Elicitation of Diosgenin Production in Trigonella foenum-graecum (Fenugreek) Seedlings by Methyl Jasmonate
Abstract
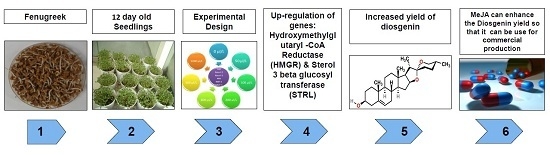
:1. Introduction
2. Results

| Primer | Primer Sequence | Reference | PE ± SE * |
|---|---|---|---|
| 18S Ribosomal RNA | F primer: 5′→3′ GTCTCAACCATAAACGATGCCGACCA | Accession ID: KJ813746 | 2.0 ± 0.0 |
| R primer: ACCTGGTAAGTTTCCCCGTGTTGAGT | |||
| HMG | R primer: 5′→3′ ACTCAACACGGGGAAACTTACCAGGT | 171 bp Contig 19639-our transcriptome data | 2.1 ± 0.0 |
| F primer: 5′→3′ CACCCGCAAGATGAGATTTT | |||
| STRL | F primer: 5′→3′ TGGAAAATGAGGATGGGGTA | 144 bp Conting 10332-our transcriptome data | 2.0 ± 0.0 |
| R primer: 3′→5′TTGCATTGGATCAGGAACAA |
| MeJA Concentration | Fenugreek Varieties (Diosgenin Yield in %) | |||||
|---|---|---|---|---|---|---|
| GM2 | Kasuri-1 | Kasuri-2 | PEB | RMT | MMT | |
| 0 µL/L | 0.9 | 0.65 | 0.55 | 0.75 | 0.85 | 0.75 |
| 50 µL/L | 1.44 | 0.78 | 0.83 | 0.23 | 1.19 | 0.83 |
| 100 µL/L | 1.8 * | 1.6 * | 1.7 * | 1.1 * | 1.01 * | 1.1 * |
| 200 µL/L | 0.72 | 1.04 | 0.77 | 0.38 | 0.97 | 0.9 |
| 300 µL/L | 1.62 | 1.01 | 0.66 | 0.45 | 1.02 | 0.83 |
| 500 µL/L | 0.72 | 0.48 | 0.61 | 0.15 | 0.94 | 0.6 |
| 1000 µL/L | 1.36 | 0.46 | 0.44 | 0.11 | 0.68 | 0.38 |

| Fenugreek Varieties | MeJA Treatment | ||||||
|---|---|---|---|---|---|---|---|
| 0 µL/L | 50 µL/L | 100 µL/L | 200 µL/L | 300 µL/L | 500 µL/L | 1000 µL/L | |
| GM-2 | 5.5 ± 2 * | 5.5 ± 3 | 5.2 ± 4 | 5.5 ± 3 | 5.8 ± 4 | 5.9 ± 2 | 5.8 ± 4 |
| K-1 | 4.8 ± 3 | 4.9 ± 2 | 4.3 ± 4 | 5.2 ± 2 | 5.1 ± 3 | 5 ± 2 | 4.8 ± 3 |
| K-2 | 4.6 ± 2 | 4.8 ± 3 | 4.2 ± 3 | 5 ± 2 | 4.9 ± 3 | 4.8 ± 2 | 4.9 ± 2 |
| PEB | 5.2 ± 3 | 5.2 ± 2 | 4.3 ± 3 | 4.9 ± 2 | 4.8 ± 2 | 4.9 ± 3 | 4.8 ± 2 |
| RMT | 7.2 ± 4 | 7.2 ± 3 | 6.4 ± 2 | 7 ± 3 | 9.8 ± 5 | 6.9 ± 3 | 7 ± 3 |
| MMT | 6.2 ± 3 | 6.2 ± 2 | 5.3± 4 | 6.2 ± 3 | 6 ± 2 | 5.9 ± 3 | 5.8 ± 4 |
| Fenugreek Varieties | MeJA Treatment | ||||||
|---|---|---|---|---|---|---|---|
| 0 µL/L | 50 µL/L | 100 µL/L | 200 µL/L | 300 µL/L | 500 µL/L | 1000 µL/L | |
| GM-2 | 175 ± 1 * | 172 ± 4 | 180 ± 8 | 179 ± 0 | 178 ± 5 | 174 ± 6 | 173 ± 5 |
| K-1 | 145 ± 3 | 151 ± 2 | 152 ± 1 | 146 ± 5 | 147 ± 0 | 142 ± 2 | 140 ± 2 |
| K-2 | 140 ± 5 | 144 ± 8 | 148 ± 8 | 142 ± 7 | 142 ± 5 | 141 ± 2 | 140 ± 9 |
| PEB | 179 ± 5 | 175 ± 1 | 182 ± 3 | 173 ± 8 | 172 ± 8 | 170 ± 5 | 168 ± 5 |
| RMT | 190 ± 8 | 185 ± 2 | 192 ± 5 | 187 ± 0 | 184 ± 8 | 183 ± 5 | 180 ± 3 |
| MMT | 174 ± 6 | 176 ± 1 | 180 ± 7 | 178 ± 2 | 177 ± 5 | 175 ± 6 | 171 ± 3 |
| Fenugreek Varieties | MeJA Treatment | ||||||
|---|---|---|---|---|---|---|---|
| 0 µL/L | 50 µL/L | 100 µL/L | 200 µL/L | 300 µL/L | 500 µL/L | 1000 µL/L | |
| GM-2 | 130 ± 8 * | 128 ±1 | 134 ±9 | 131 ± 5 | 124 ± 5 | 122 ±5 | 120 ± 8 |
| K-1 | 85 ± 5 | 88 ± 3 | 92 ± 5 | 81 ± 5 | 83 ± 0 | 82 ± 8 | 79 ± 8 |
| K-2 | 80 ± 5 | 82 ± 5 | 85 ± 9 | 84 ± 8 | 83 ± 7 | 79 ± 8 | 78 ± 3 |
| PEB | 125 ± 5 | 124 ± 9 | 136 ± 8 | 134 ± 0 | 133 ± 8 | 128 ± 8 | 122 ± 2 |
| RMT | 135 ± 5 | 136 ± 0 | 142 ± 5 | 139 ± 8 | 138 ± 5 | 132 ± 7 | 130 ± 5 |
| MMT | 133 ± 9 | 134 ± 8 | 140 ± 5 | 138 ± 7 | 137 ± 1 | 136 ± 5 | 130 ± 2 |
3. Discussion

4. Materials and Methods
4.1. Fenugreek Seed Sterilization and Sowing
4.2. Methyl Jasmonate Treatments
4.3. Growth Parameter Measurement
4.4. Diosgenin Extraction and Analysis
4.5. Primer Designing
4.6. RNA Extraction, Complementary DNA (cDNA) Preparation and Gene Expression
4.7. Statistical Analysis
5. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Acharya, S.N.; Thomas, J.E.; Basu, S.K. Fenugreek, an alternative crop for semiarid regions of North America. Crop Sci. 2008, 48, 841–853. [Google Scholar] [CrossRef]
- Mullaicharam, A.R.; Geetali, D.; Uma, M. Medicinal values of fenugreek—A review. Res. J. Pharm. Biol. Chem. Sci. 2013, 4, 1304–1313. [Google Scholar]
- Leela, N.K.; Shafeekh, K.M. Fenugreek. In Chemistry of Spices; Parthasarathy, V.A., Chempakam, B., Zachariah, T.J., Eds.; Biddles Ltd., CAB International: King’s Lynn, UK, 2008; pp. 242–259. [Google Scholar]
- Gopu, C.L.; Gilda, S.S.; Paradkar, A.R.; Mahadik, K.R. Development and validation of a densitometric TLC method for analysis of trigonelline and 4-hydroxyisoleucine in Fenugreek seeds. Acta Chromatogr. 2008, 20, 709–719. [Google Scholar] [CrossRef]
- Habori, A.; Raman, M. Pharmacological Properties. In Fenugreek, the Genus Trigonella; Petropoulos, G.A., Ed.; Taylor and Francis: London, UK, 2002; pp. 163–182. [Google Scholar]
- Tiran, D. The use of fenugreek for breast feeding women. Complement. Ther. Nurs. Midwifery 2003, 9, 155–156. [Google Scholar] [CrossRef]
- Broca, C.; Breil, V.; Cruciani, G.C.; Manteghetti, M.; Rouault, C.; Derouet, M.; Rizkalla, S.; Pau, B.; Petit, P.; Ribes, G.; et al. The insulinotrophic agent 1D1101(4-hydroxyisoleucine) activates insulin signaling in rat. Am. J. Physiol. Endocrinol. Metab. 2004, 287, E463–E471. [Google Scholar] [CrossRef] [PubMed]
- Devi, B.A.; Kamalakkannan, N.; Prince, P.S. Supplementation of fenugreek leaves to diabetic rats-effect on carbohydrate metabolic enzymes in diabetic liver and kidney. Phytother. Res. 2003, 17, 1231–1233. [Google Scholar] [CrossRef] [PubMed]
- Aasim, M.; Hussain, N.; Umer, E.M.; Zubair, M.; Hussain, B.S.; Saeed, S.; Rafique, T.S.; Sancak, C. In vitro shoot regeneration of fenugreek (Trigonella foenum-graecum L.) using different cytokinins. Afr. J. Biotechnol. 2010, 9, 7174–7179. [Google Scholar]
- Tahiliani, P.; Kar, A. Mitigation of thyroxine induced hyperglycaemia by two plant extracts. Phytother. Res. 2003, 17, 294–296. [Google Scholar] [CrossRef] [PubMed]
- Thakaran, S.; Salimuddin; Baquer, N.Z. Oral administration of orthovanadate and Trigonella foenum-graecum seed powder restore the activities of mitochondrial enzymes in tissues of alloxan-induced diabetic rats. Mol. Cell. Biochem. 2003, 247, 45–53. [Google Scholar] [CrossRef]
- Suboh, S.M.; Bilto, Y.Y.; Aburjai, T.A. Protective effects of selected medicinal plants against protein degradation, lipid peroxidation and deformability loss of oxidatively stressed human erythrocytes. Phytother. Res. 2004, 18, 280–284. [Google Scholar] [CrossRef] [PubMed]
- Thompson Coon, J.S.; Ernst, E. Herbs for serum cholesterol reduction: A systematic view. J. Fam. Pract. 2003, 52, 468–478. [Google Scholar] [PubMed]
- Venkatesan, N.; Devaraj, S.N.; Devraj, H. Increased binding of LDL and VLDL to apo B, E receptors of hepatic plasma membrane of rats treated with Fibranat. Eur. J. Nutr. 2003, 42, 262–271. [Google Scholar] [CrossRef] [PubMed]
- Devasena, T.; Menon, V.P. Fenugreek affects the activity of β-glucuronidase and mucinase in the colon. Phytother. Res. 2003, 17, 1088–1091. [Google Scholar] [CrossRef] [PubMed]
- Harsha, K.; Preeti, P.; Manish, K.P.; Harendra, K.; Amit, C.K.; Devi, D.J. Foaming glycosides: A review. IOSR J. Pharm. 2012, 2, 23–28. [Google Scholar]
- Freeman, B.C.; Beatiie, G.A. An overview of plant defenses against pathogens and herbivores. Plant Health Instruct. 2008. [Google Scholar] [CrossRef]
- Gonzalez, L.R.; Mitchell, G.; Gattuso, M.; Diarra, M.S.; Malouin, F.; Bouarab, K. Plant antimicrobial agents and their effects on plant and human pathogens. Int. J. Mol. Sci. 2009, 10, 3400–3419. [Google Scholar] [CrossRef] [PubMed]
- Agarwal, M.; Agarwal, M.; Jain, S.C. In vitro regulation of bioactive compounds in Trigonella species by mutagenic treatments. J. Plant Sci. 2015, 3, 40–44. [Google Scholar]
- Lee, J.; Jung, K.; Kim, Y.S.; Park, D. Diosgenin inhibits melanogenesis through the activation of phosphatidylinositol-3-kinase pathway (PI3K) signaling. Life Sci. 2007, 81, 249–254. [Google Scholar] [CrossRef] [PubMed]
- Tada, Y.; Kanda, N.; Haratake, A.; Tobiishi, M.; Uchiwa, H.; Watanabe, S. Novel effects of diosgenin on skin aging. Steroids 2009, 74, 504–511. [Google Scholar] [CrossRef] [PubMed]
- Yan, L.L.; Zhang, Y.J.; Gao, W.Y.; Man, S.L.; Wang, Y. In vitro and in vivo anticancer activity of steroid saponins of Paris polyphylla var. yunnanensis. Exp. Oncol. 2009, 31, 27–32. [Google Scholar]
- Gong, G.; Qin, Y.; Huang, W.; Zhous, S.; Wu, X.; Yang, X.; Zhao, Y.; Li, D. Protective effects of diosgenin in the hyperlipidemic rat model and in human vascular endothelial cells against hydrogen peroxide-induced apoptosis. Chem.-Biol. Interact. 2010, 184, 366–375. [Google Scholar] [CrossRef] [PubMed]
- Katy, G.D.; Nadja, A.C.; Kristhea, G.P. Mechanisms involved in the vasodilator effect induced by diosgenin in rat superior mesenteric artery. Eur. J. Pharmacol. 2007, 574, 172–178. [Google Scholar]
- Qin, T.C.; Zhang, Y.D.; Zhang, J.Z. Distribution and utilization of diosgenin resources in Hubei province. Biol. Resour. 1997, 13, 200–202. [Google Scholar]
- Liu, P.; Wu, X.Y.; Yue, D.Y. Comprehensive utilization of diosgenin resources. Resour. Econ. Compr. Util. 1993, 12, 47–49. [Google Scholar]
- Aradhana, M.; Rao, A.C.; Kale, R.K. Diosgenin a growth stimulator of mammary gland of ovariectomized mouse. Indian J. Exp. Biol. 1992, 30, 367–370. [Google Scholar] [PubMed]
- Patel, K.; Gadewar, M.; Tahilyani, V.; Patel, D.K. A review on pharmacological and analytical aspects of diosgenin: A concise report. Nat. Prod. Bioprospect. 2012, 2, 46–52. [Google Scholar] [CrossRef]
- Rosser, A. The day of the yam. Nurs. Times 1985, 81, 47–48. [Google Scholar] [PubMed]
- Hardman, R. Pharmaceutical products from plant steroids. Trop. Sci. 1969, 11, 196–222. [Google Scholar]
- Petropoulos, G.A. Agronomic, Genetic and Chemical Studies of Trigonella foenum-graecum L. Ph.D. Thesis, Bath University, Bath, UK, 1973. [Google Scholar]
- Kanak, V.; Arpita, G.; Vinay, K.; Spandan, C.; Navin, S.; Kalpesh, K.; Tanushree, T.; Surendra, K.C. De novo transcriptome sequencing in Trigonella foenum-graecum L. to identify genes involved in the biosynthesis of diosgenin. Plant Genome 2013, 6, 1–11. [Google Scholar]
- Paul, E.S.; Wenpei, S.; Stephen, H.H. Methyl jasmonate inhibition of root growth and induction of a leaf protein are decreased in an Arabidopsis thaliana mutant. Proc. Natl. Acad. Sci. USA 1992, 89, 6837–6840. [Google Scholar]
- Sembdner, G.; Parthier, B. The biochemistry and the physiological and molecular actions of jasmonates. Annu. Rev. Plant Physiol. Plant Mol. Biol. 1993, 44, 569–589. [Google Scholar] [CrossRef]
- Aldridge, D.C.; Galt, S.; Giles, D.; Turner, W.B. Metabolites of Lasiodiplodia theobromae. J. Chem. Soc. 1971, 1623–1627. [Google Scholar] [CrossRef]
- Goodrich-Tanrikulu, M.; Mahoney, N.E.; Rodriguez, S.B. The plant growth regulator methyl jasmonate inhibits aflatoxin production by Aspergillus flavus. Microbiology 1995, 141, 2831–2837. [Google Scholar] [CrossRef] [PubMed]
- Aerts, R.J.; Gisi, D.; Carolis, E.; Luca, V.; Baumann, T.W. Methyl jasmonate vapor increases the developmentally controlled synthesis of alkaloids in Catharanthus and Cinchona seedlings. Plant J. 1994, 5, 635–643. [Google Scholar] [CrossRef]
- Wasternack, C.; Parthier, B. Jasmonate-signalled gene expression. Trends Plant Sci. 1997, 2, 302–307. [Google Scholar] [CrossRef]
- Morrissey, J.P.; Osbourn, A.E. Fungal resistance to plant antibiotics as a mechanism of pathogenesis. Microbiol. Mol. Biol. Rev. 1999, 63, 708–724. [Google Scholar] [PubMed]
- Chen, X.; Wang, X.; Li, Z.; Kong, L.; Liu, G.; Fu, J.; Wang, A. Molecular cloning, tissue expression and protein structure prediction of the porcine 3-hydroxy-3-methylglutaryl-coenzyme A reductase (HMGR) gene. Gene 2012, 495, 170–177. [Google Scholar] [CrossRef] [PubMed]
- Heidrun, G.; Martin, J.M.; Tonim, K.; Meinhart, H.Z. Jasmonic acid is a signal transducer in elicitor-induced plant cell cultures. Proc. Natl. Acad. Sci. USA 1992, 89, 2389–2393. [Google Scholar]
- Joseph, C.; Fred, W.; Jeanne, P.; Rick, C.; Court, S. Is the reaction catalyzed by 3-hydroxy-3-methylglutaryl coenzyme A reductase a rate-limiting step for isoprenoid biosynthesis in plant. Plant Physiol. 1995, 109, 1337–1343. [Google Scholar]
- Oncina, R.; Del, R.J.A.; Gomez, P.; Ortuno, A. Effect of ethylene on diosgenin accumulation in callus cultures of Trigonella foenum-graecum L. Food Chem. 2002, 76, 475–479. [Google Scholar] [CrossRef]
- Gomez, P.; Ortuno, A.; Del, R.J.A. Ultrastructural changes and diosgenin content in cell suspensions of Trigonella foenum-graecum L. by ethylene treatment. Plant Growth Regul. 2004, 44, 93–99. [Google Scholar] [CrossRef]
- Debjani, D.; Bratati, D. Elicitation of diosgenin production in Trigonella foenum-graecum L. seedlings by heavy metals and signaling molecules. Acta Physiol. Plant 2011, 33, 1585–1590. [Google Scholar]
- Romeo, R.; Teresa, S.; Christopher, B.; Tajalli, K. Elicitation of plants and microbial cell systems. Biotechnol. Appl. Biochem. 2003, 37, 91–102. [Google Scholar]
- Namdeo, A.G. Plant cell elicitation for production of secondary metabolites: A review. Pharmacogn. Rev. 2007, 1, 69–79. [Google Scholar]
- Iriti, M.; Faoro, F. Chemical diversity and defence metabolism: How plants cope with pathogens and ozone pollution. Int. J. Mol. Sci. 2009, 10, 3371–3399. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.J.; Chen, F.; Wang, X.; Rajapakse, N.C. Effect of methyl jasmonate on secondary metabolites of sweet basil (Ocimum basilicum L.). J. Agric. Food Chem. 2006, 54, 2327–2332. [Google Scholar] [CrossRef] [PubMed]
- Keinanen, M.; Oldham, N.; Baldwin, I.T. Rapid HPLC screening of jasmonate-induced increases in tobacco alkaloids, phenolics, and diterpene glycosides in Nicotiana attenuata. J. Agric. Food Chem. 2001, 49, 3553–3558. [Google Scholar] [CrossRef] [PubMed]
- Dixon, R.A.; Paiva, N.L. Stress-induced phenylpropanoid metabolism. Plant Cell 1995, 7, 1085–1097. [Google Scholar] [CrossRef] [PubMed]
- Rebecca, M.; Deepa, S.P. Effect of methyl jasmonate and chitosan on growth characteristics of Ocimum basilicum L., Ocimum sanctum L. and Ocimum gratissimum L. cell suspension cultures. Afr. J. Biotechnol. 2012, 11, 4759–4766. [Google Scholar] [CrossRef]
- Ren, A.; Qin, L.; Shi, L.; Dong, X.; Mu da, S.; Li, Y.X.; Zhao, M.W. Methyl jasmonate induces ganoderic acid biosynthesis in the basidiomycetous fungus Ganoderma lucidum. Bioresour. Technol. 2010, 101, 6785–6790. [Google Scholar] [CrossRef] [PubMed]
- Diarra, S.T.; Ji, H.; Jianbo, W.; Jiaru, L. Ethylene treatment improves diosgenin accumulation in in vitro cultures of Dioscorea zingiberensis via up-regulation of CAS and HMGR gene expression. Electron. J. Biotechnol. 2013. [Google Scholar] [CrossRef]
- Burnett, R.J.; Maldonado-Mendoza, I.E.; McKnight, T.D.; Nessler, C.L. Expression of a 3-hydroxy-3-methylglutaryl coenzyme A reductase gene from Camptotheca acuminata is differentially regulated by wounding and methyl jasmonate. Plant Physiol. 1993, 103, 41–48. [Google Scholar] [CrossRef] [PubMed]
- Mehrafarin, A.; Qaderi, A.; Rezazadeh, S.; Naghdi Badi, H.; Noormohammadi, G.H.; Zand, E. Bioengineering of important secondary metabolites and metabolic pathways in fenugreek (Trigonella foenum-graecum L.). J. Med. Plants 2010, 9, 1–18. [Google Scholar]
- Zhao, J.; Davis, L.C.; Verpoorte, R. Elicitor signal transduction leading to production of plant secondary metabolites. Biotechnol. Adv. 2005, 23, 283–333. [Google Scholar] [CrossRef] [PubMed]
- Trivedi, P.D.; Pundarikakshudu, K.; Rathnam, S.; Shah, K.S. A validated quantitative thin-layer chromatographic method for estimation of diosgenin in various plant samples, extract, and market formulation. J. AOAC Int. 2007, 90, 358–363. [Google Scholar] [PubMed]
- Michael, W.P.; Graham, W.H.; Leo, D. Relative expression software tool (REST©) for group-wise comparison and statistical analysis of relative expression results in real-time PCR. Nucleic Acids Res. 2002, 30, e36. [Google Scholar] [CrossRef]
- Pfaffl, M.W. A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acid Res. 2001, 29, e45. [Google Scholar] [CrossRef] [PubMed]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chaudhary, S.; Chikara, S.K.; Sharma, M.C.; Chaudhary, A.; Alam Syed, B.; Chaudhary, P.S.; Mehta, A.; Patel, M.; Ghosh, A.; Iriti, M. Elicitation of Diosgenin Production in Trigonella foenum-graecum (Fenugreek) Seedlings by Methyl Jasmonate. Int. J. Mol. Sci. 2015, 16, 29889-29899. https://doi.org/10.3390/ijms161226208
Chaudhary S, Chikara SK, Sharma MC, Chaudhary A, Alam Syed B, Chaudhary PS, Mehta A, Patel M, Ghosh A, Iriti M. Elicitation of Diosgenin Production in Trigonella foenum-graecum (Fenugreek) Seedlings by Methyl Jasmonate. International Journal of Molecular Sciences. 2015; 16(12):29889-29899. https://doi.org/10.3390/ijms161226208
Chicago/Turabian StyleChaudhary, Spandan, Surendra K. Chikara, Mahesh C. Sharma, Abhinav Chaudhary, Bakhtiyar Alam Syed, Pooja S. Chaudhary, Aditya Mehta, Maulik Patel, Arpita Ghosh, and Marcello Iriti. 2015. "Elicitation of Diosgenin Production in Trigonella foenum-graecum (Fenugreek) Seedlings by Methyl Jasmonate" International Journal of Molecular Sciences 16, no. 12: 29889-29899. https://doi.org/10.3390/ijms161226208
APA StyleChaudhary, S., Chikara, S. K., Sharma, M. C., Chaudhary, A., Alam Syed, B., Chaudhary, P. S., Mehta, A., Patel, M., Ghosh, A., & Iriti, M. (2015). Elicitation of Diosgenin Production in Trigonella foenum-graecum (Fenugreek) Seedlings by Methyl Jasmonate. International Journal of Molecular Sciences, 16(12), 29889-29899. https://doi.org/10.3390/ijms161226208