2.1. Head Regeneration Is Temperature—But Not Age-Dependent
While the age of adult
Nematostella cannot yet be determined at a molecular or cellular level, one can distinguish juvenile and adult polyps from their anatomy (
Figure 1). Previous studies describing regeneration in
Nematostella have been carried out either in juveniles [
7,
34] or in adults [
12,
29,
30,
31,
32,
33,
35]. However, it is not known if the regenerative capacities/mechanisms are conserved between the two. In order to determine if the timing of oral regeneration in
Nematostella is age-dependent, we performed head amputation (bisection under the pharyngeal region; see red dotted line in
Figure 1a) experiments in
Nematostella juveniles and adults while following the timing of regeneration. This sub-pharyngeal amputation site was used in all of the following amputation experiments performed in this study. We analyzed the morphology of the regenerating animals at 22 °C (temperature used in [
7]) each day for one week. We observed that both juveniles (20 out of 20 cases) as well as adults (10 out of 10 cases) regenerate with a similar timing (
Figure 2).
We observed a specific sequence of events during the regeneration process (
Figure 2). First, the mesenteries come into tight contact with the amputation site 24–48 h post-amputation (hpa, only observable in transparent juveniles). Next, at 72–96 hpa the tentacle bulbs become visible in juveniles and adults. Finally, there is a progressive elongation and formation of those structures up to 144 hpa (
Figure 2). Interestingly, we also observed that the number of new regenerating tentacles corresponds to the number of tentacles present in the polyp before amputation. The four-tentacle juveniles regenerate four new tentacles and the 12+ tentacle adults regenerate 12+ new tentacles (
Figure 2, 168 hpa).
In order to investigate if oral regeneration following sub-pharyngeal amputation is temperature-dependent as a function of age, we performed sub-pharyngeal bisections in
Nematostella juveniles or adults and left them to regenerate at 16 °C (
Supplementary Figure S2A,B). The timing of oral regeneration for both juveniles (20 out of 20 cases,
Supplementary Figure S2A) and adults (10 out of 10 cases,
Figure S2B) is considerably slowed at 16 °C compared to 22 °C. In particular, the appearance of the tentacle bulbs is delayed by approximately 48–72 h in animals that regenerated at 16 °C. This observation is in line with a previous report that showed that the timing of regeneration for the isolated adult physa is temperature-dependent [
30]. All following experiments were carried out at 22 °C for consistency and to be able to compare our findings to those reported by Passamaneck and Martindale and Bossert and colleagues [
7,
30].
Figure 2.
Timing of oral regeneration is similar in juveniles and adults. Comparison of the duration of oral regeneration between six-week-old juveniles (upper panel—four tentacles) and adults (bottom panel—12 tentacles). From left to right: regenerating polyps at 22 °C 24 h post-amputation (hpa), 48, 72, 96, 120, 144 and 168 hpa. At 72 hpa, in both juvenile and adult polyps, the tentacles bulbs are clearly visible (white arrows), and the pharynx starts to form in some individuals (only visible in transparent juveniles). Five days post-amputation (120 hpa), the juvenile and adult polyps are regenerated as indicated by the presence of the pharynx and elongated tentacles. The white asterisk at 168 hpa indicates the tentacles: four on the regenerating juvenile and 12 on the regenerating adult polyp.
Figure 2.
Timing of oral regeneration is similar in juveniles and adults. Comparison of the duration of oral regeneration between six-week-old juveniles (upper panel—four tentacles) and adults (bottom panel—12 tentacles). From left to right: regenerating polyps at 22 °C 24 h post-amputation (hpa), 48, 72, 96, 120, 144 and 168 hpa. At 72 hpa, in both juvenile and adult polyps, the tentacles bulbs are clearly visible (white arrows), and the pharynx starts to form in some individuals (only visible in transparent juveniles). Five days post-amputation (120 hpa), the juvenile and adult polyps are regenerated as indicated by the presence of the pharynx and elongated tentacles. The white asterisk at 168 hpa indicates the tentacles: four on the regenerating juvenile and 12 on the regenerating adult polyp.
2.2. Cell Proliferation Is Required during Head Regeneration in Adult Tissue
In
Nematostella, cell proliferation is required for head regeneration in juveniles [
7]. In order to determine the earliest time-point where this cellular proliferation is detectable, we performed EdU labeling at 1.30, 6, 12, 24, and 48 hpa. We found that while no staining is detectable at the two earliest time-points (data not shown), clear cellular proliferation is observed at 12 hpa at the amputation site (
Figure 3Aa).
Cellular proliferation increases massively in this region between 24 hpa and 48 hpa, confirming a previous report (
Figure 3Ab) [
7]. Interestingly, and in contrast with previous observations, EdU-positive cells were not only detected in the ectodermal epithelium and the gastrodermis at the amputation site, but also in the oral-most regions of the mesenteries (
Figure 3Ab).
Figure 3.
(A) Localized cellular proliferation in juveniles begins at 12 hpa at the amputation site. Overlapping confocal images in which the nuclei (DNA, cyan) were stained using DAPI and proliferating cells (red) were marked using an EdU labeling kit (Aa,Ab). Oral parts of the regenerating juveniles at 12 hpa (Aa) and 48 hpa (Ab). All animals are oriented with the amputation site to the top. The white arrow in (b) shows the presence of dividing cells in the most oral part of the mesentery tissues. Number of cases for the representative phenotype are in white at the bottom right of each image. m, mesentery; (B) Cell proliferation is necessary for adult tissue regeneration. Cell division is present during regeneration in adults after sub-pharyngeal amputation (Ba–Bb’). (Ba–Bb’) Confocal stack images in which the DNA (nucleus) is labeled with DAPI (cyan) and proliferating cells (white) were marked using an EdU labeling kit. (Bc,Bc’) Blocking cell proliferation using hydroxyurea (HU) blocks regeneration in the adult amputated polyps (c’) contrary to the regenerated untreated control (c). Number of cases for the representative phenotype are in white at the bottom right of each images (Ba,Bb,Bc,Bc’). Scale bar in (Ab) is 20 μm and applies to (Aa). Scale bar in (Ba’) is 50 μm and applies to (Ba,Bb,Bb’). Scale bar in (Bc’) is 1 mm and applies to (Bc).
Figure 3.
(A) Localized cellular proliferation in juveniles begins at 12 hpa at the amputation site. Overlapping confocal images in which the nuclei (DNA, cyan) were stained using DAPI and proliferating cells (red) were marked using an EdU labeling kit (Aa,Ab). Oral parts of the regenerating juveniles at 12 hpa (Aa) and 48 hpa (Ab). All animals are oriented with the amputation site to the top. The white arrow in (b) shows the presence of dividing cells in the most oral part of the mesentery tissues. Number of cases for the representative phenotype are in white at the bottom right of each image. m, mesentery; (B) Cell proliferation is necessary for adult tissue regeneration. Cell division is present during regeneration in adults after sub-pharyngeal amputation (Ba–Bb’). (Ba–Bb’) Confocal stack images in which the DNA (nucleus) is labeled with DAPI (cyan) and proliferating cells (white) were marked using an EdU labeling kit. (Bc,Bc’) Blocking cell proliferation using hydroxyurea (HU) blocks regeneration in the adult amputated polyps (c’) contrary to the regenerated untreated control (c). Number of cases for the representative phenotype are in white at the bottom right of each images (Ba,Bb,Bc,Bc’). Scale bar in (Ab) is 20 μm and applies to (Aa). Scale bar in (Ba’) is 50 μm and applies to (Ba,Bb,Bb’). Scale bar in (Bc’) is 1 mm and applies to (Bc).
![Ijms 16 26100 g003a]()
![Ijms 16 26100 g003b]()
In order to investigate if cell proliferation is also detectable during adult regeneration [
7], we performed EdU staining on adult polyps 48 h after sub-pharyngeal amputation (
Figure 3(Ba–Bb’)). While no EdU-positive cells are detected in aboral tissues (
Figure 3(Bb,Bb’)), cellular proliferation is clearly visible at the amputation site in the adult tissue at 48 hpa (
Figure 3(Ba,Ba’)). We further tested if cellular proliferation, similarly to juveniles [
7], is required for adult regeneration. To do this we used the pharmaceutical cellular proliferation inhibitor hydroxyurea (HU). Continuous treatment of HU for six days post-amputation blocks oral regeneration in the adult polyp (
Figure 3(Bc,Bc’)). Thus, cell division is also required for oral regeneration in adult
Nematostella. 2.3. Wound Healing Occurs at 6 HPA
A recent report analyzed
Nematostella wound healing after puncturing the body column with a glass needle [
34]. The authors found that this process is visually completed after approximately 4 h (25 °C). In order to determine exactly when wound healing occurs in
Nematostella juveniles, we performed DIC and confocal imaging on regenerating juveniles following sub-pharyngeal amputation at several time-points. While in some cases we were able to visualize a clear opening using DIC optics (
Figure 4Aa), the three-dimensional folding and contraction of the tissues at the amputation site often made it hard to distinguish between a real wound or a depression that looked like an open wound (
Figure 4(Ab–Ae)). Thus, determining the wound closure simply by imaging proves to be difficult. In addition, using classical staining/imaging approaches we are unable to distinguish between a closed wound or contraction of the surrounding myo-epithelia.
Figure 4.
(A) Wound closure. Oral opening during Nematostella regeneration. Example of DIC images of the oral part, oral view (ov), at the amputation site of an example of the opening at 2 hpa (white arrows in (Aa)) or the closed wound at 6 hpa (white arrows in (Ab)); (Ac–Ae) are confocal images of the oral-most part of the same polyp in lateral view (lv) or details of the oral view (Ad,Ae); (Ad) (yellow frame) or (Ae) (green frame) correspond to the double yellow or green arrow slice, respectively, in (c); Because of the folding that occurs at the amputation site during the first hours of regeneration, the dynamics of the wound healing are hard to assess in DIC optic or confocal images. Scale bars are 20 μm in (Aa–Ac) and 10 μm in (Ad,Ae); (B) Diagram of the compression assay during regeneration. The purple dots represent the nematosomes. The red dotted line represents the amputation site. The forceps are laterally compressing the regenerating polyp body; (C) Time series of the compression assay in an opened (Ca–Cc) or a wound-closed (Cd–Cf) polyp. The dotted double arrow in (Ca) indicates the axial orientation of the animals shown in (Ca–Cf). O, Oral; AbO, Aboral.
Figure 4.
(A) Wound closure. Oral opening during Nematostella regeneration. Example of DIC images of the oral part, oral view (ov), at the amputation site of an example of the opening at 2 hpa (white arrows in (Aa)) or the closed wound at 6 hpa (white arrows in (Ab)); (Ac–Ae) are confocal images of the oral-most part of the same polyp in lateral view (lv) or details of the oral view (Ad,Ae); (Ad) (yellow frame) or (Ae) (green frame) correspond to the double yellow or green arrow slice, respectively, in (c); Because of the folding that occurs at the amputation site during the first hours of regeneration, the dynamics of the wound healing are hard to assess in DIC optic or confocal images. Scale bars are 20 μm in (Aa–Ac) and 10 μm in (Ad,Ae); (B) Diagram of the compression assay during regeneration. The purple dots represent the nematosomes. The red dotted line represents the amputation site. The forceps are laterally compressing the regenerating polyp body; (C) Time series of the compression assay in an opened (Ca–Cc) or a wound-closed (Cd–Cf) polyp. The dotted double arrow in (Ca) indicates the axial orientation of the animals shown in (Ca–Cf). O, Oral; AbO, Aboral.
![Ijms 16 26100 g004]()
In order to have a more robust way to address wound healing after sub-pharyngeal amputation, we developed a compression assay to assess the state of the opening at the amputation site. This assay uses nematosomes (
Supplementary Figure S3) as a marker to follow the fluid dynamics present in the gastric cavity of
Nematostella (
Figure 4B,C).
Nematosomes are cellular aggregates formed by cnidocytes that are freely circulating within the
Nematostella body [
36]. When compressing a juvenile with an open wound, the nematosomes will be expelled at the amputation site. On the contrary, when the wound is closed, the nematosomes will either remain in the gastric cavity or leak out of the body cavity through the aboral pore. This pore is an opening with an unknown function located at the aboral-most region of the body (
Supplementary Figure S4) [
37]. We assume that nematosomes will exit the body cavity through the wound or the aboral pore depending on the wound healing status. To use this wound healing assay, we compressed the body column of sub-pharyngeal amputated juveniles at 0, 1, 2, 4, 6, 12 hpa and followed the behavior of the nematosomes (
Figure 5).
Figure 5.
Wound healing assay.
Figure 5.
Wound healing assay.
Interestingly, at 1 and 2 hpa in the majority of cases (90%
n = 31 and 71%
n = 24, respectively), the nematosomes are forced to exit the body cavity through the amputation site (
Figure 5), showing that the wound is not healed yet. However, at 4 hpa, the majority of the juveniles (67%, 14 out of 21 cases) exhibited nematosomes leaking through the aboral pore. This is the case for 100% of juveniles (22 out of 22 cases) at 6 hpa and later time-points (
Figure 5). Thus, in
Nematostella, the wound is closed between 4 and 6 hpa following a sub-pharyngeal amputation.
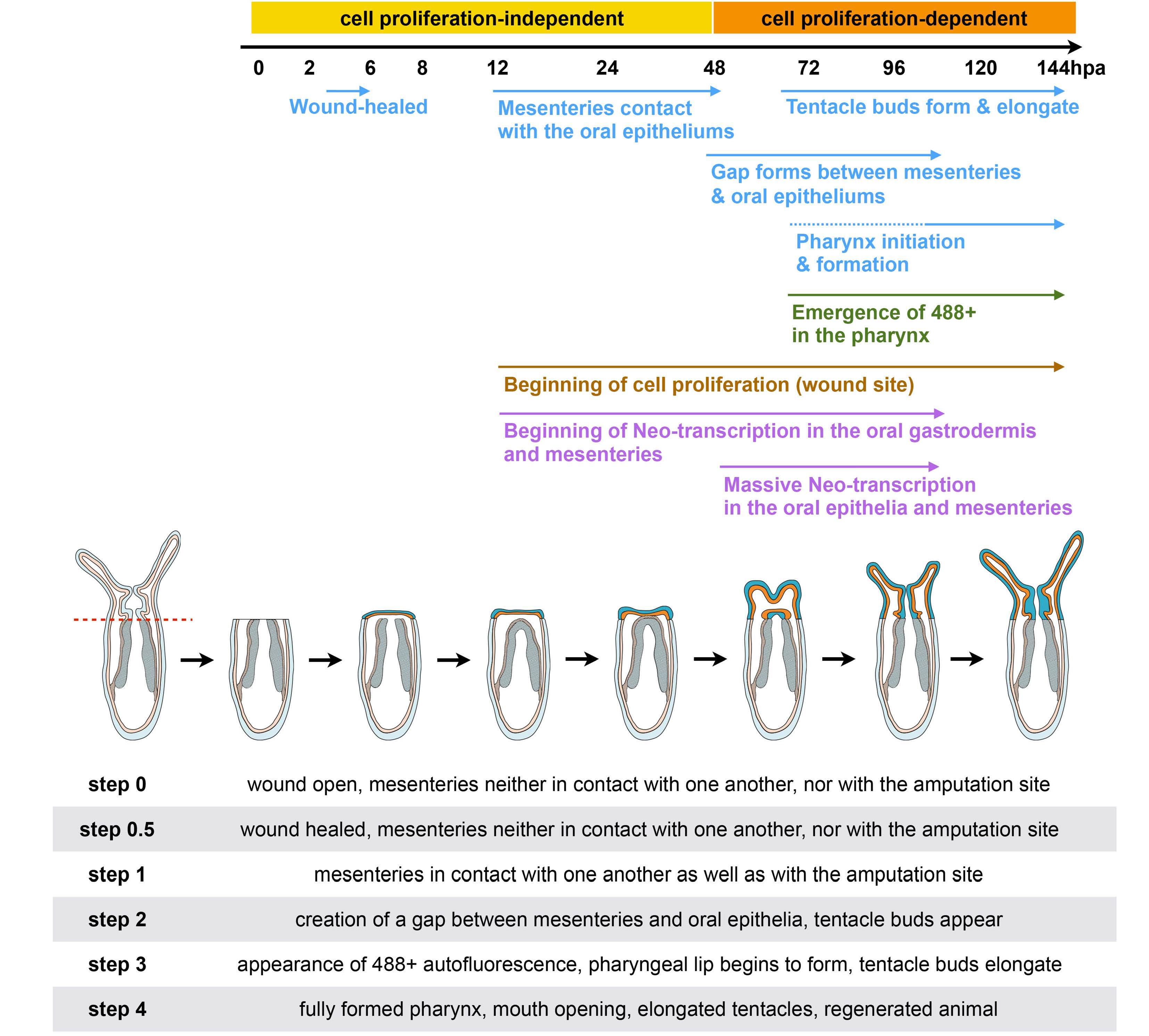
2.4. Mesentery Behavior and Pharynx Formation as Specific Landmarks for Oral Regeneration
The size and transparency of juvenile
Nematostella make them more amenable for detailed imaging experiments than adults. We thus analyzed in detail the tissue behavior during oral regeneration of juveniles. We performed DNA labeling on regenerating juveniles following sub-pharyngeal amputation and observed the sequential events every 12 h from 0 to 144 hpa using confocal imaging (
Figure 6).
Focusing on the behavior of the mesenteries and the pharynx reformation, we distinguish four main characteristic features: (Step 0) 0–12 hpa, no contact between the remaining mesenteries and the surrounding oral epithelia (
Figure 6a); (Step 1) 12–48 hpa, contact of the remaining mesenteries between each other at their most oral site and with the surrounding epithelia at the amputation site (
Figure 6b); (Step 2) 60–96 hpa, emergence of a space between the mesenteries and the epithelia at the amputation site (
Figure 6c). The epithelia of the amputation site seems dragged down towards the aboral region by the remaining mesenteries. This accentuates the protrusion of the developing tentacle bulbs; (Step 3) 72–120 hpa, the pharyngeal lip (basal part of the future pharynx) forms (
Figure 6d). Interestingly, the pharyngeal lip appears to develop first from the oral-most part of the remaining mesenteries; (Step 4) 96–144 hpa, the pharynx is fully regenerated and the tentacles elongate (
Figure 6e). Subsequently, the upper part of the pharynx forms (Step 4) progressively in the space that was previously created (
Figure 6e) and corresponds to a highly proliferative region (
Figure 3Ab).
Figure 6.
Dynamics of the oral tissue during regeneration. The dynamic behavior of the oral tissue during regeneration was analyzed using confocal microscopy. (a–e) Confocal image stacks in which the DNA (nucleus) is labeled with DAPI (cyan). Five main phenotypes were observed between 0 and 144 hpa and are represented in this figure: (Step 0) the remaining parts of the mesenteries are separated together and from the epithelia at the amputation site (white dashed circle) (a); (Step 1) the remaining parts of the mesenteries are fused together and are in tight contact with the epithelia at the amputation site (white dashed circle) (b); (Step 2) an empty space forms between the remaining part of the mesenteries and the amputation site (white dashed circle) (c); (Step 3) the pharyngeal lip forms at the oral-most region of the remaining mesenteries, and the empty space becomes filled with nuclei (white dashed circle) (d); (Step 4) the pharynx is fully formed at the oral-most region of the remaining mesenteries (e). Numbers at the bottom of the image panel indicate the total number of analyzed specimens at 12, 24, 48, 72, 96, 120, 144 hpa and the number of cases representative of one of the described five steps in relation to the regeneration time in hours post-amputation (hpa). The amputation site is represented by a red dashed line in (a). Tentacle bulbs and elongated ones are shown by the yellow arrowhead (c–e). The white asterisk is the mouth opening (e). m, mesentery; pha lip, pharyngeal lip; pha, pharynx. Scale bar in (a) is 20 μm and applies to (b–e).
Figure 6.
Dynamics of the oral tissue during regeneration. The dynamic behavior of the oral tissue during regeneration was analyzed using confocal microscopy. (a–e) Confocal image stacks in which the DNA (nucleus) is labeled with DAPI (cyan). Five main phenotypes were observed between 0 and 144 hpa and are represented in this figure: (Step 0) the remaining parts of the mesenteries are separated together and from the epithelia at the amputation site (white dashed circle) (a); (Step 1) the remaining parts of the mesenteries are fused together and are in tight contact with the epithelia at the amputation site (white dashed circle) (b); (Step 2) an empty space forms between the remaining part of the mesenteries and the amputation site (white dashed circle) (c); (Step 3) the pharyngeal lip forms at the oral-most region of the remaining mesenteries, and the empty space becomes filled with nuclei (white dashed circle) (d); (Step 4) the pharynx is fully formed at the oral-most region of the remaining mesenteries (e). Numbers at the bottom of the image panel indicate the total number of analyzed specimens at 12, 24, 48, 72, 96, 120, 144 hpa and the number of cases representative of one of the described five steps in relation to the regeneration time in hours post-amputation (hpa). The amputation site is represented by a red dashed line in (a). Tentacle bulbs and elongated ones are shown by the yellow arrowhead (c–e). The white asterisk is the mouth opening (e). m, mesentery; pha lip, pharyngeal lip; pha, pharynx. Scale bar in (a) is 20 μm and applies to (b–e).
![Ijms 16 26100 g006]()
2.5. Fluorescence in the Pharyngeal Region as a Landmark for Pharynx Reformation
Nematostella, like other cnidarians, possesses endogenous fluorescence emitted by fluorescent proteins and/or fluorescence of the tissues. The six-week-old
Nematostella juveniles display a green (excitation wave length at 488 nm) fluorescence (henceforth referred to as 488+) from a currently unknown origin (
Figure 7A).
Figure 7.
(A) Image of 488+ detection in the pharynx. Fed (a,a’), starved (b–c’), regenerating 72 hpa (d–e’) Nematostella polyp juveniles. DIC optic images (a–d). Epifluorescent images (a’–d’). The red dotted line labels the amputation site under the pharynx (c–d’). The green line in (c,c’) shows the 488+ fluorescence localized in the basal part of the pharynx. The area delimitated with the dotted line in d and d’ is the region where the 488+ re-emerged in the polyp at 72 hpa. (e,e’) Confocal images at 72 hpa labeled for DNA (nuclei in cyan) on the 488-negative (e) and 488-positive (e’) polyp juvenile. The area delimitated with the white dotted line in (e’) (488+ regenerating polyps) shows the pharyngeal lip/pharynx in formation that is absent from the 488-negative regenerating polyps in which only the contact between the two mesenteries is visible. pha, pharynx; m, mesentery. Scale bar in (Aa’) is 20 μm and applies to (Aa–Ae,Ab’–Ae’); (B) Biosorter. The dot plot in (a,b) contains the sorting results of the animal by density (Extension) vs. mass (Time Of Flight—TOF) or by 488 fluorescence intensity (Green) vs. density (Extension), respectively, using a Biosorter system. (c,d) Examples of the Biosorter profiles (signal vs. length) of the bright fluorescent group (Bright in b, which corresponds to the Alive group in (a)) or the non-fluorescent group (unlabeled group localized at the bottom left of the two dot plots (a,b)). O, Oral; AbO, Aboral. Scale bar is 100 μm in (Bc).
Figure 7.
(A) Image of 488+ detection in the pharynx. Fed (a,a’), starved (b–c’), regenerating 72 hpa (d–e’) Nematostella polyp juveniles. DIC optic images (a–d). Epifluorescent images (a’–d’). The red dotted line labels the amputation site under the pharynx (c–d’). The green line in (c,c’) shows the 488+ fluorescence localized in the basal part of the pharynx. The area delimitated with the dotted line in d and d’ is the region where the 488+ re-emerged in the polyp at 72 hpa. (e,e’) Confocal images at 72 hpa labeled for DNA (nuclei in cyan) on the 488-negative (e) and 488-positive (e’) polyp juvenile. The area delimitated with the white dotted line in (e’) (488+ regenerating polyps) shows the pharyngeal lip/pharynx in formation that is absent from the 488-negative regenerating polyps in which only the contact between the two mesenteries is visible. pha, pharynx; m, mesentery. Scale bar in (Aa’) is 20 μm and applies to (Aa–Ae,Ab’–Ae’); (B) Biosorter. The dot plot in (a,b) contains the sorting results of the animal by density (Extension) vs. mass (Time Of Flight—TOF) or by 488 fluorescence intensity (Green) vs. density (Extension), respectively, using a Biosorter system. (c,d) Examples of the Biosorter profiles (signal vs. length) of the bright fluorescent group (Bright in b, which corresponds to the Alive group in (a)) or the non-fluorescent group (unlabeled group localized at the bottom left of the two dot plots (a,b)). O, Oral; AbO, Aboral. Scale bar is 100 μm in (Bc).
![Ijms 16 26100 g007a]()
![Ijms 16 26100 g007b]()
The 488+ is randomly distributed throughout the entire body in freshly fed animals (
Figure 7(Aa,Aa’)). Interestingly, it became more and more localized to the pharynx when juvenile polyps were starved for one or two weeks (
Figure 7(Ab,Ab’,Ac,Ac’)), suggesting a correlation of this staining with a metabolic state of the animals. The varied metabolic states within one batch of animals could explain the asynchrony of regeneration. It may also contribute to differences in the cell proliferation rate observed between animals (see table below
Figure 6; Röttinger, unpublished data). After sub-pharyngeal amputation, we observed that the 488+ re-emerges in the regenerating polyp in nearly half of the cases around 72 hpa (
Table 1).
Table 1.
The 488+ in hand-sorted regenerating polyps. Counting of the 488+ polyp juveniles at 24, 48, and 72 hpa. Two different experimenters performed blind counts. Hand-sorter 1 indicates the first experimenter and Hand-sorter 2 the second.
Table 1.
The 488+ in hand-sorted regenerating polyps. Counting of the 488+ polyp juveniles at 24, 48, and 72 hpa. Two different experimenters performed blind counts. Hand-sorter 1 indicates the first experimenter and Hand-sorter 2 the second.
| – | 488 | 24 hpa | 48 hpa | 72 hpa |
|---|
| Hand-sorted | + | 0/41 | 0% | 4/66 | 6% | 44/77 | 57% |
| − | 41/41 | 100% | 62/66 | 94% | 33/77 | 43% |
| Bio-sorted | + | 3/41 | 7% | 2/66 | 3% | 31/77 | 40% |
| − | 38/41 | 93% | 64/66 | 97% | 46/77 | 60% |
We thus used the localized green fluorescence in the pharynx of the uncut animal as a proxy to harmonize a batch of polyps before a series of cutting experiments. Sorting batches of uncut animals was performed using a large particle flow cytometer (Biosorter system, Union Biometrica) that enables the analysis of animals based on their length (time of flight, TOF), density (extinction), morphology (profiler), fluorescence, and the relative localization of the fluorescence along the animal body. In order to analyze the global amount of fluorescence intensity and its localization within the animals, we first defined debris (41% of the population;
n = 264) based on morphology parameters (TOF and extinction parameters of each polyp; dot plot
Figure 7Ba). We then measured the 488+ fluorescence intensity (the mean of fluorescence intensity is
mfi = 4961.1) within the same batch (59% of the population;
n = 264; dot plot
Figure 7Bb). This 488+ can be localized within the profile of the animal using the Profiler software (Profile
Figure 7Bc). Strikingly, the highest amount of 488+ fluorescence is localized in the region of the polyp where the pharynx is supposed to be.
In order to sort them in an automatic manner with the Biosorter, we identified a profile of interest (
Figure 7Bc). We then bio-sorted 84 juveniles with this selected profile, performed sub-pharyngeal amputation, and followed their regeneration. In parallel, we hand-sorted 31 animals that size-matched and appeared in good condition without the use of the green auto-fluorescence proxy. All animals were cut below the pharynx, removing the 488+ at 0 hpa. Animals were then placed back into culturing conditions and the re-emergence of the 488+ was assessed in the regenerating polyps from hand-sorted
vs. bio-sorted batches (
Table 2).
Table 2.
Emergence of the 488+ fluorescence during oral regeneration in the hand-sorted versus bio-sorted polyps at 72 and 120 hpa.
Table 2.
Emergence of the 488+ fluorescence during oral regeneration in the hand-sorted versus bio-sorted polyps at 72 and 120 hpa.
| – | 488 | 72 hpa | 120 hpa |
|---|
| Hand-sorted | + | 14/33 | 42% | 18/31 | 58% |
| − | 19/33 | 58% | 13/31 | 42% |
| Bio-sorted | + | 38/84 | 45% | 51/84 | 61% |
| − | 46/84 | 55% | 33/84 | 39% |
Interestingly, both batches, hand-sorted
vs. bio-sorted, displayed a similar heterogeneity in their individual advancement through the regeneration steps as reflected by the numbers of 488+
vs. 488− polyps in each batch of animals (
Table 2). While the 488+-based selection did not yield a better or more synchronous regeneration, this experiment shows that the Biosorter system can be used as a tool to physically sort animals with precise criteria and/or analyze them for a specific phenotype in large-scale experiments in an unbiased manner. These data also show that the viability of the animals and their regeneration rate are not affected by the Biosorter system.
In order to determine if a correlation exists between the emergence of the 488+ and any previously described steps of regeneration, we sub-pharyngeally bisected juveniles and analyzed the phenotypes in detail of the 488+ and 488− polyps at 72 hpa. We observed that the 488+ starts to emerge in the oral-most part of the remaining mesenteries around 72 hpa (
Figure 7(Ad,Ad’)). Using confocal imaging on DAPI-stained (nucleus) animals we analyzed the detailed morphology of 488− and 488+ regenerating juveniles at 72 hpa. We observed that 488− are mainly at Step 2 (11 out of 12 cases) of the oral regeneration staging system described in
Figure 6 and
Figure 7Ae. No pharynx in formation is visible. Interestingly, the 488+ are mainly at Step 3 or 4 (10 out of 12 cases), with a clear pharyngeal lip or pharynx in formation (
Figure 7Ae’). These observations show a strong correlation between the initiation of pharynx formation (Steps 3 and 4) and the presence of 488+ in the regenerating juvenile after sub-pharyngeal amputation.
Figure 8.
Tissue tracking experiment during regeneration. Spectral confocal images of Nematostella juveniles expressing Kaede photoconvertible fluorescent protein mRNA (a–c’). The Keade photoconverted region is represented in magenta and the non-photoconverted region in grey. Endogenous fluorescence is shown in turquoise to help visualize the morphology of the polyp (a’–c’); At 24 hpa, regions of interest (the epithelium central to the amputation site (a); the epithelium lateral to the amputation site (b), or the oral tip of the mesenteries (c)) were exposed to a UV laser resulting in permanent photo-conversion of the Kaede protein (magenta); The photoconversion at the central epithelium reveals integration of the converted patch (white arrows in (a’)) into the tentacles and most oral tip of the pharynx (white dotted line); Photoconversion of the lateral epithelium shows that this tissue remains in place during regeneration (white arrow in (b’)) or is incorporated into the adjacent tentacles (white arrows in (b’’)); The photoconversion of the oral tip of the mesentery results in integration of the converted patch (white arrows in (c’)) into the pharynx (white dotted line). Scale bar is 20 μm in (a).
Figure 8.
Tissue tracking experiment during regeneration. Spectral confocal images of Nematostella juveniles expressing Kaede photoconvertible fluorescent protein mRNA (a–c’). The Keade photoconverted region is represented in magenta and the non-photoconverted region in grey. Endogenous fluorescence is shown in turquoise to help visualize the morphology of the polyp (a’–c’); At 24 hpa, regions of interest (the epithelium central to the amputation site (a); the epithelium lateral to the amputation site (b), or the oral tip of the mesenteries (c)) were exposed to a UV laser resulting in permanent photo-conversion of the Kaede protein (magenta); The photoconversion at the central epithelium reveals integration of the converted patch (white arrows in (a’)) into the tentacles and most oral tip of the pharynx (white dotted line); Photoconversion of the lateral epithelium shows that this tissue remains in place during regeneration (white arrow in (b’)) or is incorporated into the adjacent tentacles (white arrows in (b’’)); The photoconversion of the oral tip of the mesentery results in integration of the converted patch (white arrows in (c’)) into the pharynx (white dotted line). Scale bar is 20 μm in (a).
![Ijms 16 26100 g008]()
2.6. The Regenerating Pharynx Forms from the Oral Part of the Remaining Mesenteries
Taken together, our observations of the sequential events (
Figure 6) and the 488+ emergence (
Figure 7A) during regeneration led us to hypothesize that the regenerating pharynx after sub-pharyngeal amputation may come from the oral-most part of the remaining mesenteries. To test this hypothesis, we performed
in vivo tissue tracking experiments in juveniles overexpressing
Kaede mRNA. KAEDE is a fluorescent protein that can be permanently photoconverted from green to red by exposing the expressing cells of interest to UV light [
38]. For our tissue tracking experiments, we photoconverted three distinct regions of interest at 24 hpa: (1) the epithelia central to the amputation site (
Figure 8a); (2) the epithelia lateral to the amputation site (
Figure 8b); or (3) the oral tip of the mesenteries (
Figure 8c).
It is important to note that when we convert the oral tip of the mesenteries, the laser must pass through the lateral epithelia and this region is also converted. After conversion we analyzed the location of the converted red fluorescence at eight days post-amputation (dpa) using spectral confocal imaging (see materials and methods). Spectral imaging measures the complete fluorescent spectrum of each pixel and matches these to pre-calibrated profiles. In our experiments we calibrated the profiles to detect converted Kaede, unconverted Kaede, and endogenous fluorescence. We found that the central epithelia of the amputation site gave rise to the tentacles in 19 out of 19 cases (
Figure 8a,a’). Additionally, this region also gave rise to the mouth (oral-most part of the pharynx) in 15 out of 19 cases (four cases were undetermined) (
Figure 8a,a’). The lateral epithelia remained in the lateral tissues after regeneration in four out of 11 cases and in the tentacles in seven out of 11 cases (
Figure 8b,b’,b’’). In neither central epithelia nor lateral epithelia conversions did we observe converted cells contributing to the pharynx. In the case of photoconverted mesenteries we observed converted KAEDE-expressing tissues in the newly formed pharynx in seven out of seven cases (
Figure 8c,c’;
Supplementary Figure S5). These animals also displayed converted tissues in their lateral epithelia and/or tentacles corresponding to the point of the laser entry during the conversion process. Since the lateral tissue remains in the lateral regions or ends up in the tentacles during regeneration but not the pharynx, we conclude that the converted tissue observed in the pharynx is indeed from the oral tip of the mesenteries, confirming our initial hypothesis (
Figure 8c,c’;
Supplementary Figure S5).
2.7. De Novo Transcription Is Induced First in the Gastrodermis at the Amputation Site
In order to reform missing body parts, an injured organism requires rapid activation of rRNA and tRNA transcription for proper protein biosynthesis of existing or new transcribed mRNA, as well as for cellular proliferation [
39,
40]. To characterize the transcription in
Nematostella, we used EU-Click-it chemistry (Life Technologies, Carlsbad, CA, USA) to detect
de novo transcription in
Nematostella after amputation (
Figure 9).
Figure 9.
De novo transcription at the amputation site of the regenerating polyp. Overlap of confocal images showing
de novo transcription (EU) in red and nucleus (DNA) staining in cyan in the oral epitheliums (
b–
f) and gastric cavity (
b’–
f’) of the amputated juvenile polyp. The uncut control is in (
a–
a’). The white arrows in
a,
b,
b’,
c,
f show the cells that are undergoing
de novo transcription. The white dotted lines in (
c’–
f’) show the regions in the body gastric cavity that are undergoing massive
de novo transcription, the oral part of the mesenteries, and the epitheliums. All animals are oriented with the amputation site to the top. Scale bar in (
a) is 20 μm and applies to all
Figure 9 and
Supplementary Figure S6.
Figure 9.
De novo transcription at the amputation site of the regenerating polyp. Overlap of confocal images showing
de novo transcription (EU) in red and nucleus (DNA) staining in cyan in the oral epitheliums (
b–
f) and gastric cavity (
b’–
f’) of the amputated juvenile polyp. The uncut control is in (
a–
a’). The white arrows in
a,
b,
b’,
c,
f show the cells that are undergoing
de novo transcription. The white dotted lines in (
c’–
f’) show the regions in the body gastric cavity that are undergoing massive
de novo transcription, the oral part of the mesenteries, and the epitheliums. All animals are oriented with the amputation site to the top. Scale bar in (
a) is 20 μm and applies to all
Figure 9 and
Supplementary Figure S6.
This technology allows the visualization of EU (Ethynyl Uridine), a modified Uridine analog, incorporated into nascent RNA [
41]. In uncut controls,
de novo transcription is barely detectable and only a few cells were EU-positive (EU+) throughout the body epithelia (
Figure 9a,a’). After sub-pharyngeal amputation, between 1.30 and 24 hpa, the
de novo transcription pattern is similar to controls in regard to the epithelia (
Figure 9b,c;
Supplementary Figure S6(Aa,Aa’)). However, in the same time frame, some EU+ cells start to emerge in increasing numbers in the internal oral tissues such as the mesenteries and gastrodermis (
Figure 9b’,c’). At 48 hpa, a strong EU+ signal is detected in the oral epithelia, both the ectodermis and gastrodermis, and the oral-most part of the remaining mesenteries (
Figure 9d,d’;
Supplementary Figure S6(Ac,Ac’)). At 96 hpa, EU+ cells are present in the elongating tentacles with the exception of the tentacle tips (
Supplementary Figure S6Ba). Staining progressively decreases in the oral epidermis but remains dense in the oral gastrodermis and in the oral-most part of the mesenteries where the new pharynx is developing (
Figure 9e,e’). In the fully formed pharynx, at 144 hpa, only a few EU+ cells remain at the base of the tentacles and in the lower part of the pharynx (
Figure 9f,f’;
Supplementary Figure S6(Ae,Ae’)).
2.8. Inhibition of Transcription or Proliferation has Different Effects on Regeneration
In order to determine the role of
de novo transcription during regeneration in
Nematostella, we treated amputated juveniles with the antibiotic Actinomycin D (AMD), an inhibitor of DNA-primed RNA synthesis [
39]. In untreated controls, EU+ cells are massively detected at 48 hpa. As expected, at the same time-point no staining was observed in the regenerating juveniles that were treated with AMD from 36 to 48 hpa (
Supplementary Figure S7a,b). Interestingly, we also observed that cell proliferation and regeneration were inhibited in AMD-treated animals (
Supplementary Figure S7c,d).
A recent study has used hydroxyurea (HU) to efficiently block proliferation and regeneration in
Nematostella [
7]. However, nothing is known about the precise phenotype caused by the inhibition of cellular proliferation during regeneration. We thus amputated juveniles below the pharynx, treated them with either AMD or HU from 0 to 144 hpa, and scored wound healing success in addition to the exact stage at which regeneration was blocked using the above-described assays and morphological landmarks at 12, 72, and 144 hpa (
Figure 10;
Figure 11).
Figure 10.
Effect of the inhibition of cell proliferation or transcription on the wound healing process. Hydroxyurea (HU; orange) was used at 20 mM to block cell proliferation, and Actinomycin D (AMD; green) was used at 10 ug/mL to block transcription. Both drugs were used in a time window from 0 to 12 hpa. The compression assay was performed at 6, 12, or 24 hpa.
Figure 10.
Effect of the inhibition of cell proliferation or transcription on the wound healing process. Hydroxyurea (HU; orange) was used at 20 mM to block cell proliferation, and Actinomycin D (AMD; green) was used at 10 ug/mL to block transcription. Both drugs were used in a time window from 0 to 12 hpa. The compression assay was performed at 6, 12, or 24 hpa.
We used the compression assay we described above (
Figure 4) to assess wound healing under these experimental conditions. At 6 hpa, wound healing is delayed in a fraction of the HU-treated juveniles (33%,
n = 58). At 12 hpa, almost all of the animals treated with HU are completely healed (91%,
n = 47), although the process was delayed by approximately 6 h (
Figure 10). However, in AMD-treated juveniles, 29% (
n = 71) are not healed at 6 hpa, and a similar fraction remains open at 12 hpa (24%,
n = 56) as well as 24 hpa (28%,
n = 45) (
Figure 10). This observation suggests that wound healing is not blocked but delayed when cell proliferation only is inhibited. However, when both
de novo transcription and cell proliferation are blocked with the AMD treatment, wound healing does not occur in a subset of animals.
Figure 11.
Inhibition of
de novo transcription blocks regeneration at an earlier step than inhibition of cell proliferation. The experimental amputated polyps were treated with hydroxyurea (HU) to block cell proliferation or with Actinomycin D (AMD) to block
de novo transcription from 0 to 144 hpa and analyzed at the indicated time-points. Confocal images showing the morphological phenotype using nuclear (DNA, cyan) staining in regenerating control (
a,
d,
g) or experimental (
b,
c,
e,
f,
h) polyps at 12 hpa (
a–
c); 72 hpa (
d–
f); or 144 hpa (
g,
h); The white arrows in (
a,
b,
h) show the characteristic depression of the epithelium at the amputated site that correlates with the initiation of the contact between the remaining mesenteries and the surrounding epithelia (circle white dotted line in (
a,
b,
h)); This depression and contact initiation are absent in the polyps treated with AMD (
c). The green arrow in (
d) indicates the forming pharyngeal lip. The white dotted lines in (
d) to (
f) indicate the mesenteries. The yellow arrowheads in (
g) indicate the tentacles.
m, mesenteries;
pha, pharynx. All animals are oriented with the amputation site to the top. Number of cases for the representative phenotype are in white at the bottom right of each image. Scale bar in (
a) is 20 μm and applies to all
Figure 11.
Figure 11.
Inhibition of
de novo transcription blocks regeneration at an earlier step than inhibition of cell proliferation. The experimental amputated polyps were treated with hydroxyurea (HU) to block cell proliferation or with Actinomycin D (AMD) to block
de novo transcription from 0 to 144 hpa and analyzed at the indicated time-points. Confocal images showing the morphological phenotype using nuclear (DNA, cyan) staining in regenerating control (
a,
d,
g) or experimental (
b,
c,
e,
f,
h) polyps at 12 hpa (
a–
c); 72 hpa (
d–
f); or 144 hpa (
g,
h); The white arrows in (
a,
b,
h) show the characteristic depression of the epithelium at the amputated site that correlates with the initiation of the contact between the remaining mesenteries and the surrounding epithelia (circle white dotted line in (
a,
b,
h)); This depression and contact initiation are absent in the polyps treated with AMD (
c). The green arrow in (
d) indicates the forming pharyngeal lip. The white dotted lines in (
d) to (
f) indicate the mesenteries. The yellow arrowheads in (
g) indicate the tentacles.
m, mesenteries;
pha, pharynx. All animals are oriented with the amputation site to the top. Number of cases for the representative phenotype are in white at the bottom right of each image. Scale bar in (
a) is 20 μm and applies to all
Figure 11.
![Ijms 16 26100 g011]()
Interestingly, we observed two strikingly different phenotypes at 12 hpa in the HU-
versus AMD-treated juveniles. Similar to controls at 12 hpa, HU-treated polyps progress to Step 1 when the mesenteries are fused together and enter in contact with the surrounding epithelia at the amputation site (
Figure 11a,b). In addition, a characteristic depression is present in the epithelia at the amputation site in the control as well as in HU-treated polyps (
Figure 11a,b). However, the AMD-treated regenerating juveniles appear to have been blocked in Step 0, right after the amputation, when the mesenteries are neither in contact with one another, nor with the epithelia of the amputation site (
Figure 11c). The characteristic depression in the epithelia at the amputation site is absent as well.
To further characterize the phenotypes resulting from AMD or HU treatment, we assessed pharynx formation using the appearance of 488+ as a proxy at 72 hpa. In untreated regenerating juveniles, the 488+ fluorescence is observed in 62% (38 out of 61 cases). In AMD-treated juveniles, we never observed 488+ in 100% (52 out of 52 cases). We obtained similar results in HU-treated polyps in which 488+ never becomes detectable in 85% (53 out of 62 cases). In addition, no pharyngeal lip or tentacle bulbs were visible in either of the treatments (
Figure 11e,f) as the polyps resulting from AMD or HU treatment remain blocked at Step 0 or Step 1, respectively. These data show that for both the inhibition of
de novo transcription or cell proliferation, the pharynx never starts to form, suggesting that cell proliferation is required for pharynx formation.
At 144 hpa, HU-treated polyps still remain blocked at Step 1 (
Figure 11h) compared to the controls in which a fully formed pharynx is present (
Figure 11g). At 144 hpa, AMD-treated polyps were highly opaque and degraded (data not shown), suggesting a lethal effect of long-term inhibition of transcription. All together these results show that cell proliferation is required for both pharynx formation and tentacle elongation.