Exposure to Non-Extreme Solar UV Daylight: Spectral Characterization, Effects on Skin and Photoprotection
Abstract
:1. Introduction
2. Conditions of Solar Exposure
2.1. Solar Standard Spectra/Zenithal Solar Spectra
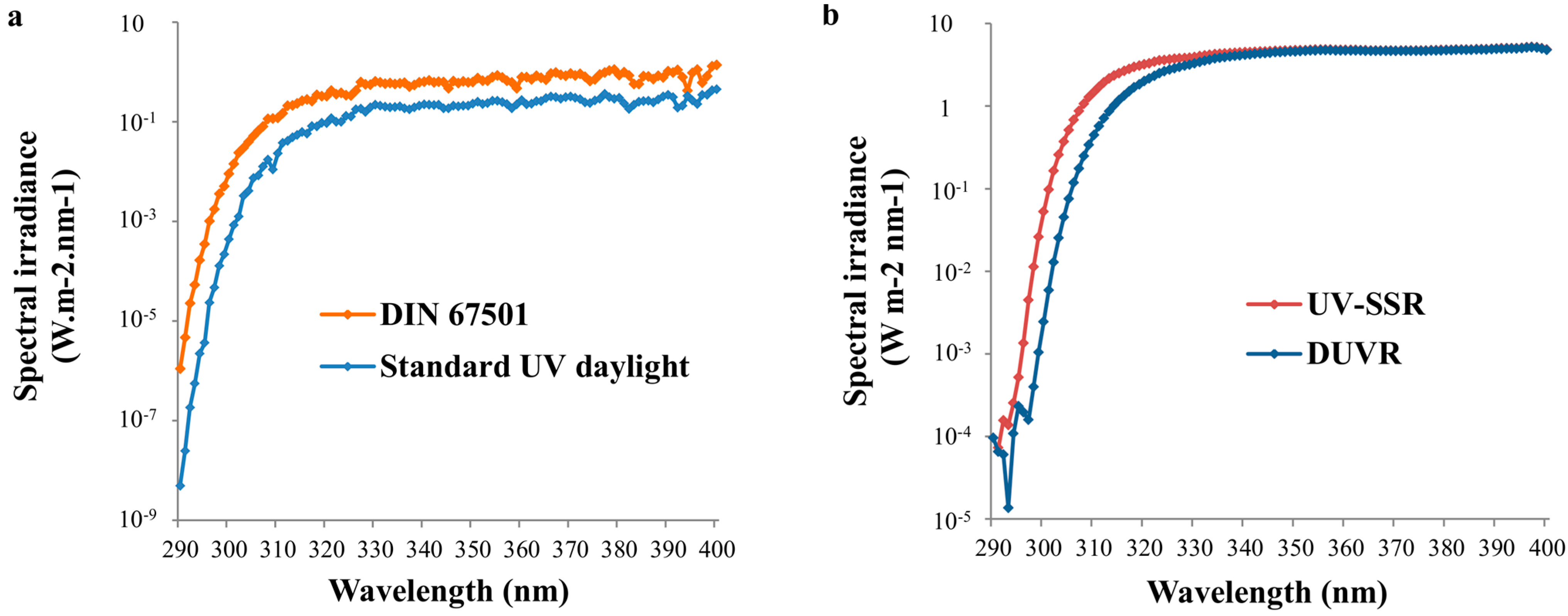
2.2. Standard Spectra Representing Daily Solar UV Exposure Conditions
| City | Country | Latitude (Decimal Degrees) | UV Dose (J/cm2) | UV Daylight Dose (J/cm2) | UV Daylight Proportion (%) |
|---|---|---|---|---|---|
| Oslo | Norway | 59.9 | 112.45 | 57.97 | 52% |
| Copenhagen | Denmark | 55.7 | 122.38 | 64.35 | 53% |
| Moscow | Russia | 55.8 | 122.77 | 64.6 | 53% |
| Berlin | Germany | 52.5 | 129.64 | 69.03 | 53% |
| London | England | 51.5 | 131.94 | 70.5 | 53% |
| Paris | France | 48.9 | 137.59 | 68.31 | 50% |
| Lausanne | Switzerland | 46.5 | 141.98 | 59.55 | 42% |
| Nice | France | 43.7 | 148.12 | 47.33 | 32% |
| Sapporo | Japan | 43.1 | 148.59 | 46.39 | 31% |
| Chicago | USA | 41.9 | 150.48 | 42.63 | 28% |
| Roma | Italy | 41.9 | 150.8 | 41.99 | 28% |
| New York | USA | 40.7 | 152.93 | 37.75 | 25% |
| Madrid | Spain | 40.4 | 153.46 | 36.69 | 24% |
| Lisbon | Portugal | 38.7 | 156.19 | 34.54 | 22% |
| Tunis | Tunisia | 36.8 | 158.92 | 33.73 | 21% |
| Tokyo | Japan | 35.6 | 161.3 | 33.03 | 20% |
| Los Angeles | USA | 34.1 | 163 | 32.52 | 20% |
| Miami | USA | 25.8 | 172.42 | 25.18 | 15% |
| Mexico City | Mexico | 19.4 | 176.82 | 18.3 | 10% |
| Hanoï | Vietnam | 21.0 | 175.99 | 19.57 | 11% |
| Saint Lucia | West-Indies | 13.9 | 177.25 | 18.05 | 10% |
| Bangkok | Thaïland | 13.8 | 177.27 | 18.04 | 10% |
| Darwin | Australia | −12.5 | 154.57 | 11.08 | 7% |
| Brasilia | Brazil | −15.8 | 147.72 | 14.15 | 10% |
| Saint Denis | Reunion | −20.9 | 137.56 | 17.76 | 13% |
| Johannesburg | South Africa | −26.2 | 125.54 | 18.3 | 15% |
| Brisbane | Australia | −27.5 | 122.43 | 18.44 | 15% |
| Sydney | Australia | −33.9 | 106.31 | 22.55 | 21% |
| Cape Town | South Africa | −33.9 | 105.85 | 22.72 | 21% |
| Auckland | New Zealand | −36.5 | 101.5 | 26.7 | 26% |
| Melbourne | Australia | −37.8 | 95.55 | 26.56 | 28% |
3. Effects of Exposure to Daily UV Radiation
3.1. Effects of DUVR in Human Skin in Vivo
| Parameters | DUVR Spread over 2 Weeks | DUVR Spread over 4 Weeks | ||||
|---|---|---|---|---|---|---|
| 9 × 0.25 MED | 9 × 0.50 MED | 9 × 0.75 MED | 19 × 0.5 MED | |||
| Clinical Parameters | ||||||
| Pigmentation | ||||||
| Δa* | + | ++ | +++ | ++ | ||
| Δb* | ns | + | ++ | + | ||
| ΔL* | − | −−− | −−− | − | ||
| Erythema | ns | + | ++ | + | ||
| Hydration | − | − | − | ns | ||
| Biomechanical properties | ||||||
| Elasticity | ns | − | − | ns | ||
| Residual deformation | ns | ns | ns | ND | ||
| Microtopography | ||||||
| Number of wrinkles | ns | ns | − | + | ||
| Coefficient of developed profile | ns | ns | − | ns | ||
| Loss of skin density (densiscore) § | ND | ND | + | ND | ||
| Biological parameters | ||||||
| Epidermis | ||||||
| Histology | ||||||
| Epidermal thickness | ns | ns | + | + | ||
| Langerhans cells | ||||||
| Number of Langerhans cells | − | −− | −−− | −− | ||
| Size of Langerhans cells | + | ++ | +++ | ns | ||
| Urocanic acid isomerization | + | ND | ND | ND | ||
| Melanocytes | ||||||
| Number of melanocytes | + | + | + | + | ||
| Size of melanocytes | + | ++ | +++ | + | ||
| Melanin deposition | + | ++ | +++ | + | ||
| Proliferation | ||||||
| Ki-67 + cells | + | ++ | +++ | ns | ||
| Cellular damage | ||||||
| sunburn cell formation | ns | + | + | + | ||
| p53 accumulation | ns | ++ | +++ | + | ||
| Dermis | ||||||
| Tenascin | ns | ns | ++ | + | ||
| Elastin | ns | ns | ns | ns | ||
| Fibrillin | ns | − | − | ND | ||
| Lyzozyme/elastin | ns | ns | ns | + | ||
| Pro-collagen I | − | −− | −−− | ns | ||
| Pro-collagen III/Pro-collagen I | ns | ns | + | ns | ||
| Glycosaminoglycan deposition | − | − | − | −− | ||

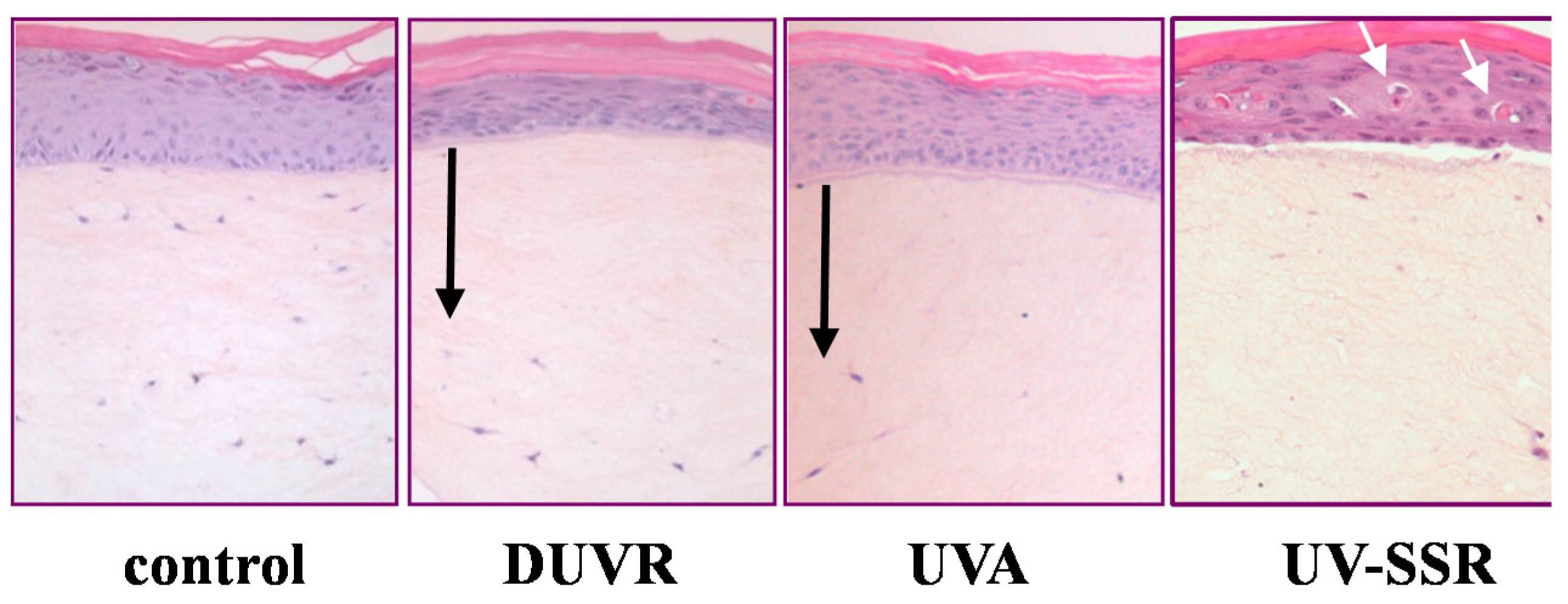
3.2. In Vitro Effects of DUVR in Reconstructed Human Skin Model
3.2.1. Biological Efficient Dose and Histologic Changes
3.2.2. Modulation of Gene Expression
3.2.3. Contribution of UVA Wavelengths to DUVR Biological Effects
3.2.4. Focus on Oxidative Stress Induced by DUVR and Characterization of the Fibroblast and Keratinocyte Response

4. Photoprotection against DUVR
4.1. Photoprotection Assessed in Vivo

4.2. Photoprotection Assessed in Vitro
5. Conclusions
Acknowledgments
Conflicts of Interest
References
- Tewari, A.; Sarkany, R.P.; Young, A.R. UVA1 induces cyclobutane pyrimidine dimers but not 6-4 photoproducts in human skin in vivo. J. Investig. Dermatol. 2012, 132, 394–400. [Google Scholar] [CrossRef] [PubMed]
- Bruls, W.A.; Slaper, H.; van der Leun, J.C.; Berrens, L. Transmission of human epidermis and stratum corneum as a function of thickness in the ultraviolet and visible wavelengths. Photochem. Photobiol. 1984, 40, 485–494. [Google Scholar] [CrossRef] [PubMed]
- Lim, H.W.; Naylor, M.; Honigsmann, H.; Gilchrest, B.A.; Cooper, K.; Morison, W.; Deleo, V.A.; Scherschun, L. American Academy of Dermatology Consensus Conference on UVA protection of sunscreens: Summary and recommendations: Washington, DC, Feb 4, 2000. J. Am. Acad. Dermatol. 2001, 44, 505–508. [Google Scholar] [CrossRef] [PubMed]
- Krutmann, J. Ultraviolet A radiation-induced biological effects in human skin: Relevance for photoaging and photodermatosis. J. Dermatol. Sci. 2000, 23, S22–S26. [Google Scholar] [CrossRef] [PubMed]
- D’Orazio, J.; Jarrett, S.; Maro-Ortiz, A.; Scott, T. UV Radiation and the skin. Int. J. Mol. Sci. 2013, 14, 12222–12248. [Google Scholar] [CrossRef] [PubMed]
- Naylor, E.C.; Watson, R.E.; Sherratt, M.J. Molecular aspects of skin ageing. Maturitas 2011, 69, 249–256. [Google Scholar] [CrossRef] [PubMed]
- Aydinly, S.; Justus, C.G.; Kaase, H.; Kasten, F.; Kockot, D.; Kok, C.J.; Richmond, J.V.; Zerlant, G.A. Solar Spectral Irradiance; Technical Report for Commission Internationale de l'Éclairage (CIE): Vienna, Austria, 1989. [Google Scholar]
- Frederick, J.E.; Lubin, D. Possible long-term changes in biologically active ultraviolet radiation reaching the ground. Photochem. Photobiol. 1988, 47, 571–578. [Google Scholar] [CrossRef] [PubMed]
- Frederick, J.E.; Snell, H.E.; Haywood, E.K. Solar ultraviolet radiation at the Earth’s surface. Photochem. Photobiol. 1989, 50, 443–450. [Google Scholar] [CrossRef]
- Lubin, D.; Jensen, E.H. Effects of clouds and stratospheric ozone depletion on ultraviolet radiation trends. Nature 1995, 377, 710–713. [Google Scholar] [CrossRef]
- Sabziparvar, A.A.; Shine, K.P.; Forster, P.M. A model-derived global climatology of UV irradiation at the Earth’s surface. Photochem. Photobiol. 1999, 69, 193–202. [Google Scholar]
- Tewari, A.; Grage, M.M.; Harrison, G.I.; Sarkany, R.; Young, A.R. UVA1 is skin deep: Molecular and clinical implications. Photochem. Photobiol. Sci. 2013, 12, 95–103. [Google Scholar] [CrossRef] [PubMed]
- Wilkinson, F.J. Solar simulators for sunscreen testing. In Measurements of Optical Radiation Hazards; Matthes, R., Sliney, D.H., Eds.; Märkl-Druck: München, Germany, 1998; p. 653. [Google Scholar]
- Fourtanier, A.; Moyal, D.; Seite, S. Sunscreens containing the broad-spectrum UVA absorber, Mexoryl SX, prevent the cutaneous detrimental effects of UV exposure: A review of clinical study results. Photodermatol. Photoimmunol. Photomed. 2008, 24, 164–174. [Google Scholar] [CrossRef] [PubMed]
- Seite, S.; Christiaens, F.; Bredoux, C.; Compan, D.; Zucchi, H.; Lombard, D.; Fourtanier, A.; Young, A.R. A broad-spectrum sunscreen prevents cumulative damage from repeated exposure to sub-erythemal solar ultraviolet radiation representative of temperate latitudes. J. Eur. Acad. Dermatol. Venereol. 2010, 24, 219–222. [Google Scholar] [CrossRef] [PubMed]
- The European Cosmetic and Toiletry Association (Colipa). Sun Protection Factor Test Method. 1994. Available online: http://www.colipa.com (accessed on 18 November 2014).
- Diffey, B.; Robson, J. A new substrate to measure sunscreen protection factors throughout the ultraviolet spectrum. J. Soc. Cosmet. Chem. 1989, 40, 127–133. [Google Scholar]
- Commission Internationale de l’Éclairage (CIE). Spectral Weighting of Solar Ultraviolet Radiation. 2003. Available online: http://www.cie.co.at/cie/ (accessed on 18 November 2014).
- Cosmetic Toiletry & Fragrance Association of South Africa (CTFA-SA); The European Cosmetic and Toiletry Association (Colipa); Japan Cosmetic Industry Association (JCIA). International Sun Protection Factor (SPF) Test Method. 2003. Available online: http://www.cie.co.at/cie/ (accessed on 18 November 2014).
- Deutsches Institut für Normung e.V. (DIN). Experimentelle Bewertung des Erythemschutzes von externen Sonnenschutzmitteln. für Die Menschliche Haut (Experimental evaluation of the protection from erythema by external sunscreen products for the human skin). 1999. Available online: http://www.en.din.de (accessed 18 November 2014).
- Sklar, L.R.; Almutawa, F.; Lim, H.W.; Hamzavi, I. Effects of ultraviolet radiation, visible light, and infrared radiation on erythema and pigmentation: A review. Photochem. Photobiol. Sci. 2013, 12, 54–64. [Google Scholar] [CrossRef] [PubMed]
- Sayre, R.M.; Cole, C.A.; Billhimer, W.L.; Stanfield, J.; Ley, R.D. Spectral comparison of solar simulators and sunlight. Photodermatol. Photoimmunol. Photomed. 1990, 7, 159–165. [Google Scholar] [PubMed]
- Seite, S.; Fourtanier, A.; Moyal, D.; Young, A.R. Photodamage to human skin by suberythemal exposure to solar ultraviolet radiation can be attenuated by sunscreens: a review. Br. J. Dermatol. 2010, 163, 903–914. [Google Scholar] [CrossRef] [PubMed]
- Grothmann, K.; Kaase, H. Testung von Lichtschutzmitteln—Vorschlag zur Definition einer Referenz-Spektralverteilung für UV-Sonnensimulatoren (Sun protection measurement—Proposal for a definition of a UV solar simulator standard spectrum). Dermatol. Monatsschr. 1993, 179, 108–111. [Google Scholar]
- Christiaens, F.J.; Chardon, A.; Fourtanier, A.; Frederick, J.E. Standard ultraviolet daylight for nonextreme exposure conditions. Photochem. Photobiol. 2005, 81, 874–878. [Google Scholar] [CrossRef] [PubMed]
- Pearse, A.D.; Gaskell, S.A.; Marks, R. Epidermal changes in human skin following irradiation with either UVB or UVA. J. Investig. Dermatol. 1987, 88, 83–87. [Google Scholar] [CrossRef] [PubMed]
- Lavker, R.M.; Veres, D.A.; Irwin, C.J.; Kaidbey, K.H. Quantitative assessment of cumulative damage from repetitive exposures to suberythemogenic doses of UVA in human skin. Photochem. Photobiol. 1995, 62, 348–352. [Google Scholar] [CrossRef] [PubMed]
- Séité, S.; Moyal, D.; Richard, S.; de Rigal, J.; Léveque, J.L.; Hourseau, C.; Fourtanier, A. Mexoryl SX: A broad absorption UVA filter protects human skin from the effects of repeated suberthemal doses of UVA. J. Photochem. Photobiol. B 1998, 44, 69–76. [Google Scholar] [CrossRef] [PubMed]
- Liardet, S.; Scaletta, C.; Panizzon, R.; Hohlfeld, P.; Laurent-Applegate, L. Protection against pyrimidine dimers, p53, and 8-hydroxy-2'-deoxyguanosine expression in ultraviolet-irradiated human skin by sunscreens: Difference between UVB + UVA and UVB alone sunscreens. J. Investig. Dermatol. 2001, 117, 1437–1441. [Google Scholar] [CrossRef] [PubMed]
- Sander, C.S.; Chang, H.; Salzmann, S.; Muller, C.S.; Ekanayake-Mudiyanselage, S.; Elsner, P.; Thiele, J.J. Photoaging is associated with protein oxidation in human skin in vivo. J. Investig. Dermatol. 2002, 118, 618–625. [Google Scholar] [CrossRef] [PubMed]
- Sheehan, J.M.; Cragg, N.; Chadwick, C.A.; Potten, C.S.; Young, A.R. Repeated ultraviolet exposure affords the same protection against DNA photodamage and erythema in human skin types II and IV but is associated with faster DNA repair in skin type IV. J. Investig. Dermatol. 2002, 118, 825–829. [Google Scholar] [CrossRef] [PubMed]
- Norval, M.; McLoone, P.; Lesiak, A.; Narbutt, J. The effect of chronic ultraviolet radiation on the human immune system. Photochem. Photobiol. 2008, 84, 19–28. [Google Scholar] [CrossRef] [PubMed]
- Holloway, L. Atmospheric sun protection factor on clear days: Its observed dependence on solar zenith angle and its relevance to the shadow rule for sun protection. Photochem. Photobiol. 1992, 56, 229–234. [Google Scholar] [CrossRef] [PubMed]
- Frederick, J.E.; Lubin, D. The budget of biologically active ultraviolet radiation in the Earth-atmosphere system. J. Geophys. Res. 1988, 93, 3825–3832. [Google Scholar] [CrossRef]
- Christiaens, F.; Chardon, A. Ultraviolet Radiation for non-Extreme Exposure Conditions: Definition and indoor reproduction. In the Official Newsletter of the Thematic Network for Ultraviolet Measurements, Davos, Switzerland, 20–21 October 2005; pp. 11–13.
- Seite, S.; Medaisko, C.; Christiaens, F.; Bredoux, C.; Compan, D.; Zucchi, H.; Lombard, D.; Fourtanier, A. Biological effects of simulated ultraviolet daylight: A new approach to investigate daily photoprotection. Photodermatol. Photoimmunol. Photomed. 2006, 22, 67–77. [Google Scholar] [CrossRef] [PubMed]
- Krutmann, J. The interaction of UVA and UVB wavebands with particular emphasis on signalling. Prog. Biophys. Mol. Biol. 2006, 92, 105–107. [Google Scholar] [CrossRef] [PubMed]
- Muthusamy, V.; Piva, T.J. A comparative study of UV-induced cell signalling pathways in human keratinocyte-derived cell lines. Arch. Dermatol. Res. 2013, 305, 817–833. [Google Scholar] [CrossRef] [PubMed]
- Chardon, A.M.; Crétois, I.; Hourseau, C. Skin colour typology and suntanning pathways. Int. J. Cosm. Sci. 1991, 13, 191–208. [Google Scholar] [CrossRef]
- Bernerd, F.; Asselineau, D. An organotypic model of skin to study photodamage and photoprotection in vitro. J. Am. Acad. Dermatol. 2008, 58, S155–S159. [Google Scholar] [CrossRef] [PubMed]
- Vioux-Chagnoleau, C.; Lejeune, F.; Sok, J.; Pierrard, C.; Marionnet, C.; Bernerd, F. Reconstructed human skin: From photodamage to sunscreen photoprotection and anti-aging molecules. J. Dermatol. Sci. Suppl. 2006, 2, S1–S12. [Google Scholar]
- Bernerd, F.; Asselineau, D. Successive alteration and recovery of epidermal differentiation and morphogenesis after specific UVB-damages in skin reconstructed in vitro. Dev. Biol. 1997, 183, 123–138. [Google Scholar] [CrossRef] [PubMed]
- Bernerd, F.; Asselineau, D. UVA exposure of human skin reconstructed in vitro induces apoptosis of dermal fibroblasts: subsequent connective tissue repair and implications in photoaging. Cell. Death Differ. 1998, 5, 792–802. [Google Scholar] [CrossRef] [PubMed]
- Lejeune, F.; Christiaens, F.; Bernerd, F. Evaluation of sunscreen products using a reconstructed skin model exposed to simulated daily ultraviolet radiation: Relevance of filtration profile and SPF value for daily photoprotection. Photodermatol. Photoimmunol. Photomed. 2008, 24, 249–255. [Google Scholar] [CrossRef] [PubMed]
- Marionnet, C.; Pierrard, C.; Lejeune, F.; Sok, J.; Thomas, M.; Bernerd, F. Different oxidative stress response in keratinocytes and fibroblasts of reconstructed skin exposed to non extreme daily-ultraviolet radiation. PLoS One 2010, 5, e12059. [Google Scholar] [CrossRef] [PubMed]
- Bernerd, F.; Vioux, C.; Lejeune, F.; Asselineau, D. The sun protection factor (SPF) inadequately defines broad spectrum photoprotection: Demonstration using skin reconstructed in vitro exposed to UVA, UVBor UV-solar simulated radiation. Eur. J. Dermatol. 2003, 13, 242–249. [Google Scholar] [PubMed]
- Marionnet, C.; Lejeune, F.; Pierrard, C.; Vioux-Chagnoleau, C.; Bernerd, F. Biological contribution of UVA wavelengths in non extreme daily UV exposure. J. Dermatol. Sci. 2012, 66, 238–240. [Google Scholar] [CrossRef] [PubMed]
- Marionnet, C.; Pierrard, C.; Lejeune, F.; Bernerd, F. Modulations of gene expression induced by daily ultraviolet light can be prevented by a broad spectrum sunscreen. J. Photochem. Photobiol. B 2012, 116, 37–47. [Google Scholar] [CrossRef] [PubMed]
- Wlaschek, M.; Tantcheva-Poor, I.; Naderi, L.; Ma, W.; Schneider, L.A.; Razi-Wolf, Z.; Schuller, J.; Scharffetter-Kochanek, K. Solar UV irradiation and dermal photoaging. J. Photochem. Photobiol. B 2001, 63, 41–51. [Google Scholar] [CrossRef] [PubMed]
- Quan, T.; Qin, Z.; Xia, W.; Shao, Y.; Voorhees, J.J.; Fisher, G.J. Matrix-degrading metalloproteinases in photoaging. J. Investig. Dermatol. Symp. Proc. 2009, 14, 20–24. [Google Scholar] [CrossRef] [PubMed]
- Fisher, G.J.; Datta, S.; Wang, Z.; Li, X.Y.; Quan, T.; Chung, J.H.; Kang, S.; Voorhees, J.J. c-Jun-dependent inhibition of cutaneous procollagen transcription following ultraviolet irradiation is reversed by all-trans retinoic acid. J. Clin. Investig. 2000, 106, 663–670. [Google Scholar] [CrossRef] [PubMed]
- Castanet, J.; Ortonne, J.P. Pigmentary changes in aged and photoaged skin. Arch. Dermatol. 1997, 133, 1296–1299. [Google Scholar] [CrossRef] [PubMed]
- Kovacs, D.; Cardinali, G.; Aspite, N.; Cota, C.; Luzi, F.; Bellei, B.; Briganti, S.; Amantea, A.; Torrisi, M.R.; Picardo, M. Role of fibroblast-derived growth factors in regulating hyperpigmentation of solar lentigo. Br. J. Dermatol. 2010, 163, 1020–1027. [Google Scholar] [CrossRef] [PubMed]
- Chen, N.; Hu, Y.; Li, W.H.; Eisinger, M.; Seiberg, M.; Lin, C.B. The role of keratinocyte growth factor in melanogenesis: A possible mechanism for the initiation of solar lentigines. Exp. Dermatol. 2010, 19, 865–872. [Google Scholar] [CrossRef] [PubMed]
- Hirobe, T. Role of keratinocyte-derived factors involved in regulating the proliferation and differentiation of mammalian epidermal melanocytes. Pigment. Cell. Res. 2005, 18, 2–12. [Google Scholar] [CrossRef] [PubMed]
- Norval, M.; Halliday, G.M. The consequences of UV-induced immunosuppression for human health. Photochem. Photobiol. 2011, 87, 965–977. [Google Scholar] [CrossRef] [PubMed]
- Nasti, T.H.; Timares, L. Inflammasome activation of IL-1 family mediators in response to cutaneous photodamage. Photochem. Photobiol. 2012, 88, 1111–1125. [Google Scholar] [CrossRef] [PubMed]
- Trautinger, F. Heat shock proteins in the photobiology of human skin. J. Photochem. Photobiol. B 2001, 63, 70–77. [Google Scholar] [CrossRef] [PubMed]
- Jonak, C.; Klosner, G.; Trautinger, F. Significance of heat shock proteins in the skin upon UV exposure. Front. Biosci. (Landmark Ed.) 2009, 14, 4758–4768. [Google Scholar] [CrossRef]
- Brunet, S.; Giacomoni, P.U. Heat shock mRNA in mouse epidermis after UV irradiation. Mutat. Res. 1989, 219, 217–224. [Google Scholar] [CrossRef] [PubMed]
- Trautinger, F.; Kokesch, C.; Klosner, G.; Knobler, R.M.; Kindas-Mugge, I. Expression of the 72-kD heat shock protein is induced by ultraviolet A radiation in a human fibrosarcoma cell line. Exp. Dermatol. 1999, 8, 187–192. [Google Scholar] [CrossRef] [PubMed]
- Marionnet, C.; Pierrard, C.; Golebiewski, C.; Bernerd, F. Diversity of Biological Effects Induced by Longwave UVA Rays (UVA1) in Reconstructed Skin. PLoS One 2014, 9, e105263. [Google Scholar] [CrossRef] [PubMed]
- Matsuda, M.; Hoshino, T.; Yamashita, Y.; Tanaka, K.; Maji, D.; Sato, K.; Adachi, H.; Sobue, G.; Ihn, H.; Funasaka, Y.; et al. Prevention of UVB radiation-induced epidermal damage by expression of heat shock protein 70. J. Biol. Chem. 2010, 285, 5848–5858. [Google Scholar] [CrossRef] [PubMed]
- Matsuda, M.; Hoshino, T.; Yamakawa, N.; Tahara, K.; Adachi, H.; Sobue, G.; Maji, D.; Ihn, H.; Mizushima, T. Suppression of UV-induced wrinkle formation by induction of HSP70 expression in mice. J. Investig. Dermatol. 2013, 133, 919–928. [Google Scholar] [CrossRef] [PubMed]
- Gordon, J.R.; Brieva, J.C. Images in clinical medicine. Unilateral dermatoheliosis. N. Engl. J. Med. 2012, 366. [Google Scholar] [CrossRef] [PubMed]
- Moskovitz, J.; Bar-Noy, S.; Williams, W.M.; Requena, J.; Berlett, B.S.; Stadtman, E.R. Methionine sulfoxide reductase (MsrA) is a regulator of antioxidant defense and lifespan in mammals. Proc. Natl. Acad. Sci. USA 2001, 98, 12920–12925. [Google Scholar] [CrossRef] [PubMed]
- Picot, C.R.; Moreau, M.; Juan, M.; Noblesse, E.; Nizard, C.; Petropoulos, I.; Friguet, B. Impairment of methionine sulfoxide reductase during UV irradiation and photoaging. Exp. Gerontol. 2007, 42, 859–863. [Google Scholar] [CrossRef] [PubMed]
- Salmon, A.B.; Perez, V.I.; Bokov, A.; Jernigan, A.; Kim, G.; Zhao, H.; Levine, R.L.; Richardson, A. Lack of methionine sulfoxide reductase A in mice increases sensitivity to oxidative stress but does not diminish life span. FASEB J. 2009, 23, 3601–3608. [Google Scholar] [CrossRef] [PubMed]
- Cosmetic Toiletry & Fragrance Association of South Africa (CTFA–SA); The European Cosmetic and Toiletry Association (COLIPA); Japan Cosmetic Industry Association (JCIA); Cosmetic Toiletry & Fragrance Association (CTFA). International Sun Protection factor (SPF) test method. 2006. Available online: http://www.colipa.com (accessed on 18 November 2014).
- Moyal, D.; Chardon, A.; Kollias, N. Determination of UVA protection factors using the persistent pigment darkening (PPD) as the end point. (Part 1). Calibration of the method. Photodermatol. Photoimmunol. Photomed. 2000, 16, 245–249. [Google Scholar] [CrossRef] [PubMed]
- Japan Cosmetic Industry Association (JCIA). Measurement Standards for UVA Efficacy; Tokyo, Japan, 21 November 1995. [Google Scholar]
- Young, A.R.; Boles, J.; Herzog, B.; Osterwalder, U.; Baschong, W. A sunscreen’s labeled sun protection factor may overestimate protection at temperate latitudes: A human in vivo study. J. Investig. Dermatol. 2010, 130, 2457–2462. [Google Scholar] [CrossRef] [PubMed]
- Moyal, D. Need for a well-balanced sunscreen to protect human skin from both Ultraviolet A and Ultraviolet B damage. Indian J. Dermatol. Venereol. Leprol. 2012, 78, S24–S30. [Google Scholar] [CrossRef] [PubMed]
- Moyal, D.; Battie, C.; Tricaud, C. Prevention of Skin Pigmentation in Darker Skin: Experimental Evidence in India, Proceedings of Poster International Congress of Dermatology, New Delhi, India, 24–26 January 2013.
- Bernerd, F.; Moyal, D.; Pai, S.B.; Srinivas, C.R. Ultraviolet-induced skin damage and its prevention with sunscreen. In Basic Science for Modern Cosmetic Dermatology; Srinivas, C.R., Verschoore, M., Eds.; Jaypee Brothers Medical Publishers: New Dehli, India, 2014; p. 91. [Google Scholar]
- Diffey, B. Sunscreens: Expectation and realization. Photodermatol. Photoimmunol. Photomed. 2009, 25, 233–236. [Google Scholar] [CrossRef] [PubMed]
- Damour, O.; Augustin, C.; Black, A.F. Applications of reconstructed skin models in pharmaco-toxicological trials. Med. Biol. Eng. Comput. 1998, 36, 825–832. [Google Scholar] [CrossRef] [PubMed]
© 2014 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Marionnet, C.; Tricaud, C.; Bernerd, F. Exposure to Non-Extreme Solar UV Daylight: Spectral Characterization, Effects on Skin and Photoprotection. Int. J. Mol. Sci. 2015, 16, 68-90. https://doi.org/10.3390/ijms16010068
Marionnet C, Tricaud C, Bernerd F. Exposure to Non-Extreme Solar UV Daylight: Spectral Characterization, Effects on Skin and Photoprotection. International Journal of Molecular Sciences. 2015; 16(1):68-90. https://doi.org/10.3390/ijms16010068
Chicago/Turabian StyleMarionnet, Claire, Caroline Tricaud, and Françoise Bernerd. 2015. "Exposure to Non-Extreme Solar UV Daylight: Spectral Characterization, Effects on Skin and Photoprotection" International Journal of Molecular Sciences 16, no. 1: 68-90. https://doi.org/10.3390/ijms16010068
APA StyleMarionnet, C., Tricaud, C., & Bernerd, F. (2015). Exposure to Non-Extreme Solar UV Daylight: Spectral Characterization, Effects on Skin and Photoprotection. International Journal of Molecular Sciences, 16(1), 68-90. https://doi.org/10.3390/ijms16010068