The Formation of pH-Sensitive Wormlike Micelles in Ionic Liquids Driven by the Binding Ability of Anthranilic Acid
Abstract
:1. Introduction
2. Results and Discussion
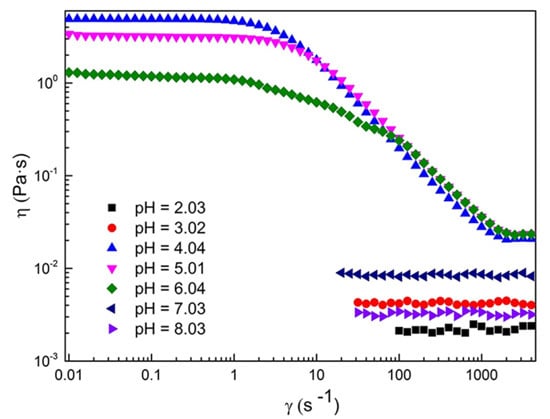
2.1. Rheological Properties of C16MPBr-AA Complex System




2.2. Morphological Variation of Self-Assembly Induced by pH


2.3. Possible Mechanism of the Morphological Variation Induced by pH


2.4. pH-Sensitive Ability of C16MPBr-AA Complex System

3. Materials and Methods
3.1. Materials

3.2. Rheological Measurements
3.3. Nuclear Magnetic Resonance (NMR) Spectroscopy
3.4. Dynamic Light Scattering (DLS) Measurements
3.5. Cryogenic-Transmission Electron Microscopy (Cryo-TEM)
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Whitesides, G.M.; Mathias, J.P.; Seto, C.T. Molecular self-assembly and nanochemistry: A chemical strategy for the synthesis of nanostructures. Science 1991, 254, 1312–1319. [Google Scholar] [CrossRef] [PubMed]
- Muhammad, N.; Gao, Y.; Khan, M.; Khan, I.; Rahim, A.; Lqbal, F.; Lqbal, J. Effect of ionic liquid on thermo-physical properties of bamboo biomass. Wood Sci. Technol. 2015, 49, 897–913. [Google Scholar] [CrossRef]
- Yan, H.; Long, Y.; Song, K.; Tung, C.H.; Zheng, L. Photo-induced transformation from wormlike to spherical micelles based on pyrrolidinium ionic liquids. Soft Matter 2014, 10, 115–121. [Google Scholar] [CrossRef] [PubMed]
- Zhao, M.; Yan, Z.; Dai, C.; Du, M.; Li, H.; Zhao, Y.; Ding, Q. Formation and rheological properties of wormlike micelles by N-hexadecyl-N-methylpiperidinium bromide and sodium salicylate. Colloid Polym. Sci. 2015, 293, 1073–1082. [Google Scholar] [CrossRef]
- Cheng, N.; Wang, P.; Sheng, T.; Bi, X.; Gong, Y.; Yu, L. Self-aggregation of new alkylcarboxylate-based anionic surface active ionic liquids: Experimental and theoretical investigations. J. Phys. Chem. B 2014, 118, 2758–2768. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Wei, X.; Liu, J.; Sun, J.; Du, P.; Ping, A. Study on the aqueous two-phase systems composed of surfactant, ionic liquid and water. Fluid Phase Equilib. 2013, 347, 1–7. [Google Scholar] [CrossRef]
- Feng, Y.; Chu, Z.; Dreiss, C.A. Other types of smart wormlike micelles. In SpringerBriefs in Molecular Science; Springer: Berlin, Germany, 2015. [Google Scholar]
- Shrestha, R.G.; Shrestha, L.K.; Aramaki, K. Formation of wormlike micelle in a mixed amino-acid based anionic surfactant and cationic surfactant systems. J. Colloid Interface Sci. 2007, 311, 276–284. [Google Scholar] [CrossRef] [PubMed]
- Zou, M.; Dong, J.; Yang, G.; Li, X. A Comprehensive study on micellization of dissymmetric pyrrolidinium headgroups based gemini surfactants. Phys. Chem. Chem. Phys. 2015, 17, 10265–10273. [Google Scholar] [CrossRef] [PubMed]
- Shrestha, R.G.; Rodriguez-Abreu, C.; Aramaki, K. Wormlike micelles in mixed amino acid surfactant/nonionic surfactant aqueous systems and the effect of added electrolytes. J. Oleo Sci. 2009, 58, 243–254. [Google Scholar] [CrossRef] [PubMed]
- Yu, H.; Shi, X.; Yu, P.; Zhou, J.; Zhang, Z.; Wu, H.; Li, Y. pH-Responsive wormlike micelles for intracellular delivery of hydrophobic drugs. J. Control. Release 2013, 172, e33–e34. [Google Scholar] [CrossRef]
- Shrestha, L.K.; Yamamoto, M.; Arima, S.; Aramaki, K. Charge-free reverse wormlike micelles in nonaqueous media. Langmuir 2011, 27, 2340–2348. [Google Scholar] [CrossRef] [PubMed]
- Yu, H.; Xu, Z.; Wang, D.; Chen, X.; Zhang, Z.; Yin, Q.; Li, Y. Intracellular pH-activated PEG-b-PDPA wormlike micelles for hydrophobic drug delivery. Polym. Chem. 2013, 4, 5052–5055. [Google Scholar] [CrossRef]
- Sakai, K.; Nomura, K.; Shrestha, R.G.; Endo, T.; Sakamoto, K.; Sakai, H.; Abe, M. Wormlike micelle formation by acylglutamic acid with alkylamines. Langmuir 2012, 28, 17617–17622. [Google Scholar] [CrossRef] [PubMed]
- Feng, Y.; Chu, Z.; Dreiss, C.A. Applications of smart wormlike micelles. In SpringerBriefs in Molecular Science; Springer: Berlin, Germany, 2015; pp. 79–91. [Google Scholar]
- Ramanathan, M.; Shrestha, L.K.; Mori, T.; Ji, Q.; Hill, J.P.; Ariga, K. Amphiphile nanoarchitectonics: From basic physical chemistry to advanced applications. Phys. Chem. Chem. Phys. 2013, 15, 10580–10611. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.; Han, X.; Huang, J.; Fu, H.; Yu, C. A facile route to design pH-responsive viscoelastic wormlike micelles: Smart use of hydrotropes. J. Colloid Interface Sci. 2009, 330, 449–455. [Google Scholar] [CrossRef] [PubMed]
- Plechkova, N.V.; Seddon, K.R. Applications of ionic liquids in the chemical industry. Chem. Soc. Rev. 2007, 37, 123–150. [Google Scholar] [CrossRef] [PubMed]
- Spenley, N.A.; Cates, M.E.; McLeish, T.C. Nonlinear rheology of wormlike micelles. Phys. Rev. Lett. 1993, 71, 939–942. [Google Scholar] [CrossRef] [PubMed]
- Candau, S.J.; Oda, R. Linear viscoelasticity of salt-free wormlike micellar solutions. Colloids Surf. A Physicochem. Eng. Asp. 2001, 183, 5–14. [Google Scholar] [CrossRef]
- Berret, J.F.; Appell, J.; Porte, G. Linear rheology of entangled wormlike micelles. Langmuir 1993, 9, 2851–2854. [Google Scholar] [CrossRef]
- Acharya, D.P.; Kunieda, H. Wormlike micelles in mixed surfactant solutions. Adv. Colloid Interface Sci. 2006, 123, 401–413. [Google Scholar] [CrossRef] [PubMed]
- Kern, F.; Lequeux, F.; Zana, R.; Candau, S.J. Dynamic properties of salt-free viscoelastic micellar solutions. Langmuir 1994, 10, 1714–1723. [Google Scholar] [CrossRef]
- Song, A.; Hao, J. Highly viscous wormlike micellar phases formed from the mixed AOT/C14DMAO/H2O system. J. Colloid Interface Sci. 2011, 353, 231–236. [Google Scholar] [CrossRef] [PubMed]
- Hassan, P.A.; Candau, S.J.; Kern, F.; Manohar, C. Rheology of wormlike micelles with varying hydrophobicity of the counterion. Langmuir 1998, 14, 6025–6029. [Google Scholar] [CrossRef]
- Dong, B.; Zhang, J.; Zheng, L.; Wang, S.; Li, X.; Inoue, T. Salt-induced viscoelastic wormlike micelles formed in surface active ionic liquid aqueous solution. J. Colloid Interface Sci. 2008, 319, 338–343. [Google Scholar] [CrossRef] [PubMed]
- Shikata, T.; Hirata, H.; Kotaka, T. Micelle formation of detergent molecules in aqueous media. 2. Role of free salicylate ions on viscoelastic properties of aqueous cetyltrimethylammonium bromide-sodium salicylate solutions. Langmuir 2002, 4, 354–359. [Google Scholar] [CrossRef]
- Bachofer, S.J.; Simonis, U.; Nowicki, T.A. Orientational binding of substituted naphthoate counterions to the tetradecyltrimethylammonium bromide micellar interface. J. Phys. Chem. 1991, 95, 480–488. [Google Scholar] [CrossRef]
- Shikata, T.; Hirata, H.; Kotaka, T. Micelle formation of detergent molecules in aqueous media: Viscoelastic properties of aqueous cetyltrimethylammonium bromide solutions. Langmuir 1987, 3, 1081–1086. [Google Scholar] [CrossRef]
- Zhao, M.; Gao, Y.; Zheng, L. Liquid crystalline phases of the amphiphilic ionic liquid N-hexadecyl-N-methylpyrrolidinium bromide formed in the ionic liquid ethylammonium nitrate and in water. J. Phys. Chem. B 2010, 114, 11382–11389. [Google Scholar] [CrossRef] [PubMed]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
You, Q.; Zhang, Y.; Wang, H.; Fan, H.; Guo, J.; Li, M. The Formation of pH-Sensitive Wormlike Micelles in Ionic Liquids Driven by the Binding Ability of Anthranilic Acid. Int. J. Mol. Sci. 2015, 16, 28146-28155. https://doi.org/10.3390/ijms161226096
You Q, Zhang Y, Wang H, Fan H, Guo J, Li M. The Formation of pH-Sensitive Wormlike Micelles in Ionic Liquids Driven by the Binding Ability of Anthranilic Acid. International Journal of Molecular Sciences. 2015; 16(12):28146-28155. https://doi.org/10.3390/ijms161226096
Chicago/Turabian StyleYou, Qing, Yan Zhang, Huan Wang, Hongfu Fan, Jianping Guo, and Ming Li. 2015. "The Formation of pH-Sensitive Wormlike Micelles in Ionic Liquids Driven by the Binding Ability of Anthranilic Acid" International Journal of Molecular Sciences 16, no. 12: 28146-28155. https://doi.org/10.3390/ijms161226096
APA StyleYou, Q., Zhang, Y., Wang, H., Fan, H., Guo, J., & Li, M. (2015). The Formation of pH-Sensitive Wormlike Micelles in Ionic Liquids Driven by the Binding Ability of Anthranilic Acid. International Journal of Molecular Sciences, 16(12), 28146-28155. https://doi.org/10.3390/ijms161226096