the extracellular space and affect plasma metabolites. Most of these oxidizing metabolites have extremely short biological half-lives and are difficult to detect in vivo. Plasma levels of secondary end-products, such as isoprostanes, carbonyls, MDA and HNE-modified compounds have been used as surrogate indicators of oxidative stress. In the following sections we will present evidence suggesting that plasma lipoproteins may not only serve as sensitive physiologic biosensors of oxidative stress but also contribute to the propagation of oxidative damage throughout the vasculature [2].
2. Oxidized Low-Density Lipoproteins (LDL) and Atherosclerosis
Atherosclerosis is recognized as an inflammatory process characterized by the accumulation of cholesterol in macrophages trapped in the sub-endothelium and extracellular matrix [3,4]. While epidemiologic and intervention studies have implicated plasma low-density lipoproteins (LDL) as the primary source of cholesterol, in vitro studies with cell cultures would suggest that LDL in its native form could not contribute to this accumulation, and that only chemically modified LDL could lead to the formation of foam cells [5,6,7]. Oxidatively modified LDL is one form of modified LDL that has been demonstrated to be present in vivo [8], specifically in atherosclerotic lesions [9,10], and has been implicated in the pathogenesis of atherosclerotic diseases [11,12,13,14]. These observations and other data from animal and human studies are summarized in the oxidation hypothesis of atherosclerosis [15,16,17,18]. According to this hypothesis, native LDL does not accumulate in monocyte-derived macrophages, independent of concentration. LDL has to be modified before it can be taken up via scavenger receptors and initiate the lipid accumulation process. Delayed clearance of LDL in individuals with hypercholesterolemia increases the propensity for circulating LDL to be modified and thus contribute to the initiation and progression of atherosclerosis [17]. Indeed individuals with risk factors for cardiovascular disease tend to have higher levels of oxidized LDL (oxLDL) [19] and high concentrations of oxLDL predict future cardiovascular events [20]. Under this hypothesis, the presence of a pro-inflammatory state serves as fuel to accelerate the oxidative modification of LDL [4,21,22,23,24].
Alternatives to the oxidation hypothesis of atherosclerosis include the response-to-injury hypothesis which suggests that the initial step in atherogenesis is endothelial denudation resulting in compensatory responses that alter normal vascular function [25]. The response-to-retention hypothesis is based on the observation that, due its size, LDL particles can be delivered to the subendothelium by transcytosis, retained within the arterial wall, forming microaggregates that are subsequently taken up by macrophages and smooth muscle cells [26,27].
While the impact of oxLDL in atherogenesis is well recognized, the exact process for the in vivo modification of plasma LDL remains unclear. Given the endogenous antioxidant defenses present in human blood [28], it has been suggested that plasma LDL must be trapped in some microenvironment with excess oxidants for the modification to occur. According to the oxidation hypothesis, LDL, being smaller in size, is trapped in the subendothelium where it could be modified by resident macrophages. Once modified, oxLDL can play a central role in progression of atherosclerosis by (i) Promoting the recruitment of circulating monocytes into the intimal space via monocyte chemotactic protein-1, MCP-1 [29]; (ii) Inhibiting the movement of resident macrophages to leave the intima [30]; (iii) Accelerating the rate of uptake and accumulation of lipoproteins resulting in the formation of foam cells [5,31,32,33,34]; and (iv) Being cytotoxic to endothelial cells leading to loss of endothelial integrity [32,33,34].
3. Biomarkers of Oxidative Modification
Oxidative stress occurs when cellular antioxidant defense processes are inadequate to inactivate reactive oxygen species (ROS) [35] and reactive nitrogen species (RNS) [36], either because of excess production of ROS/RNS or a dysfunctional antioxidant system. Direct detection of ROS/RNS remains a challenge due to the instability of these reactive species and the need of for special equipment to detect ROS/RNS by electron spin resonance [37]. Surrogate markers of oxidation include stable metabolites (e.g., nitrate or nitrite) and oxidation target products such as lipid peroxidation end products [38,39] and oxidized proteins [40,41].
Exposure of PUFA to ROS or RNS may lead to lipid peroxidation. Lipid peroxidation, in turn, can result in changes in membrane properties that might affect fluidity and activity of membrane-bound receptors as well as generation of secondary oxidized products that are chemically reactive. Lipid peroxidation generates a number of relatively stable endproducts such as reactive aldehydes, e.g., malondialdehyde (MDA) 4-hydroxy-2-nonenal (HNE) and 2-propenal (acrolein) [42] and isoprostanes [38,43].
MDA is produced by peroxidative decomposition of polyunsaturated lipids and reacts mainly with lysine residues of plasma proteins. MDA-modified proteins are characteristically immunogenic and autoantibodies against MDA-modified proteins have been detected in vivo [44]. Increased levels of MDA have been reported in plasma and atherosclerotic plaques of patients with type 2 diabetes mellitus (T2DM) [45]. Adducts of apoB-100 lysine residues with MDA and HNE have been characterized in human atherosclerotic lesions [46]. Apolipoprotein B-100 (apoB-100), constitutes 95% of the protein component in LDLHNE is generated from free radical modification of ω6 polyunsaturated fatty acids and is a toxic second messenger of oxygen free radicals [42]. It can undergo reactions with proteins, peptides, phospholipids, and nucleic acids resulting in changes in a wide range of biological activities [47]. Acrolein is present in environmental sources, specifically cigarette smoke. Acrolein reacts with lysine residues of apolipoprotein A-I (apoA-I), the primary structural apolipoprotein of plasma high-density lipoproteins (HDL) resulting in impairment of the function of HDL in cholesterol efflux and acrolein-modified apoA-I has been localized in human atherosclerotic plaques [48].
F2-Isoprostanes (F2-IsoP) are a family of prostaglandin-like compounds generated in vivo by free radical-catalyzed non-enzymatic peroxidation of esterified arachidonic acid, released into the circulation by phospholipases and subsequently excreted in the urine [38,43]. One form of F2-IsoPs, 8-iso-PGF2a, is found in plasma primarily esterified to lipids with only a minor component as free acids. F2-IsoPs are chemically stable in vivo but are rapidly metabolized and excreted as free acids in the urine.
Just as with lipid peroxidation, a number of modifications could occur. Under conditions of moderate oxidative stress, oxidation of cysteine residues lead to the formation of mixed disulfides between protein thiol groups and GSH, a process referred to as S-glutathionylation. GSSG:GSH ratio (glutathione disulfide versus reduced glutathione) in blood has been used as an indicator of oxidative status in humans [49]. S-glutathionylated proteins have been assessed as potential biomarkers of oxidative/nitrosative stress in human diseases. For instance, glutathionylated hemoglobin has been reported to be increased in patients with diabetes, hyperlipidemia, and in patients on hemodialysis or peritoneal dialysis [50].
Tyrosine moieties on proteins can be modified resulting in the formation of 3-nitrotyrosine (NO2-Tyr), 3-chlorotyrosine (Cl-Tyr), or 3-bromotyrosine [51,52]. High levels of HDL apoA-I modified with NO2-Tyr and Cl-Tyr have been reported [53] as isolated from whole plasma or from atherosclerotic plaques [54,55].
4. Oxidative Susceptibility of Plasma Lipoproteins
Once the concept that (1) oxLDL does play a critical role in the initiation and progression of atherosclerosis and (2) LDL must be retained in an oxidant-rich microenvironment for oxidative modification to occur, is accepted, the actual process of lipoprotein oxidation remains to be understood. Several forms of LDL oxidation have been characterized in animal models [56,57] as well as in human subjects [9,10,14,29].
The continuous monitoring of the formation of lipid hydroperoxides on isolated LDL in the presence of Cu2+, as detected at 234 nm, (Figure 1A) has proven to be a useful assay for oxidative susceptibility [58]. For this assay LDL is isolated from freshly collected plasma either by density ultracentrifugation (d: 1.020–1.063 g/mL) or by a combination of ultracentrifugation and column chromatography [59]. When controlled for the ratio of LDL-cholesterol and Cu2+, plasma LDL isolated from different individuals could have a wide range of lag times (Figure 1B), with longer lag time being indicative of reduced oxidative susceptibility. The lag time for LDL depends on the ratio of Cu2+ to LDL-cholesterol (LDL-C) and temperature of the incubation. Shorter lag time would be obtained with either higher concentrations of Cu2+ or higher temperature. In our laboratory, lag times were most reproducible at room temperature and 9 mmol of Cu2+ with 45 mg of LDL-C [59].
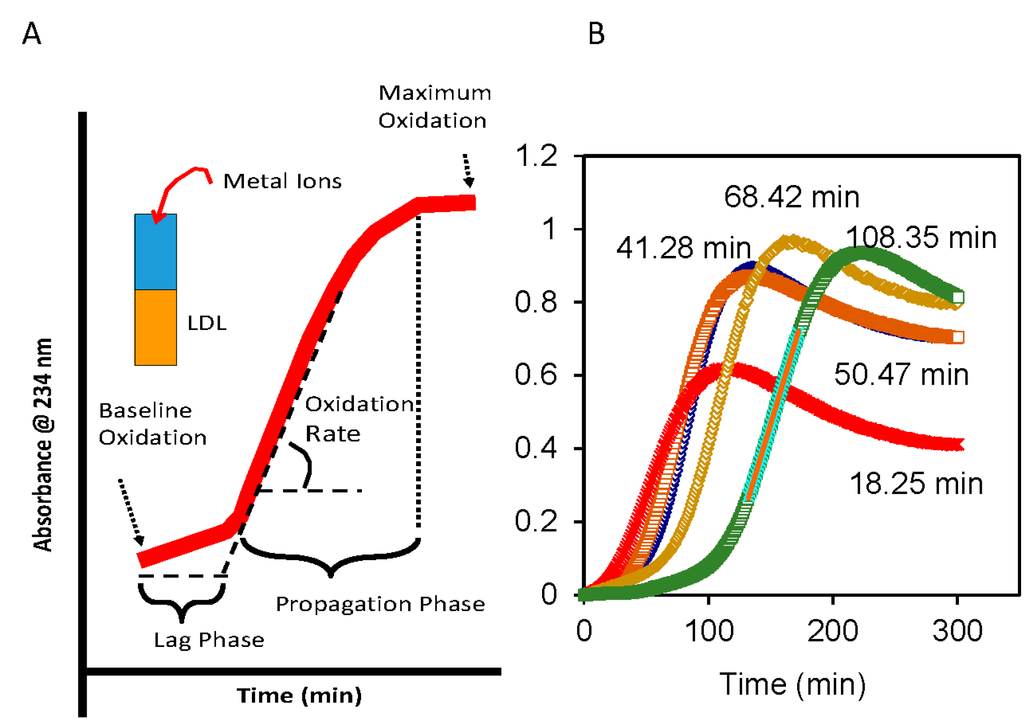
Figure 1.
Oxidative susceptibility of plasma Low-Density Lipoproteins (LDL) in the presence of Cu2+ as catalyst. (A) Kinetics of conjugated dienes formation as assessed by absorbance (234 nm) include three phases: Lag phase, propagation phase and degradation phase. Degradation phase is characterized by loss of signal after the maximum oxidation is reached; and (B) Under comparable incubation conditions, the lag phases for LDL isolated from fasting plasma of different individuals can vary widely from 18 to 108 min.
It should be noted that this oxidative process is not limited to plasma LDL but all plasma lipoproteins are susceptible to oxidative modification (Figure 2).
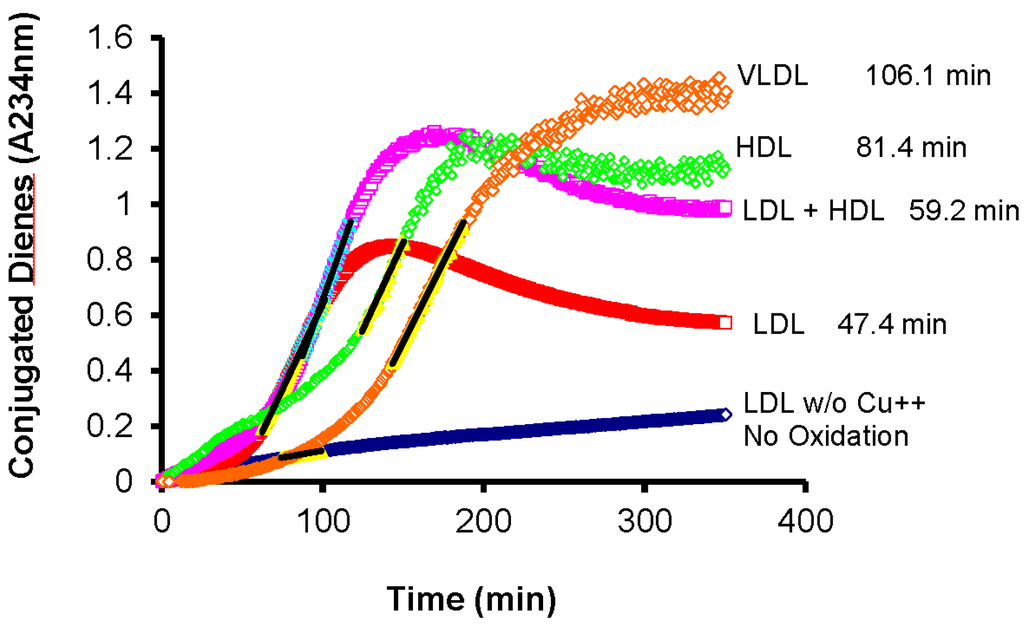
Figure 2.
All lipoproteins isolated from fasting plasma are susceptible to oxidative modification in the presence of Cu2+ as a catalyst. (VLDL: Very-low density lipoproteins; LDL: Low-density lipoproteins; HDL: High-density lipoproteins; See [59] for details on the isolation procedure and incubation conditions).
Several investigators have reported on both the variability in lag times among individuals as well as the fact that all plasma lipoproteins may be subjected to oxidative modification using similar conditions. For instance, McEneny et al. [60], have reported that VLDL isolated from fasting plasma of patients with type 2 diabetes mellitus (T2DM) have significantly shorter lag times, i.e., more susceptible to oxidative modification, than VLDL isolated from healthy control subjects. They also noted that different VLDL subfractions isolated by density ultracentrifugation from the same plasma may also have different lag times, with the smaller particles being more susceptible to oxidative modification [60]. This is in spite of the fact that the subpopulation of larger VLDL tends to have more pre-formed lipid hydroperoxides than the smaller VLDL subpopulation [60]. The simple explanation is that smaller particles have less space for antioxidants, both on the surface and in the core, while larger particles having more lipids would have more lipid hydroperoxides per particle. The degree of oxidizability has also been shown to correlate with the cytotoxicity of the lipoproteins when exposed to endothelial cells [34].
Oxidative susceptibility of plasma LDL as assessed by the lag time of Cu2+-induced formation of conjugated dienes (Figure 1A) has been reported to predict the presence of coronary artery disease (CAD), independent of traditional risk factors [61]. Reduced lag times have been reported in patients with cardiovascular disease (CVD) risk, including patients with ischemic stroke [60,62], T2DM [60], and patients with chronic renal failure undergoing regular hemodialysis [63]. Longer lag time, i.e., resistance to oxidation, was associated with increased β-carotene and vitamin E contents in the LDL particle [64]. Oxidative susceptibility of plasma LDL could be affected by daily activities such as exercise [65] and diet [66]. Diets high in polyunsaturated fatty acid, e.g., linolenic acid (18:2) resulted in lower resistance to oxidation as compared to diets with monounsaturated fatty acid, oleic acid (18:1) [67,68,69].
5. Plasma Lipoproteins and the Arterial Wall: Fasting versus Postprandial
While the focus of cardiovascular risk has been on LDL which accounts for 60% of the cholesterol concentration in plasma, triglycerides transported by the triglyceride-rich lipoproteins (TRL) can contribute significantly to the PUFA flux. For a typical individual with an LDL-C concentration of 130 mg/dL, a plasma volume of 2000 mL, and a fractional clearance rate of 0.4 pool/day, only 1 gram of cholesterol is transported during a 24-h period. For the same individual with a caloric intake of 2000 kcal/day and 30% from fat, the TRL is responsible for the delivery of 55 grams of triglycerides (TG) per 24 h. In addition to this flux of dietary TG carried by the intestinal chylomicrons, there is an additional 8–10 grams of TG/day that is carried by the very-low density lipoproteins (VLDL) secreted by the liver.
In the circulation, TRL becomes attached to the arterial wall via heparan sulfate proteoglycans and remains attached during interactions with lipoprotein lipase (LPL) which is responsible for the hydrolysis of the TG cargo [70,71]. The released free fatty acids (FFA) and monoglycerides can now diffuse through the endothelial cell layer and are re-constituted as TG for storage [72]. Available in vitro data suggests that, as the concentration of FFA in the immediate microenvironment reaches a critical level, LPL action is interrupted, and the partially hydrolyzed TRL is released back into the circulation and moves on to the next site [73,74]. An alternate hypothesis is based on the activation/inhibition of LPL by specific apolipoproteins present on TRL. While apolipoprotein B (apoB) is the primary structural apolipoprotein for TRL, the interactions of these TG-rich lipoproteins with LPL are modulated by two other smaller apolipoproteins, apoC-II and apoC-III [75,76]. It has been suggested that TG hydrolysis will proceed with apoC-II as the required activator. However, with the loss of core TG, apoC-III may redistribute on the surface of the particle and interfere with the action of LPL, causing the particle to be released into the circulation, and thus allowing interactions of partially delipidated TRL with other LPL molecules at downstream sites [77,78,79].
It is our hypothesis that during the interactions of TRL with LPL that is anchored on the arterial wall, plasma lipoproteins could be seeded with ROS generated in the subendothelium. This transfer of oxidative epitopes onto plasma lipoproteins would account for the acute and transient reduction in circulating autoantibodies against oxidatively modified LDL observed during postprandial lipemia in patients with diseased endothelium [80]. In normolipidemic controls without excess ROS, there would be negligible transfer of oxidative epitopes onto plasma lipoproteins, and reduction in circulating autoantibodies against oxLDL would not be observed. [80]. This meal-induced response is also specific for PUFA which is highly susceptible to oxidative modification and could not be demonstrated when test meals containing other fatty acids are used [81].
A corollary to this hypothesis addresses the oxidative susceptibility of the lipoproteins in the circulation. In the current scheme (Figure 3), once the lipoprotein particle is seeded with ROS it can follow a number of possible metabolic pathways [82].
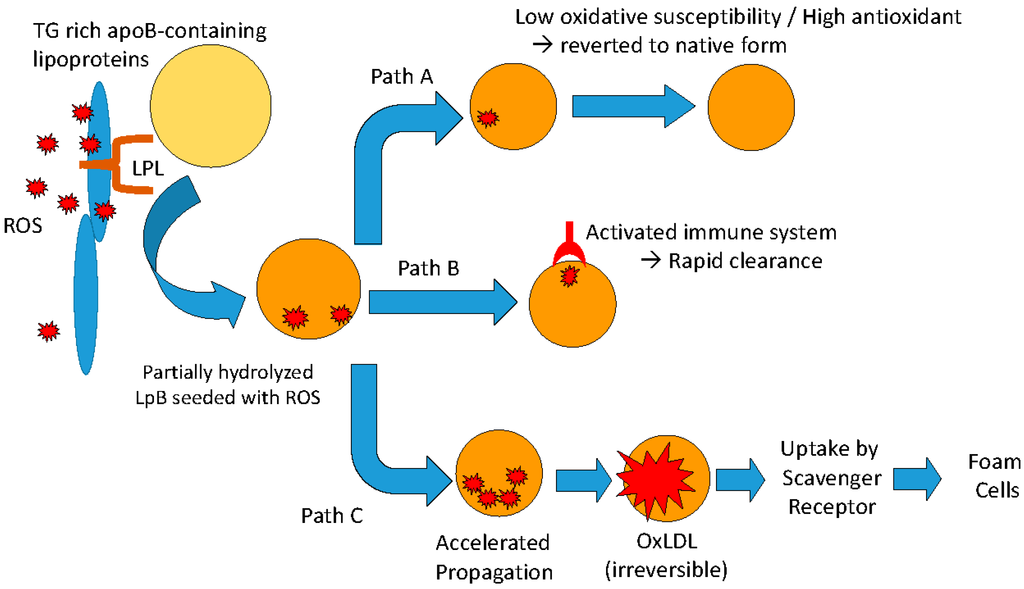
Figure 3.
Possible metabolic fates of apoB-containing lipoproteins that have been seeded with reactive oxygen species (ROS) following interactions with lipoprotein lipase anchored along the arterial wall.
Path A: In the normal individual, ROS newly transferred onto the plasma lipoprotein particle is rapidly quenched by antioxidants present on the particle and the lipoprotein particle is restored to its native state. One would expect that a lipoprotein particle that is resistant to oxidative modification, i.e., longer lag time (low oxidative susceptibility), would be more likely to be restored to its native state.
Path B: The lipoprotein particle seeded with ROS may be recognized by autoantibodies specific of oxidized epitopes and is rapidly cleared from the circulation. This would be analogous to the case of Watanabe Heritable HyperLipidemia (WHHL) which has been reported to have reduced atherosclerotic lesions after being immunized with malondialdehyde-modified LDL, in spite of elevated LDL cholesterol levels [83]. Another example of anti-atherogenic effect of autoantibodies against oxLDL is the marked reduction in neointimal area after balloon injury in cholesterol-fed rabbits despite severe hypercholesterolemia [84].
Path C: In the presence of excess generation of ROS and/or insufficient antioxidant capacity, or both, oxidative modification will proceed to the propagation phase (see Figure 1) resulting in the conversion of all polyunsaturated fatty acids to lipid hydroperoxides and subsequent irreversible modification of the lipoprotein particle, including fragmentation of the apolipoprotein moieties. The damaged lipoprotein particle would be taken up by the scavenger receptor, ultimately transforming monocyte-derived macrophages into foam cells.
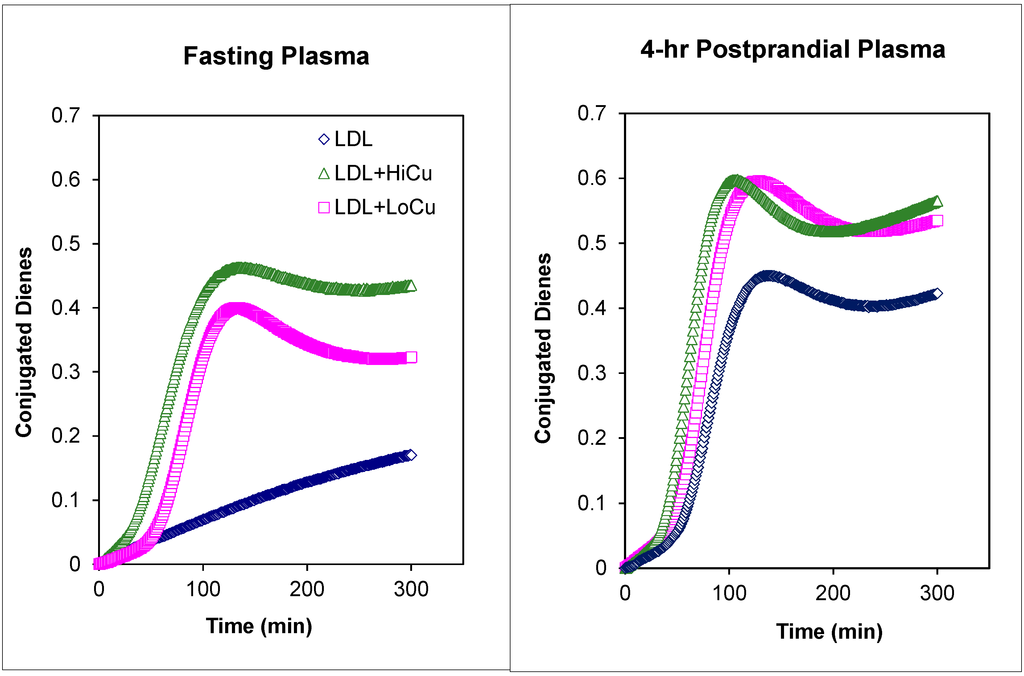
Figure 4.
Meal-induced changes in oxidative susceptibility of LDL isolated from fasting versus LDL isolated from postprandial plasma of a patient with T2DM under dietary management. Blue symbols: LDL in the absence of Cu2+ as catalyst. Green symbols: LDL in the presence of high Cu2+ concentrations (9.0 mmol Cu2+ per 45 mg of LDL-C). Pink symbols: LDL in the presence of low Cu2+ concentrations (4.5 mmol Cu2+ per 45 mg of LDL-C).
Figure 4 illustrates the changes in oxidative susceptibility for LDL isolated from a patient with T2DM under control (normal fasting plasma lipids and glucose, HbA1c of 7.8) with insulin glargine (Lantus®; Sanofi-Aventis, Paris, France) administered subcutaneously once daily at bedtime. Postprandial plasma was obtained at 4-h following the consumption of a standardized commercially available test meal breakfast containing 39 g fat, 78 g carbohydrate, 24 g protein and 750 kcCal (1 bacon, egg, and cheese biscuit + 1 hash brown patty + 8 fl oz (237 mL) of orange juice) [85].
In the absence of Cu2+ (blue symbols), the formation of conjugated dienes in fasting LDL was slow for 300 min without the characteristic propagation phase (see Figure 1A). In contrast, even in the absence of Cu2+ to initiate the oxidation, LDL isolated from postprandial plasma underwent spontaneous oxidative modification with a lag time of 101.4 min. This is suggestive of greater presence of ROS on postprandial plasma as compared to fasting plasma. In the presence of low concentrations of Cu2+ (4.5mmol Cu2+ per 45 mg of LDL-C), the lag times for fasting and postprandial LDL were comparable, 21.1 versus 20.4 min, respectively. These lag times are short when compared to lag times obtained in our laboratory for normolipidemic controls without any CVD risk factors (range: 45–85 min). In the presence of high concentrations of Cu2+ (9.0 mmol Cu2+ per 45 mg of LDL-C), the lag times for both fasting and postprandial plasma were shorter that those obtained with low Cu2+ concentrations. The lag time for fasting LDL was slightly longer than that observed for postprandial plasma, 8.0 versus 5.4 min, respectively. Details for the in vitro oxidative susceptibility assay have been published previously [59].
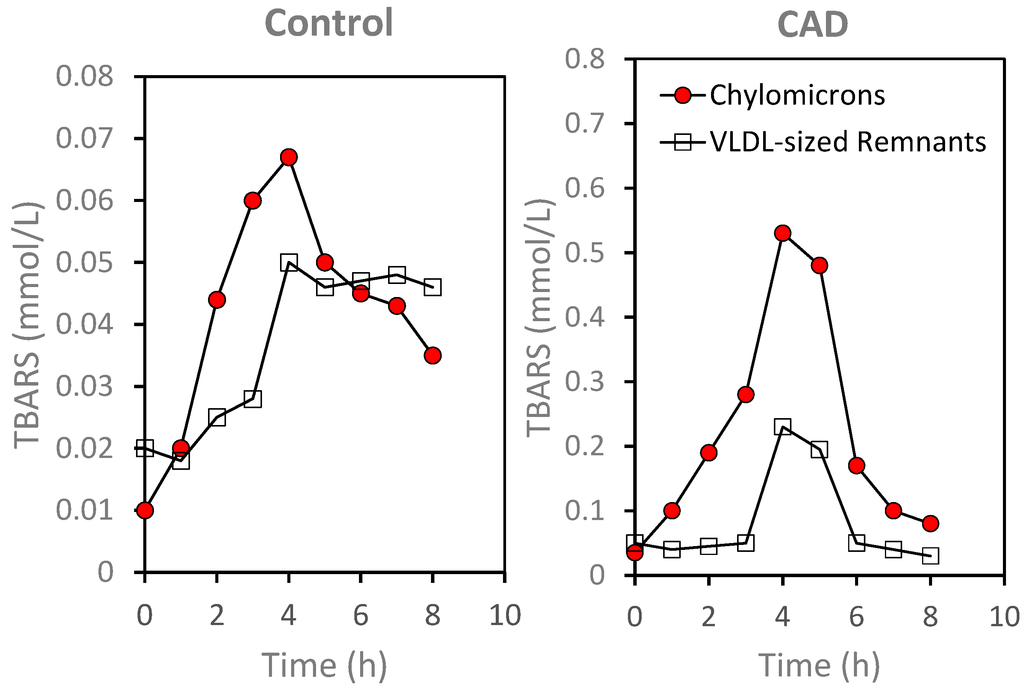
Figure 5.
Time-dependent changes in lipid hydroperoxides as assessed by TBARS in lipoprotein fractions isolated by density ultracentrifugation following meal consumption. Note the differences in scale for the y-axis. Closed circles: Chylomicron fraction. Open squares: VLDL-sized remnants (for details on the isolation methods see [78]).
The increased presence of ROS on plasma lipoproteins following meal consumption was suggested by increases in lipid hydroperoxides, as estimated by thiobarbituric active substances (TBARS), on lipoprotein fractions (Figure 5). Chylomicrons and VLDL-sized remnants were isolated from plasma by sequential density ultracentrifugation as previously described [77] and TBARS were determined by fluorescence spectroscopy using a modified method of Yagi [86,87]. As illustrated in Figure 5, TBARS levels were lower in the control subject as compared to the patient with documented CAD for both chylomicrons, 0.01 versus 0.03 mmol/L, respectively, and for VLDL-sized remnants, 0.02 versus 0.05 mmol/L. Following meal consumption, there were increases in TBARS levels in both chylomicrons and VLDL-sized remnants for control as well as for the CAD patient. Relative to fasting level, there was an approximately 15-fold increase in TBARS level in the chylomicron fraction at 4 h after meal consumption in the CAD subject, as compared to only a five-fold increase in the control subject. When compared to fasting levels, TBARS levels in the VLDL-sized remnants at 4 h after the test meal was 4.6-fold higher for the CAD subject and 2.5-fold higher for the control subject. While the difference in meal-induced changes in TBARS on isolated plasma lipoproteins need to be confirmed, similar changes were observed in a small group of four CAD patients and three normolipidemic controls.
6. Oxidative Stress: Protective Response Leading to Disease
Among the recommendations that have been stressed as part of a healthy lifestyle are physical activity and dietary regimens enriched in polyunsaturated fatty acids. Both of these, however, are associated with significant generation of ROS. It has been suggested that although excess production of ROS may lead to oxidative stress causing damage to lipids, proteins and nucleic acids, at moderate levels ROS play an important role as regulatory mediators in signaling processes.
Generation of ROS and reactive nitrogen species (RNS) by skeletal muscle has been well documented during both the resting state and contractile activity when production is increased [88,89,90]. Many of the ROS-mediated responses actually protect the cells against oxidative stress and re-establish redox homeostasis [91]. For instance, exercise conditioning has been reported to induce ROS production which affects transcription factors such as nuclear factor kappa B (NF-κB), activator protein 1 (AP1) [92,93], and mitogen-activated protein kinases (MAPKs) [94] with the net result being a reduction in oxidative stress [95]. Without proper conditioning, physical activity that requires high-power output levels, such as sprint exercise, have been reported to cause oxidative stress leading to muscle damage [96].
With respect to dietary polyunsaturated fatty acids (PUFA), the data reviewed here would support an analogous process. Moderate consumption of polyunsaturated fatty acids with each meal would allow seeding of plasma TRL with ROS which could induce an immune response by facilitating the production of antibodies specific against oxidized epitopes on plasma lipoproteins. In other words, with each meal, we are immunizing ourselves against oxidized epitopes and thus the beneficial effects of polyunsaturated fatty acids [66,68,69,84]. A review of the literature on the use of oxidative epitopes to develop a vaccine against atherosclerosis is available [97]. In the present schema, we are proposing that autoimmunization would occur in vivo by providing intermittent flux of oxidizable PUFA as part of the diet [68,69]. Under normal circumstances, we would expect low concentrations of oxLDL to be bound to circulating antibodies and removed via the Kupffer cells and rapidly removed. Thus, a priori, we would expect high titers of autoantibodies against oxidatively modified LDL to be associated with reduced atherosclerotic risk. This is not demonstrated, however, in a number of case-control studies which have reported higher autoantibody titers in patients with documented CAD [98,99]. It is possible that, in patients with existing atherosclerotic disease, the presence of monocyte-derived macrophages in the arterial wall with up-regulated scavenger receptors would actually shift the balance to accumulation oxLDL and LDL-IgG complexes and subsequent transformation into foam cells. In this instance, consumption of highly oxidizable PUFA may not be beneficial unless accompanied by significant reductions in inflammatory/oxidative status.
7. Discussion
In summary, in spite of the well-documented efficacy of lipid-lowering statins and significant reductions in cardiovascular events, atherosclerotic progression remains a problem in some individuals [100,101]. For instance, in the landmark clinical trial CARE, Cholesterol And Recurrent Events with a significant 24% reduction in risk, the frequency of the primary endpoint was 10.2% in the treated group as compared to 13.2% in the placebo group [102].
There is an increasing interest in understanding other processes that might contribute to this progression, the so-called residual risks. It has been suggested that inflammation, as assessed by hsCRP and lipoprotein-associated phospholipase A2, may be contributory factors [103,104,105]. In a survey of 30 novel biomarkers, Blankenberg and colleagues have identified hsCRP as a key player [106]. The clinical significance of these markers of inflammation remains, however, unclear [107].
Plasma levels of oxidized lipoproteins, in particular oxLDL, have also been suggested to be an independent contributing factor [82,108]. A number of prospective studies have suggested a potential role of oxidized lipoproteins, though the exact measure of oxidation is not well understood [82,108]. While progression study in nonhuman primates does suggest increases in the plasma levels of oxidized phospholipids (oxPL), the contents of oxPL in the atherogenic apoB-containing lipoproteins was actually decreased by 31% [109]. In the same report, reduction of total cholesterol from 418 to 64 mg/dL with concomitant reduction of apoB from 115 to 18 mg/dL as part of the regression study resulted in a 47% increase in oxPL contents in apoB-containing lipoproteins [109]. In a small group of hypercholesterolemic patients, reductions in total cholesterol (19%–32%) with concomitant reduction in apoB (15%–31%) were accompanied by reductions in oxLDL (23%–35%) using one assay and by increases in oxPL contents of apoB-containing lipoproteins (20%–25%) using a different assay [110]. In the REVERSAL trial (Reversal of Atherosclerosis with Aggressive Lipid Lowering) in human subjects, in spite of 39% reductions in apoB with atorvastatin and lack of disease progression as assessed by percentage changes in atheroma volume using intravascular ultrasound [111], there was significant increase in oxPL contents in apoB-containing lipoproteins (48%), and malondialdehyde contents in apoB-containing lipoproteins (21%) [112]. Neither fasting levels of antibodies against MDA-modified LDL nor fasting levels of immune complexes were predictive of the observed changes in lipids and atheroma volume [112].
The present review presents data in support of a functional assay of oxidative susceptibility of plasma LDL both in the fasting state and in response to a physiological bout of oxidative stress as potential risk factor contributing to the progression of atherosclerotic disease. We have shown that LDL oxidative susceptibility is increased during postprandial lipemia in patients with metabolic syndrome [59], a finding consistent with an earlier report by Anderson and colleagues [113]. They also noted meal-induced increases in TBARS levels [113] which have been linked to coronary heart disease risk [114] as well as future cardiovascular events in patients with stable coronary artery disease [115]. Together with the transient increases in TBARS during postprandial lipemia (Figure 5), the increase in oxidative susceptibility of plasma lipoproteins during postprandial lipemia (Figure 4) may account for increased production of oxidatively modified lipoproteins. While we have not presented data demonstrating that this meal-induced increase in oxidative susceptibility of plasma LDL is transient, we have reported that the meal-induced generation of oxidatively modified epitopes is a transient process with fasting baseline levels being observed by 6–8 h after meal consumption [80,81]. A possible explanation is that LDL particles with higher TBARS contents are more susceptible to oxidative modification and would be more likely to become oxidatively modified and thus contribute to the disease process to a greater extent. Direct data linking changes in oxidative susceptibility of plasma lipoproteins and cardiovascular disease risk would be needed to support this concept.
Acknowledgments
This work was supported in part by an investigator-initiated grant to Ngoc-Anh Le from Abbott Laboratories and by development funds available to the Biomarker Core Laboratory. Appreciation is extended to Monica Farkas-Epperson and Jeanne Dow for their assistance in different phases of this project.
Conflicts of Interest
Ngoc-Anh Le is supported in part by investigator-initiated grants from Merck, AstraZeneca. Ngoc-Anh Le is a consultant for Liposcience. The original project was supported by an investigator-initiated grant from Abbott Laboratories and the results have been previously published. Abbott Laboratories had no role in the design of the study; in the collection, analysis, or interpretation of the data; in the writing of the manuscript, and in the decision to publish the results.
References
- Zorov, D.B.; Juhaszova, M.; Sollott, S.J. Mitochondrial reactive oxygen species (ROS) and ROS-induced ROS release. Physiol. Rev. 2014, 94, 909–950. [Google Scholar] [CrossRef] [PubMed]
- Farkas-Epperson, M.; Le, N.-A. Lipoproteins as biosensors of endothelial oxidative stress. Clin. Lipidol. 2012, 7, 49–63. [Google Scholar] [CrossRef]
- Libby, P.; Ridker, P.M.; Maseri, A. Inflammation and atherosclerosis. Circulation 2002, 105, 1135–1143. [Google Scholar] [CrossRef] [PubMed]
- Van Oostrom, A.J.H.H.M.; van Wijk, J.P.H.; Castro-Cabezas, M. Lipemia, inflammation and atherosclerosis: Novel opportunities in the understanding and treatment of atherosclerosis. Drugs 2004, 64, 19–24. [Google Scholar]
- Goldstein, J.L.; Ho, Y.K.; Basu, S.K.; Brown, M.S. Binding sites on macrophages that mediates uptake and degradation of acetylated LDL, producing massive cholesterol deposition. Proc. Natl. Acad. Sci. USA 1979, 76, 333–337. [Google Scholar] [CrossRef] [PubMed]
- Fogelman, A.M.; Schechter, I.; Seager, J.; Hokom, M.; Child, J.S.; Edwards, P.A. Malondialdehyde alteration of low density lipoproteins leads to cholesteryl ester accumulation in human monocyte-macrophages. Proc. Natl. Acad. Sci. USA 1980, 77, 2214–2218. [Google Scholar] [CrossRef] [PubMed]
- Navab, M.; Hama, S.H.; Ready, S.T.; Ng, C.J.; van Lenten, B.J.; Laks, H.; Fogelman, A.M. Oxidized lipids as mediators of coronary heart disease. Curr. Opin. Lipidol. 2002, 13, 363–372. [Google Scholar] [CrossRef] [PubMed]
- Palinski, W.; Rosenfeld, M.E.; Yla-Herttuala, S.; Gurtner, G.C.; Socher, S.S.; Butler, S.W.; Parthasarathy, S.; Carew, T.E.; Steinberg, D.; Witztum, J.L. LDL undergoes oxidative modification in vivo. Proc. Natl. Acad. Sci. USA 1989, 86, 1372–1376. [Google Scholar] [CrossRef] [PubMed]
- Avogaro, P.; Bon, G.B.; Cazzolato, G. Presence of a modified LDL in humans. Arteriosclerosis 1988, 8, 79–87. [Google Scholar] [CrossRef] [PubMed]
- Yla-Herttuala, S.; Palinski, W.; Rosenfeld, M.E.; Parthasarathy, S.; Carew, T.E.; Butler, S.; Witztum, J.L.; Steinberg, D. Evidence for the presence of oxidatively modified low density lipoprotein in atherosclerotic lesions of rabbit and man. J. Clin. Investig. 1989, 84, 1086–1095. [Google Scholar] [CrossRef] [PubMed]
- Holvoet, P.; Vanhaecke, J.; Janssens, S.; van de Werf, F.; Collen, D. Oxidized LDL and malondialdehyde-modified LDL in patients with acute coronary syndromes and stable coronary artery disease. Circulation 1998, 98, 1487–1494. [Google Scholar] [CrossRef] [PubMed]
- Hulthe, J.; Fagerberg, B. Circulating oxidized LDL is associated with subclinical atherosclerosis development and inflammatory cytokines (AIR study). Arterioscler. Thromb. Vasc. Biol. 2002, 22, 1162–1167. [Google Scholar] [CrossRef] [PubMed]
- Ishigaki, Y.; Oka, Y.; Katagiri, H. Circulating oxidized LDL: A biomarker and a pathogenic factor. Curr. Opin. Lipidol. 2009, 20, 363–369. [Google Scholar] [CrossRef] [PubMed]
- Itabe, H. Oxidized low-density lipoproteins: What is understood and what remains to be clarified. Biol. Pharm. Bull. 2003, 26, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Steinberg, D.; Parthasarathy, S.; Carew, T.E.; Khoo, J.C.; Witztum, J.L. Beyond cholesterol: Modifications of low-density lipoprotein that increase its atherogenecity. N. Engl. J. Med. 1989, 320, 915–924. [Google Scholar] [CrossRef] [PubMed]
- Witztum, J.L.; Steinberg, D. Role of oxidized low density lipoprotein in atherogenesis. J. Clin. Investig. 1991, 88, 1785–1792. [Google Scholar] [CrossRef] [PubMed]
- Steinberg, D.; Witztum, J.L. Oxidized low-density lipoprotein and atherosclerosis. Arterioscler. Thromb. Vasc. Biol. 2010, 30, 2311–2316. [Google Scholar] [CrossRef] [PubMed]
- Chisolm, G.M.; Steinburg, D. The oxidative modification hypothesis of atherogenesis: An overview. Free Radic. Biol. Med. 2000, 28, 1815–1826. [Google Scholar] [CrossRef] [PubMed]
- Sigurdardottir, V.; Fagerberg, B.; Hulthe, J. Circulating oxidized low-density lipoprotein (LDL) is associated with risk factors of the metabolic syndrome and LDL size in clinically healthy 58-year-old men (AIR study). J. Intern. Med. 2002, 252, 440–447. [Google Scholar] [CrossRef] [PubMed]
- Shimada, K.; Mokuno, H.; Matsunaga, E.; Miyazaki, T.; Sumiyoshi, K.; Miyauchi, K.; Daida, H. Circulating oxidized LDL is an independent predictor for cardiac event in patients with coronary artery disease. Atherosclerosis 2004, 174, 343–347. [Google Scholar] [CrossRef] [PubMed]
- Memon, R.; Staprans, I.; Noor, M. Infection and inflammation induce LDL oxidation in vivo. Arterioscler. Thromb. Vasc. Biol. 2000, 20, 1536–1540. [Google Scholar] [CrossRef] [PubMed]
- Pearson, T.A.; Mensah, G.A.; Alexander, R.W.; Anderson, J.L.; Canno, R.O., III; Criqui, M.; Fadl, Y.Y.; Fortmann, S.P.; Hong, Y.; Myer, G.L.; et al. Markers of inflammation and cardiovascular disease: Application to clinical and public health practice: A statement for healthcare professionals from the centers for disease control and prevention and the american heart association. Circulation 2003, 107, 499–511. [Google Scholar] [CrossRef] [PubMed]
- Szmitko, P.E.; Wang, C.H.; Weisel, R.D.; Jeffries, G.A.; Anderson, T.J.; Verma, S. Biomarkers of vascular disease linking inflammation to endothelial activation: Part II. Circulation 2003, 108, 2041–2048. [Google Scholar] [CrossRef] [PubMed]
- Zhang, R.; Brennan, M.L.; Shen, Z.; MacPherson, J.C.; Schmitt, D.; Molenda, C.E.; Hazen, S.L. Myeloperoxidase functions as a major enzymatic catalyst for initiation of lipid peroxidation at sites of inflammation. J. Biol. Chem. 2002, 277, 46116–46122. [Google Scholar] [CrossRef] [PubMed]
- Ross, R.; Glomset, J.A. Atherosclerosis and the arterial smooth muscle cell: Proliferation of smooth muscle cell is a key event in the genesis of the lesions of atherosclerosis. Science 1973, 180, 1332–1339. [Google Scholar] [CrossRef] [PubMed]
- Williams, K.J.; Tabas, I. The response-to-retention hypothesis of early atherogenesis. Arterioscler. Thromb. Vasc. Biol. 1995, 15, 551–561. [Google Scholar] [CrossRef] [PubMed]
- Proctor, S.; Vine, D.; Mamo, J. Arterial retention of apolipoprotein B(48)- and B(100)-containing lipoproteins in atherogenesis. Curr. Opin. Lipidol. 2002, 13, 461–470. [Google Scholar] [CrossRef] [PubMed]
- Stocker, R.; Frei, B. Endogenous antioxidant defenses in human blood plasma. In Oxidative Stress: Oxidants and Antioxidants; Sies, H., Ed.; Academic Press: London, UK, 1991; pp. 213–243. [Google Scholar]
- Cushing, S.D.; Berliner, J.A.; Valente, A.J.; Navab, M.; Parhami, F.; Gerrity, R.; Schwartz, C.J.; Fogelman, A.M. Minimally modified low density lipoprotein induces monocyte chemotactic protein 1 in human endothelial cells and smooth muscle cells. Proc. Natl. Acad. Sci. USA 1990, 87, 5134–5138. [Google Scholar] [CrossRef] [PubMed]
- Quinn, M.T.; Parthasarathy, S.; Fong, L.G.; Steinberg, D. Oxidatively modified low density lipoproteins: A potential role in recruitment and retention of monocyte/macrophages during atherogenesis. Proc. Natl. Acad. Sci. USA 1987, 84, 2995–2998. [Google Scholar] [CrossRef] [PubMed]
- Shechter, I.; Fogelman, A.M.; Haberland, M.E.; Seager, J.; Hokom, M.; Edwards, P.A. The metabolism of native and MDA-altered LDL by human monocyte-macrophages. J. Lipid Res. 1981, 22, 63–71. [Google Scholar] [PubMed]
- Hessler, J.R.; Robertson, A.L., Jr.; Chisolm, G.M., III. LDL-induced cytotoxicity and its inhibition by HDL in human vascular smooth muscle and endothelial cells in culture. Atherosclerosis 1979, 32, 213–229. [Google Scholar] [CrossRef] [PubMed]
- Cathcart, M.K.; McNally, A.K.; Chisolm, G.M. Lipoxygenase-mediated transformation of human LDL to an oxidized and cytotoxic complex. J. Lipid Res. 1991, 32, 63–70. [Google Scholar] [PubMed]
- Mabile, L.; Salvayre, R.; Bonnafe, M.J.; Negre-Salvayre, A. Oxidizability and subsequent cytotoxicity of chylomicrons to monocytic U937 and endothelial cells are dependent on dietary fatty acid composition. Free Radic. Biol. Med. 1995, 19, 599–607. [Google Scholar] [CrossRef] [PubMed]
- Stadtman, E.; Berlett, B.S. Reactive oxygen-mediated protein oxidation in ageing and disease. Chem. Res. Toxicol. 1997, 10, 485–494. [Google Scholar] [CrossRef] [PubMed]
- Dedon, P.C.; Tannenbaum, S.R. Reactive nitrogen species in the chemical biology of inflammation. Arch. Biochem. Biophys. 2004, 433, 12–22. [Google Scholar] [CrossRef]
- Tarpey, M.M.; Wink, D.A.; Grisham, M.B. Methods for detection of reactive metabolites of oxygen and nitrogen: In vitro and in vivo considerations. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2004, 286, R431–R444. [Google Scholar] [CrossRef] [PubMed]
- Cracowski, J.L.; Durand, T.; Bessard, G. Isoprostanes as a biomarker of lipid peroxidation in humans: Physiology, pharmacology and clinical implications. Trends Pharmacol. Sci. 2002, 23, 230–236. [Google Scholar] [CrossRef]
- Draper, H.H.; Csallany, A.S.; Hadley, M. Urinary aldehydes as indicators of lipid peroxidation in vivo. Free Radic. Biol. Med. 2000, 29, 1071–1077. [Google Scholar] [CrossRef] [PubMed]
- Dean, R.T.; Fu, S.; Stocker, R.; Davies, M.J. Biochemistry and pathology of radical-mediated protein oxidation. Biochem. J. 1997, 324, 1–18. [Google Scholar] [PubMed]
- Requena, J.R.; Levine, R.L.; Stadtman, E.R. Recent advances in the analysis of oxidized proteins. Amino Acids 2003, 25, 221–226. [Google Scholar] [CrossRef] [PubMed]
- Esterbauer, H.; Schaur, R.; Zollner, H. Chemistry and biochemistry of 4-hydroxynonenal, malondialdehyde and related aldehydes. Free Radic. Biol. Med. 1991, 11, 81–128. [Google Scholar] [CrossRef] [PubMed]
- Montuschi, P.; Barnes, P.J.; Roberts, L.J., II. Isoprostanes: Markers and mediators of oxidative stress. FASEB J. 2004, 18, 1791–1800. [Google Scholar] [CrossRef] [PubMed]
- Stocker, R.; Keaney, J.F., Jr. Role of oxidative modifications in atherosclerosis. Physiol. Rev. 2004, 84, 1381–1478. [Google Scholar] [CrossRef] [PubMed]
- Slatter, D.A.; Bolton, C.H.; Bailey, A.J. The importance of lipid-derived malondialdehyde in diabetes mellitus. Diabetologia 2000, 43, 550–557. [Google Scholar] [CrossRef] [PubMed]
- Heinecke, J.W. Oxidized amino acids: Culprits in human atherosclerosis and indicators of oxidative stress. Free Radic. Biol. Med. 2002, 32, 1090–1101. [Google Scholar] [CrossRef] [PubMed]
- Parola, M.; Bellamo, G.; Robino, G.; Barrera, G.; Dianzani, M.U. 4-Hydroxynonenal as a biological signal: Molecular basis and pathophysiological implications. Antioxid. Redox Signal. 1999, 1, 255–284. [Google Scholar] [CrossRef] [PubMed]
- Shao, B.; O’Brien, K.D.; McDonald, T.O.; Fu, X.; Oram, J.F.; Uchida, K.; Heinecke, J.W. Acrolein modifies apolipoprotein A–I in the human artery wall. Ann. N. Y. Acad. Sci. 2005, 1043, 396–403. [Google Scholar] [CrossRef] [PubMed]
- Dalle-Donne, I.; Rossi, R.; Colombo, R.; Giustarini, D.; Milzani, A. Biomarkers of oxidative damage in human disease. Clin. Chem. 2006, 52, 601–623. [Google Scholar] [CrossRef] [PubMed]
- Giustarini, D.; Rossi, R.; Milzani, A.; Colombo, R.; Dalle-Donne, I. S-Glutathionylation: From redox regulation of protein functions to human diseases. J. Cell. Mol. Med. 2004, 8, 201–212. [Google Scholar] [CrossRef] [PubMed]
- Gaut, J.; Yeh, G.; Tran, H.; Byun, J.; Henderson, J.P.; Richter, G.M.; Brennan, M.-L.; Lusis, A.J.; Belaaouaj, A.; Hotchkiss, R.S.; et al. Neutrophils employ the myeloperoxidase system to generate antimicrobial brominating and chlorinating oxidants during sepsis. Proc. Natl. Acad. Sci. USA 2001, 98, 11961–11966. [Google Scholar] [CrossRef] [PubMed]
- Brennan, M.L.; Wu, W.; Fu, X.; Shen, Z.; Song, W.; Frost, H.; Vadseth, C.; Narine, L.; Lenkiewicz, E.; Borchers, M.T.; et al. A tale of two controversies: Defining both the role of peroxidases in nitrtyrosine formation in vivo using eosinophil peroxidase nad myeloperoxidase-deficient mice, and the nature of peroxidase-generated reactive nitrogen species. J. Biol. Chem. 2002, 277, 17415–17422. [Google Scholar] [CrossRef] [PubMed]
- Zheng, L.; Nukuna, B.; Brennan, M.; Brennan, M.L.; Sun, M.; Goormastic, M.; Settle, M.; Schmitt, D.; Fu, X.; Thomson, L.; Fox, P.L.; et al. Apolipoprotein A–I is a selective target for myeloperoxidase-catalyzed oxidation and functional impairment in subjects with cardiovascular disease. J. Clin. Investig. 2004, 114, 529–541. [Google Scholar] [CrossRef] [PubMed]
- Pennathur, S.; Bergt, C.; Shao, B.; Byun, J.; Kassim, S.Y.; Singh, P.; Green, P.S.; McDonald, T.O.; Brunzell, J.; Chait, A.; et al. Human atherosclerotic intima and blood of patients with established coronary artery disease contain high density lipoprotein damaged by reactive nitrogen species. J. Biol. Chem. 2004, 279, 42977–42983. [Google Scholar] [CrossRef] [PubMed]
- Bergt, C.; Pennathur, S.; Fu, X.; Byun, J.; O’Brien, K.; McDonald, T.O.; Singh, P.; Anantharamaiah, G.M.; Chait, A.; Brunzell, J.; et al. The myeloperoxidase product hypochlorous acid oxidizes hdl in the human artery wall and impairs ABCA1 cholesterol transport. Proc. Natl. Acad. Sci. USA 2004, 101, 13032–13037. [Google Scholar] [CrossRef] [PubMed]
- Palinski, W.; Horkko, S.; Miller, E.; Steinbrecher, U.P.; Powell, H.C.; Curtiss, L.K.; Witztum, J.L. Cloning of monoclonal autoantibodies to epitopes of oxidized lipoproteins from apolipoprotein E-deficient mice. Demonstration of epitopes of oxidized low density lipoprotein in human plasma. J. Clin. Investig. 1996, 98, 800–814. [Google Scholar] [CrossRef] [PubMed]
- Palinski, W.; Ord, V.A.; Plump, A.S.; Breslow, J.L.; Steinberg, D.; Witztum, J.L. ApoE-deficient mice are a model of lipoprotein oxidation in atherogenesis. Demonstration of oxidation-specific epitopes in lesions and high titers of autoantibodies to malondialdehyde-lysine in serum. Arterioscler. Thromb. 1994, 14, 605–616. [Google Scholar] [CrossRef] [PubMed]
- Esterbauer, H.; Striegl, G.; Puhl, H.; Rotheneder, M. Continuous monitoring of in vitro oxidation of human LDL. Free Radic. Biol. Med. 1989, 6, 67–75. [Google Scholar] [CrossRef]
- Le, N.-A.; Farkas-Epperson, M.; Sweeney, M.; Wilson, P.; Brown, W. Effect of ABT-335 (fenofibric acid) on meal-induced oxidative stress in patients with metabolic syndrome. 2013, 231, 268–273. [Google Scholar]
- McEneny, J.; O’Kane, M.J.; Moles, K.W.; McMaster, C.; McMaster, D.; Mercer, C.; Trimble, E.R.; Young, I.S. Very low density lipoprotein subfractions in type II diabetes mellitus: Alterations in composition and susceptibility to oxidation. Diabetologia 2000, 43, 485–493. [Google Scholar] [CrossRef] [PubMed]
- Hendrickson, A.; McKinstry, L.A.; Lewis, J.K.; Lum, J.; Louie, A.; Schellenberg, G.D.; Hatsukami, T.S.; Chait, A.; Jarvik, G.P. Ex vivo measures of LDL oxidative susceptibility predict carotid artery disease. Atherosclerosis 2005, 179, 147–153. [Google Scholar] [CrossRef] [PubMed]
- Ryglewicz, D.; Rodo, M.; Roszczynko, M.; Baranska-Gieruszczak, M.; Szirkowiec, W.; Swiderska, M.; Wehr, H. Dynamics of ldl oxidation in ischemic stroke patients. Acta Neurol. Scand. 2002, 105, 185–188. [Google Scholar] [CrossRef] [PubMed]
- McEneny, J.; Loughrey, C.M.; McNamee, P.T.; Trimble, E.R.; Young, I.S. Susceptibility of VLDL to oxidation in patients on regular haemodialysis. Atherosclerosis 1997, 129, 215–220. [Google Scholar] [CrossRef] [PubMed]
- Parks, E.J.; German, J.B.; Davis, P.A.; Frankel, E.N.; Kappagoda, C.T.; Rutledge, J.C.; Hyson, D.A.; Schneeman, B.O. Reduced oxidative susceptibility of LDL from patients participating in an intensive atherosclerosis treatment program. Am. J. Clin. Nutr. 1998, 68, 778–785. [Google Scholar] [PubMed]
- Kaikkonen, J.; Porkkala-Sarataho, E.; Tuomainen, T.P.; Nyyssonen, K.; Kosonen, L.; Ristonmaa, U.; Lakka, H.M.; Salonen, R.; Korpela, H.; Salonen, J.T. Exhaustive exercise increases plasma/serum total oxidation resistance in moderately trained men and women, whereas their VLDL + LDL lipoprotein fraction is more susceptible to oxidation. Scand. J. Clin. Lab. Investig. 2002, 62, 599–608. [Google Scholar] [CrossRef]
- Nielsen, N.S.; Marckmann, P.; Hoy, C.E. Effect of meal fat quality on oxidation resistance of postprandial VLDL and LDL particles and plasma triacylglycerol level. Br. J. Nutr. 2000, 84, 855–863. [Google Scholar] [PubMed]
- Parthasarathy, S.; Khoo, J.C.; Miller, E.; Barnett, J.; Witztum, J.L.; Steinberg, D. Low density lipoprotein rich in oleic acid is protected against oxidative modification: Implications for dietary prevention of atherosclerosis. Proc. Natl. Acad. Sci. USA 1990, 87, 3894–3898. [Google Scholar] [CrossRef] [PubMed]
- Reaven, P.; Parthasarathy, S.; Grasse, B.J.; Miller, E.; Almazan, F.; Mattson, F.H.; Khoo, J.C.; Steinberg, D.; Witztum, J.L. Feasibility of using an oleate-rich diet to reduce the susceptibility of low-density lipoprotein to oxidative modification in humans. Am. J. Clin. Nutr. 1991, 54, 701–706. [Google Scholar] [PubMed]
- Reaven, P.; Parthasarathy, S.; Grasse, B.J.; Miller, E.; Steinberg, D.; Witztum, J.L. Effects of oleate-rich and linoleate-rich diets on the susceptibility of LDL to oxidative modification in mildly hypercholesterolemic subjects. J. Clin. Investig. 1993, 91, 668–676. [Google Scholar] [CrossRef] [PubMed]
- Olivecrona, T.; Egelrud, T. Lipoprotein lipase and its interaction with heparin. Horm. Metab. Res. 1974, 4, 23–28. [Google Scholar] [PubMed]
- Fuki, I.V.; Blanchard, N.; Jin, W.; Marchadier, D.H.; Millar, J.S.; Glick, J.M.; Rader, D.J. Endogenously produced endothelial lipase enhances binding and cellular procesin of plasma lipoproteins via heparan sulfate proteoglycan-mediated pathway. J. Biol. Chem. 2003, 278, 34331–34338. [Google Scholar] [CrossRef] [PubMed]
- Quinn, D.; Shirai, K.; Jackson, R.L. Lipoprotein lipase: Mechanism of action and role in lipoprotein metabolism. Prog. Lipid Res. 1983, 22, 35–78. [Google Scholar] [CrossRef] [PubMed]
- Brown, W.W.; Baginsky, M.L. Some functional aspects of apolipoproteins: ApoLp-Ala inhibition of lipoprotein lipase and deinhibition by monoolein. Horm. Metab. Res. 1974, 4, 11–16. [Google Scholar] [PubMed]
- Bengtsson, G.; Olivecrona, T. Lipoprotein lipase. Mechanism of product inhibition. Eur. J. Biochem. 1980, 106, 557–562. [Google Scholar] [CrossRef] [PubMed]
- Krauss, R.M.; Herbert, P.N.; Levy, R.I.; Fredrickson, D.S. Further observations on the activation and inhibition of lipoprotein lipase byapolipoproteins. Circ. Res. 1973, 33, 403–411. [Google Scholar] [CrossRef] [PubMed]
- Lambert, D.A.; Catapano, A.L.; Smith, L.C.; Sparrow, J.T.; Gotto, A.M., Jr. Effect of the apolipoprotein C-II/C-III1 ratio on the capacity of purified milk lipoprotein lipase to hydrolyze triglycerides in monolayer vesicles. Atherosclerosis 1996, 127, 205–212. [Google Scholar] [CrossRef] [PubMed]
- Le, N.A.; Cortner, J.A.; Breslow, J.L. Metabolism of intestinal lipoproteins during the postprandial state. In Diabetes; Rifkin, H., Colwell, J.A., Taylor, S.I., Eds.; Elsevier Science Publishers: New York, NY, USA, 1991; pp. 601–608. [Google Scholar]
- Le, N.A.; Coates, P.M.; Gallagher, P.R.; Cortner, J.A. Kinetics of retinyl esters during postprandial lipemia in man: A compartmental model. Metabolism 1997, 46, 584–594. [Google Scholar] [CrossRef] [PubMed]
- Berman, M.; Hall, M., III; Levy, R.I.; Eisenberg, S.; Bilheimer, D.W.; Phair, R.D.; Goebel, R.H. Metyabolism of apoB and apoC lipoproteins in man: Kinetic studies in normal and hyperlipoproteinemic subjects. J. Lipid Res. 1978, 19, 38–56. [Google Scholar] [PubMed]
- Le, N.A.; Li, X.; Kyung, S.; Brown, W.V. Evidence for the in vivo generation of oxidatively modified epitopes in patients with atherosclerotic endothelium. Metabolism 2000, 49, 1271–1277. [Google Scholar] [CrossRef] [PubMed]
- Gradek, W.Q.; Harris, M.T.; Yahia, N.; Davis, W.W.; Le, N.-A.; Brown, W.V. Polyunsaturated fatty acids acutely suppress antibodies to malondialdehyde-modified LDL in patients with vascular disease. Am. J. Cardiol. 2004, 93, 881–885. [Google Scholar] [CrossRef] [PubMed]
- Le, N.-A. Oxidized lipids and lipoproteins: Indices of risk or targets for management. Clin. Lipidol. 2009, 4, 41–54. [Google Scholar] [CrossRef]
- Palinski, W.; Miller, E.; Witztum, J.L. Immunization of LDL receptor-deficient rabbits with homologous malondialdehyde-modified LDL reduces atherosclerosis by mechanisms other than induction of high titers of antibodies to oxidative neoepitopes. Proc. Natl. Acad. Sci. USA 1995, 92, 821–825. [Google Scholar] [CrossRef] [PubMed]
- Nilsson, J.; Calara, F.; Regnstrom, J.; Hultgardh-Nilsson, A.; Ameli, S.; Cecek, B.; Shah, P.K. Immunization with homologous oxidized LDL reduces neointimal formation after balloon injury in hypercholesterolemic rabbits. J. Am. Coll. Cardiol. 1997, 30, 1886–1891. [Google Scholar] [CrossRef] [PubMed]
- Beisswenger, P.J.; Brown, W.V.; Ceriello, A.; Le, N.A.; Goldberg, R.B.; Cooke, J.P.; Robbins, D.C.; Sarwat, S.; Yuan, H.; Jones, C.A.; et al. Meal-induced increases in C-reactive protein, interleukin-6, and tumour necrosis factor α are attenuated by prandial + basal insulin in patients with Type 2 diabetes. Diabet Med. 2011, 28, 1088–1095. [Google Scholar] [CrossRef] [PubMed]
- Yagi, K. A simple fluorometric assay for lipoperoxide in blood plasma. Biochem. Med. 1976, 15, 212–216. [Google Scholar] [CrossRef] [PubMed]
- Yagi, K. Simple assay for the level of total lipid peroxides in serum or plasma. Methods Mol. Biol. 1998, 108, 101–106. [Google Scholar] [PubMed]
- Davies, K.J.; Qunitanilha, A.T.; Brooks, G.A.; Packer, L. Free radicals and tissue damage produced by exercise. Biochem. Biophys. Res. Commun. 1982, 107, 1198–1205. [Google Scholar] [CrossRef] [PubMed]
- Close, G.L.; Ashton, T.; McArdle, A.; Maclaren, D.P.M. The emerging role of free radicals in delayed onset muscle soreness and contraction-induced muscle injury. Comp. Biochem Physiol Part A 2005, 142, 257–266. [Google Scholar] [CrossRef]
- Palomero, J.; Pye, D.; Kabayo, T.; Spiller, D.; Jackson, M. In situ detectioon and measurement of intracellular reactive oxygen species in single isolated mature skeletal muscle fiber by real time fluorescence microscopy. Antioxid Redox Signal 2008, 10, 1463–1474. [Google Scholar] [CrossRef] [PubMed]
- Trachootham, D.; Lu, D.; Ogasawara, M.A.; Nilsa, R.D.; Huang, P. Redox regulation of cell survival. Antioxid. Redox Signal. 2008, 10, 1343–1374. [Google Scholar] [CrossRef] [PubMed]
- Ji, L.L.; Gomez-Cabrera, M.C.; Vina, J. Role of free radicals and antioxidant signaling in skeletal muscle health and pathology. Infect. Disord. Drug Targets 2009, 9, 428–444. [Google Scholar] [CrossRef] [PubMed]
- Powers, S.K.; Talbert, E.E.; Adhihetty, P.J. Reactive oxygen and nitrogen species as intracellular signals in skeletal muscle. J. Physiol. 2011, 589, 2129–2138. [Google Scholar] [CrossRef] [PubMed]
- Allen, R.G.; Tresini, M. Oxidative stress and gene regulation. Free Rad. Biol. Med. 2000, 28, 463–499. [Google Scholar] [CrossRef] [PubMed]
- Ji, L.L.; Zhang, Y. Antioxidant and anti-inflammatory effects of exercise: Role of redox signaling. Free Radic. Res. 2014, 48, 3–11. [Google Scholar] [CrossRef] [PubMed]
- Morales-Alamo, D.; Calbet, J.A. Free radicals and sprint exercise in humans. Free Radic. Res. 2014, 48, 30–42. [Google Scholar] [CrossRef] [PubMed]
- Chyu, K.Y.; Shah, P. Can we vaccinate against atherosclerosis? J. Cardiovasc. Pharmacol. Ther. 2014, 19, 77–82. [Google Scholar] [CrossRef] [PubMed]
- Salonen, J.T.; Yla-Herttuala, S.; Yamamoto, R.; Butler, S.; Korpola, H.; Salonen, R.; Nyyssonen, K.; Palinski, W.; Witztum, J.L. Autoantibody against LDL and progression of carotid atherosclerosis. Lancet 1992, 339, 883–887. [Google Scholar] [CrossRef] [PubMed]
- Maggi, E.; Finardi, G.; Poli, M.; Bollati, P.; Filipponi, M.; Stefano, P.L.; Paolini, G.; Grossi, A.; Clot, P.; Albano, E. Specificity of autoantibodies against oxLDL predicting myocardial infarction. Carotid Artery Dis. 1993, 4, 1119–1122. [Google Scholar] [CrossRef]
- Thavendiranathan, P.; Bagai, A.; Brookhart, M.; Choudhry, N. Primary prevention of cardiovascualr diseases with statin therapy: A meta-analysis of randomized controlled trials. Arch. Intern. Med. 2006, 166, 2307–2313. [Google Scholar] [CrossRef] [PubMed]
- Cholesterol Treatment Trialists’ (CTT) Collaborators; Kearney, P.M.; Blackwell, L.; Collins, R.; Keech, A.; Simes, J.; Peto, R.; Armitage, J.; Baigent, C. Cholesterol treatmnet trialists’ collaborators: Efficacy of cholesterol-lowering therapy in 18,686 people with diabetes in 14 randomised trials: A meta-analysis. Lancet 2008, 371, 117–125. [Google Scholar] [CrossRef] [PubMed]
- Sacks, F.M.; Pfeffer, M.A.; Moye, L.A.; Rouleau, J.L.; Rutherford, J.D.; Cole, T.G.; Brown, L.; Warnica, J.W.; Arnold, J.M.; Wun, C.C.; et al. The effect of pravastatin on coronary events after myocardial infarction in patients with average cholesterol levels: Cholesterol and Recurrent Events Trial investigators. N. Engl. J. Med. 1996, 335, 1001–1009. [Google Scholar] [CrossRef] [PubMed]
- Albert, M.A.; Danielson, E.; Rifai, N.; Ridker, P.M. Effect of statin therapy on C-reactive protein levels: The pravastatin inflammation/CRP evaluation (PRINCE): A randomized trial and cohort study. JAMA 2001, 286, 64–70. [Google Scholar] [CrossRef] [PubMed]
- Ballantyne, C.M.; Hoogeveen, R.C.; Bang, H.; Coresh, J.; Folsom, A.R.; Heiss, G.; Sha, A.R. Lipoprotein-associated phospholipase A2, high-sensitivity C-reactive protein, and risk for incident coronary heart disease in middle-aged men and women in the Atherosclerosis Risk in Communities (aric) study. Circulation 2004, 109, 837–842. [Google Scholar] [CrossRef] [PubMed]
- Koenig, W.; Khuseyinova, N.; Lowel, H.; Trischler, G.; Meisenger, C. Lipoprotein-associated phospholipase A2 adds to risk prediction of incident coronary events by C-reactive protein in apparently healthy middle-aged men from the general population: Results from the 14-year follow-up of a large cohort from southern Germany. Circulation 2004, 110, 1903–1908. [Google Scholar] [CrossRef] [PubMed]
- Blankenberg, S.; Zeller, T.; Saarela, O.; Havulinna, A.S.; Kee, F.; Tunstall-Pedoe, H.; Kuulasmaa, K.; Yarnell, J.; Schnabel, R.B.; Wild, P.S.; et al. Contribution of 30 biomarkers to 10-year cardiovascular risk estimation in 2 poplation cohorts. Circulation 2010, 121, 2388–2397. [Google Scholar] [CrossRef] [PubMed]
- Yousuf, O.; Mohanty, B.D.; Martin, S.S.; Joshi, P.H.; Blaha, M.J.; Nasir, K.; Blumenthal, R.S.; Budoff, M.J. High-sensitivity C-reactive protein and cardiovascualr disease: A resolute belief or an elusive link. J. Am. Coll. Cardiol. 2013, 62, 397–408. [Google Scholar] [CrossRef] [PubMed]
- Le, N.A. Reducing oxidized lipids to prevent cardiovascular disease. Curr. Treatment. Options Cardiovasc. Med. 2008, 10, 263–272. [Google Scholar] [CrossRef]
- Tsimikas, S.; Aikawa, M.; Miller, F.J., Jr.; Miller, E.R.; Torzewski, M.; Lentz, S.R.; Bergmark, C.; Heistad, D.D.; Libby, P.; Witztum, J.L. Increased plasma oxidized phospholipid:apolipoprotein B-100 ratio with concomitant depletion of oxidized phospholipids from atherosclerotic lesions after dietary lipid-lowering: A potential biomarker of early atherosclerosis regression. Arterioscler. Thromb. Vasc. Biol. 2007, 27, 175–181. [Google Scholar] [CrossRef] [PubMed]
- Ky, B.; Burke, A.; Tsimikas, S.; Wolfe, M.L.; Tadesse, M.G.; Szapary, P.O.; Witztum, J.L.; Fitzgerald, G.A.; Rader, D.J. The influence of pravastatin and atorvastatin on markers of oxidative stress in hypercholesterolemic humans. J. Am. Coll. Cardiol. 2008, 51, 1653–1662. [Google Scholar] [CrossRef] [PubMed]
- Nissen, S.E.; Tuzcu, E.M.; Schoenhagen, P.; Brown, B.G.; Ganz, P.; Vogel, R.A.; Crowe, T.; Howard, G.; Cooper, C.J.; Brodie, B.; et al. Effect of intensive compared with moderate lipid-lowering therapy on progression of coronary atherosclerosis: A randomized controlled trial. JAMA 2004, 291, 1071–1080. [Google Scholar] [CrossRef] [PubMed]
- Choi, S.H.; Chae, A.; Miller, E.; Messig, M.; Ntanios, F.; DeMaria, A.N.; Nissen, S.E.; Witztum, J.L.; Tsimikas, S. Relationship between biomarkers of oxidized low-density lipoprotein, statin therapy, quantitative coronary angiography, and atheroma: Volume observations from the REVERSAL (Reversal of Atherosclerosis with Aggressive Lipid Lowering) study. J. Am. Coll. Cardiol. 2008, 52, 24–32. [Google Scholar] [CrossRef] [PubMed]
- Diwadkar, V.A.; Anderson, J.W.; Bridges, S.R.; Gowri, M.S.; Oelgten, P.R. Postprandial low-density lipoproteins in type 2 diabetes are oxidized more extensively than fasting diabetes and control samples. Proc. Soc. Exp. Biol. Med. 1999, 222, 178–184. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, S.; Miki, T.; Sha, S.; Hirata, K.; Ishikawa, Y.; Yokohama, M. Serum levels of TBARS are associated with risk of coronary heart disease. J. Atheroscler. Thromb. 2011, 18, 584–591. [Google Scholar] [CrossRef] [PubMed]
- Walter, M.F.; Jacobs, R.F.; Jeffers, B.; Ghadanfar, M.M.; Gm Preston, J.B.; Mason, R.P. Serum levels of thiobarbituric acid reactive substances predict cardiovascular events in patients with stable coronary artery disease: A longitudinal analysis of the PREVENT study. J. Am. Coll. Cardiol. 2004, 44, 1996–2002. [Google Scholar] [CrossRef] [PubMed]
© 2014 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).