In Vitro Investigation of Self-Assembled Nanoparticles Based on Hyaluronic Acid-Deoxycholic Acid Conjugates for Controlled Release Doxorubicin: Effect of Degree of Substitution of Deoxycholic Acid
Abstract
:1. Introduction
2. Results and Discussion
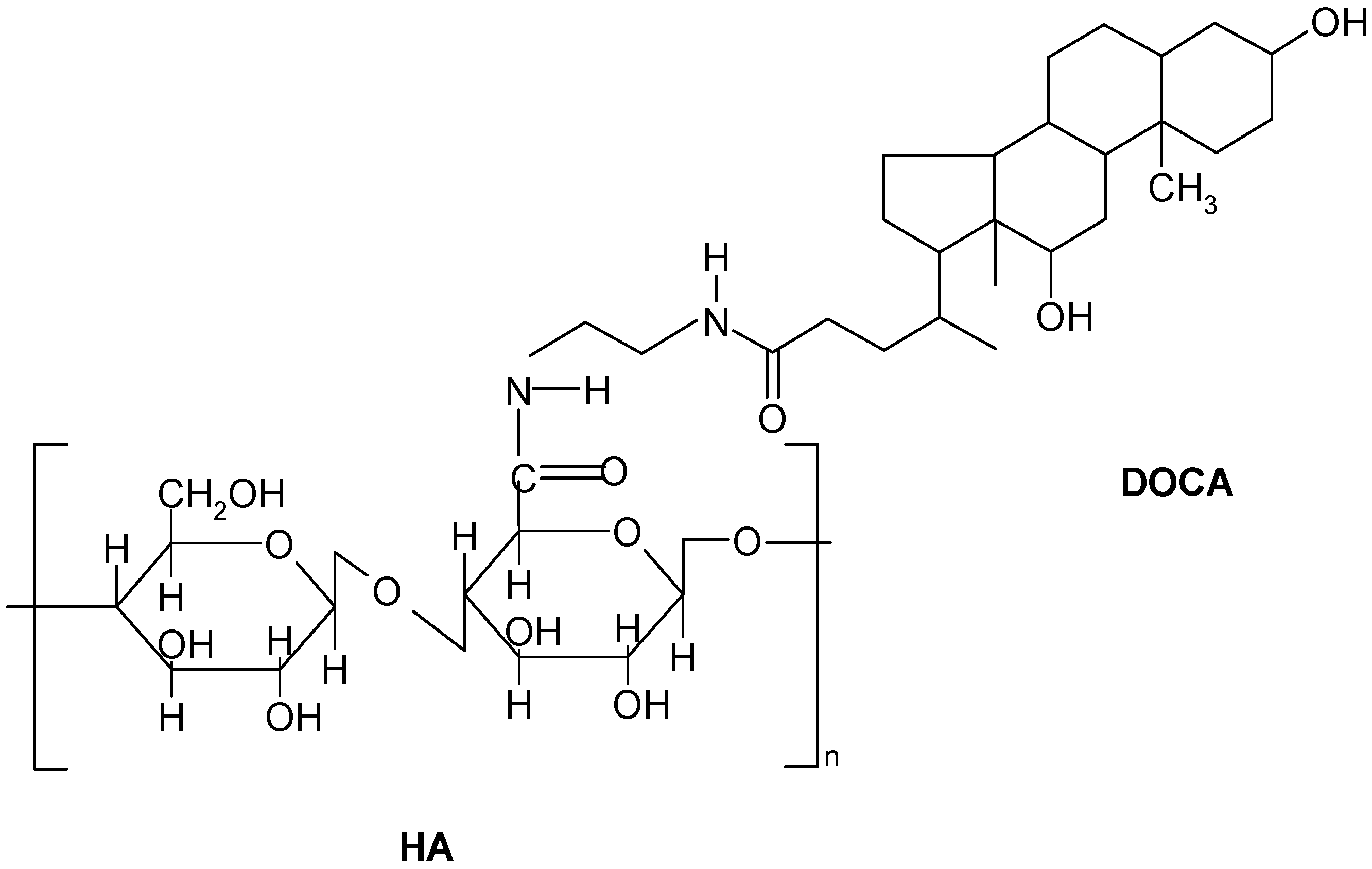
2.1. Preparation and Characterization of Hyaluronic Acid-Deoxycholic Acid (HD) Self-Assembled Nanoparticles
| Sample a | Feed Ratio b | Mn c | DS | X d (%) | d (nm) |
|---|---|---|---|---|---|
| HD6 | 1:10 | 18,925 | 5.9 | 12.3 ± 1.2 | 1044 ± 70.2 |
| HD7 | 1:20 | 19,594 | 7.6 | 15.3 ± 2.3 | 447 ± 31.8 |
| HD9 | 1:40 | 20,304 | 9.4 | 18.2 ± 3.1 | 208 ± 20.1 |
2.2. Preparation and Characterization Doxorubicin (DOX)-Loaded HD Nanoparticles
| Loading Amount of DOX (wt. %) a | 0 | 5 | 10 | 15 |
|---|---|---|---|---|
| Particle size (nm) | 208 ± 20.1 | 212 ± 23.1 | 220 ± 27.1 | 228 ± 30.1 |
| PDI b | 0.290 | 0.300 | 0.350 | 0.367 |
| Samples | Loading Amount of DOX (wt. %) a | Loading Content of DOX (%) | Loading Efficiency (%) |
|---|---|---|---|
| HD6 | 5 | 4.75 ± 0.21 | 95.00 ± 2.51 |
| HD6 | 10 | 7.88 ± 0.87 | 78.75 ± 6.13 |
| HD6 | 20 | 11.58 ± 1.12 | 57.50 ± 3.81 |
| HD7 | 5 | 4.89 ± 0.53 | 98.08 ± 9.42 |
| HD9 | 5 | 4.97 ± 0.57 | 99.33 ± 10.91 |
2.3. In Vitro Release of Doxorubicin



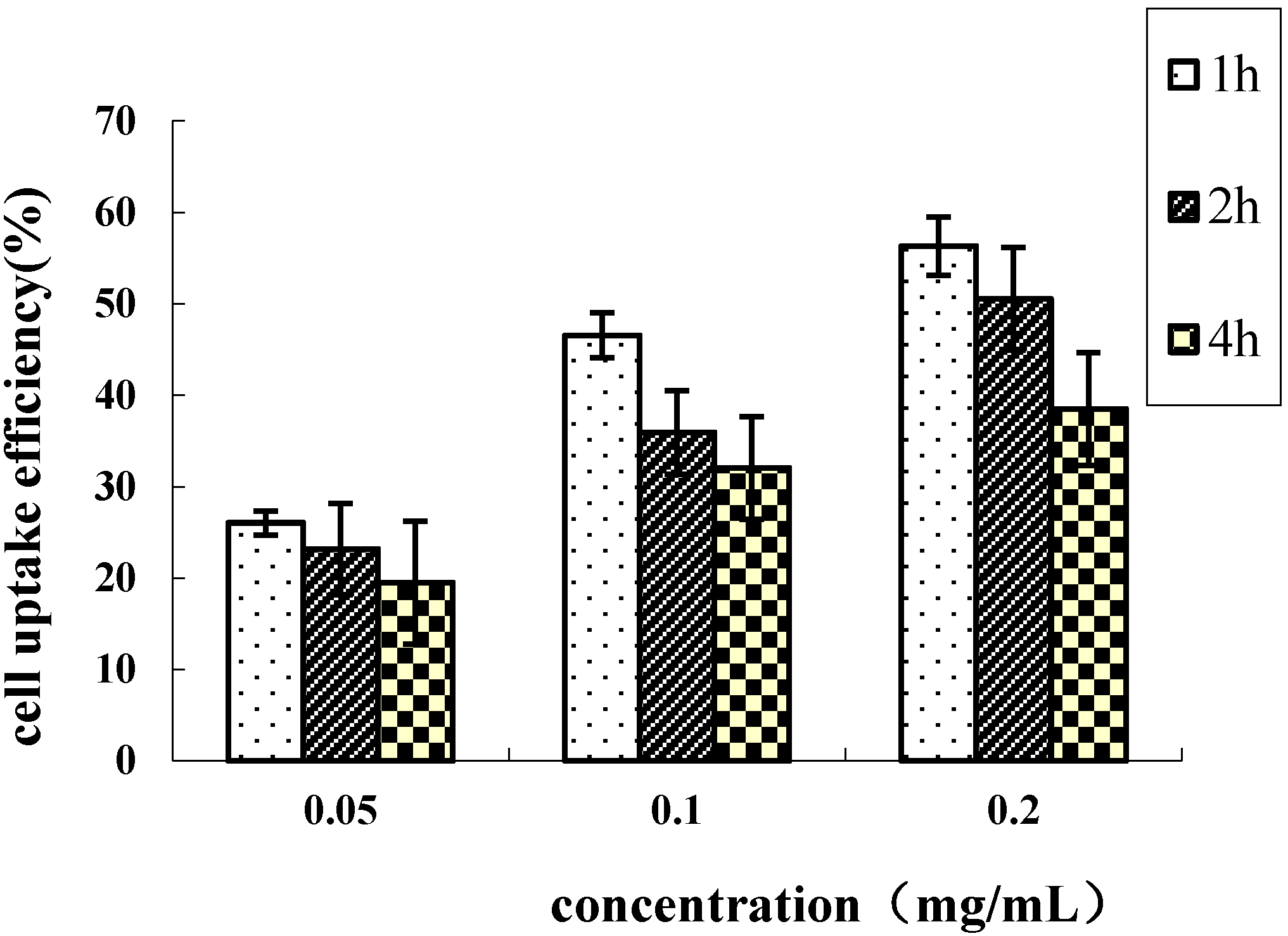
2.4. Cellular Uptake of HD Nanoparticles
2.5. In Vitro Cytotoxicity Assay

3. Materials and Methods
3.1. Materials
3.2. Synthesis of N-Deoxycholyl-ethylenediamine (DOCA-NH2)
3.3. Preparation of HA-DOCA (HD) Conjugate
3.4. Preparation and Characterization Doxorubicin-Loaded HD Nanoparticles
3.5. Doxorubicin Release from HD Nanoparticles
3.6. Cell Culture
3.7. Preparation of Fluorescein Isothiocyanate (FITC)-Labeled HD Conjugates
3.8. In Vitro Cellular Uptake of Nanoparticles
3.9. The in Vitro Cytotoxicity Assay
3.10. Statistical Methods
4. Conclusions
Author Contributions
Conflicts of Interest
References
- Uchegbu, I.F.; Lalatsa, A.; Wong, D. Fundamentals of Pharmaceutical Nanoscience; Springer Science Business Media New York: New York, NY, USA, 2013; pp. 211–234. [Google Scholar]
- Soppimath, K.S.; Aminabhavi, T.M.; Kulkarni, A.R.; Rudzinski, W.E. Biodegradable polymeric nanoparticles as drug delivery devices. J. Control. Release 2001, 70, 1–20. [Google Scholar] [CrossRef] [PubMed]
- Lai, P.; Daear, W.; Lobenberg, R.; Prenner, E.J. Overview of the preparation of organic polymeric nanoparticles for drug delivery based on gelatine, chitosan, poly(d,l-lactide-co-glycolic acid) and polyalkylcyanoacrylate. Colloids Surf. B 2014, 118, 154–163. [Google Scholar] [CrossRef]
- Shelke, N.B.; James, R.; Laurencin, C.T.; Kumbar, S.G. Polysaccharide biomaterials for drug delivery and regenerative engineering. Polym. Adv. Technol. 2014, 25, 448–460. [Google Scholar] [CrossRef]
- Hamidi, M.; Azadi, A.; Rafiei, P. Hydrogel nanoparticles in drug delivery. Adv. Drug Deliv. Rev. 2008, 60, 1638–1649. [Google Scholar] [CrossRef] [PubMed]
- Ohya, Y.; Takahashi, A.; Nagahama, K. Biodegradable polymeric assemblies for biomedical Materials. Adv. Polym. Sci. 2012, 247, 65–114. [Google Scholar]
- Liu, Z.H.; Jiao, Y.P.; Wang, Y.F.; Zhou, C.R.; Zhang, Z.Y. Polysaccharides-based nanoparticles as drug delivery systems. Adv. Drug Deliv. Rev. 2008, 60, 1650–1662. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.; Park, K. Polymeric micelles and alternative nanonized delivery vehicles for poorly soluble drugs. Int. J. Pharm. 2013, 453, 198–214. [Google Scholar] [CrossRef] [PubMed]
- Akiyoshi, K.; Sunamoto, J. Supramolecular assembly of hydrophobized polysaccharides. Supramol. Sci. 1996, 3, 157–163. [Google Scholar]
- Bourin, M.C.; Lindahl, U. Glycosaminoglycans and the regulation of blood-coagulation. Biochem. J. 1993, 289, 313–330. [Google Scholar] [PubMed]
- Scott, J.E. Extracellular-matrix, supramolecular organization and shape. J. Anat. 1995, 187, 259–269. [Google Scholar] [PubMed]
- Collis, L.; Hall, C.; Lange, L.; Ziebell, M.; Prestwich, R.; Turley, E.A. Rapid hyaluronan uptake is associated with enhanced motility: Implications for an intracellular mode of action. Febs. Lett. 1998, 440, 444–449. [Google Scholar] [CrossRef] [PubMed]
- Knudson, W. Tumor-associated hyaluronan-Providing an extracellular matrix that facilitates invasion. Am. J. Pathol. 1996, 148, 1721–1726. [Google Scholar] [PubMed]
- Gerdin, B.; Hallgren, R. Dynamic role of hyaluronan (HYA) in connective tissue activation and inflammation. J. Int. Med. 1997, 242, 49–55. [Google Scholar] [CrossRef]
- Toole, B.P. Hyaluronan: From extracellular glue to pericellular cue. Nat. Rev. Cancer 2004, 4, 528–539. [Google Scholar] [CrossRef] [PubMed]
- Leach, J.B.; Schmidt, C.E. Characterization of protein release from photocrosslinkable hyaluronic acid-polyethylene glycol hydrogel tissue engineering scaffolds. Biomaterials 2005, 26, 125–135. [Google Scholar] [CrossRef] [PubMed]
- Jia, X.Q.; Yeo, Y.; Clifton, R.J.; Jiao, T.; Kohane, D.S.; Kobler, J.B.; Zeitels, S.M.; Langer, R. Hyaluronic acid-based microgels and microgel networks for vocal fold regeneration. Biomacromolecules 2006, 7, 3336–3344. [Google Scholar] [CrossRef] [PubMed]
- Aguiar, D.J.; Knudson, W.; Knudson, C.B. Internalization of the hyaluronan receptor CD44 by chondrocytes. Exp. Cell. Res. 1999, 252, 292–302. [Google Scholar] [CrossRef] [PubMed]
- Knudson, W.; Chow, G.; Knudson, C.B. CD44-mediated uptake and degradation of hyaluronan. Matrix. Biol. 2002, 21, 15–23. [Google Scholar] [CrossRef] [PubMed]
- Luo, Y.; Bernshaw, N.J.; Lu, Z.R.; Kopecek, J.; Prestwich, G.D. Targeted delivery of doxorubicin by HPMA copolymer-hyaluronanbioconjugates. Pharm. Res. 2002, 19, 396–402. [Google Scholar] [CrossRef] [PubMed]
- Platt, V.M.; Szoka, F.C. Anticancer therapeutics: Targeting macromolecules and nanocarriers to hyaluronan or CD44, a hyaluronan receptor. Mol. Pharm. 2008, 5, 474–486. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.; Lee, K.; Park, T.G. Hyaluronic acid-paclitaxel conjugate micelles: Synthesis, characterization, and antitumor activity. Bioconjug. Chem. 2008, 19, 1319–1325. [Google Scholar] [CrossRef] [PubMed]
- Norbedo, S.; Dinon, F.; Bergamin, M.; Bosi, S.; Aroulmoji, V.; Khan, R.; Murano, E. Synthesis of 6-amino-6-deoxyhyaluronan as an intermediate for conjugation with carboxylate-containing compounds: Application to hyaluronan-camptothecin conjugates. Carbohydr. Res. 2009, 344, 98–104. [Google Scholar] [CrossRef] [PubMed]
- Eliaz, R.E.; Nir, S.; Szoka, F.C. Interactions of hyaluronan-targeted liposomes with cultured cells: Modeling of binding and endocytosis. Methods Enzymol. 2004, 387, 16–33. [Google Scholar] [PubMed]
- Choi, K.Y.; Lee, S.; Park, K.; Kim, K.; Park, J.H.; Kwon, I.C.; Jeong, S.Y. Preparation and characterization of hyaluronic acid-based hydrogel nanoparticles. J. Phys. Chem. Solids 2008, 69, 1591–1595. [Google Scholar] [CrossRef]
- Wei, X.; Senanayake, T. H.; Warren, G.; Vinogradov, S. V. Hyaluronic acid-based nanogel-drug conjugates with enhanced anticancer activity designed for the targeting of CD44-positive and drug-resistant tumors. Bioconjug. Chem. 2013, 24, 658–668. [Google Scholar] [CrossRef] [PubMed]
- Cho, H.J.; Yoon, I.S.; Yoon, H.Y.; Koo, H.; Jin, Y.J.; Ko, S.H.; Shim, J.S.; Kim, K.; Kwon, I.C.; Kim, D.D. Polyethylene glycol-conjugated hyaluronic acid-ceramide self-assembled nanoparticles for targeted delivery of doxorubicin. Biomaterials 2012, 33, 1190–1200. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.B.; Zhang, H.; Yu, Y.; Chen, Y.; Wang, D.; Zhang, G.Q.; Zhou, G.C.; Liu, J.J.; Sun, Z.G.; Sun, D.X.; et al. Biodegradable self-assembled nanoparticles of poly (d,l-lactide-co-glycolide)/hyaluronic acid block copolymers for target delivery of docetaxel to breast cancer. Biomaterials 2014, 35, 550–566. [Google Scholar]
- Dong, X.M.; Liu, C.G. Preparation and characterization of self-assembled nanoparticles of hyaluronic acid-deoxycholic acid conjugates. J. Nanomater. 2010. [Google Scholar] [CrossRef]
- Gao, Y.; Zhang, Z.W.; Chen, L.L.; Gu, W.W.; Li, Y.P. Chitosan N-betainates/DNA self-assembly nanoparticles for gene delivery: In vitro uptake and transfection efficiency. Int. J. Pharm. 2009, 371, 156–162. [Google Scholar] [CrossRef] [PubMed]
- Tsai, H.C.; Chang, W.H.; Lo, C.L.; Tsai, C.H.; Chang, C.H.; Ou, T.W.; Yen, T.C.; Hsiue, G.H. Graft and diblock copolymer multifunctional micelles for cancer chemotherapy and imaging. Biomaterials 2010, 31, 2293–2301. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.Y.; Li, L.; Zhou, H.P.; Lu, S.Y.; Yang, H.; Yin, X.J.; Ren, J.S. Preparation and characterization of N-succinyl-N-octyl chitosan micelles as doxorubicin carriers for effective anti-tumor activity. Colloids Surf. B 2007, 55, 222–228. [Google Scholar] [CrossRef]
- Bally, M.B.; Nayar, R.; Masin, D.; Cullis, P.R.; Mayer, L.D. Studies on the myelosuppressive activity of doxorubicin entrapped in liposomes. Cancer Chemother. Pharm. 1990, 27, 13–19. [Google Scholar] [CrossRef]
- Nori, A.; Kopecek, J. Intracellular targeting of polymer-bound drugs for cancer chemotherapy. Adv. Drug Deliv. Rev. 2005, 57, 609–636. [Google Scholar] [CrossRef] [PubMed]
- Hu, F.Q.; Ren, G.F.; Yuan, H.; Du, Y.Z.; Zeng, S. Shell cross-linked stearic acid grafted chitosan oligosaccharide self-aggregated micelles for controlled release of paclitaxel. Colloids Surf. B 2006, 50, 97–103. [Google Scholar] [CrossRef]
- Laroui, H.; Grossin, L.; Leonard, M.; Stoltz, J.F.; Gillet, P.; Netter, P.; Dellacherie, E. Hyaluronate-covered nanoparticles for the therapeutic targeting of cartilage. Biomacromolecules 2007, 8, 3879–3885. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.H.; Kim, Y.S.; Kim, S.; Park, J. H.; Kim, K.; Choi, K.; Chung, H.; Jeong, S.Y.; Park, R.W.; Kim, I.S.; Kwon, I.C. Hydrophobically modified glycol chitosan nanoparticles as carriers for paclitaxel. J. Control. Release 2006, 111, 228–234. [Google Scholar] [CrossRef] [PubMed]
- Mosmann, T. Rapid colorimetric assay for cellular growth and survival: Application to proliferation and cytotoxicity assays. J. Immunol. Methods 1983, 95, 55–63. [Google Scholar] [CrossRef]
- Xu, J.P.; Ji, J.; Chen, W.D.; Shen, J.C. Novel biomimetic polymersomes as polymer therapeutics for drug delivery. J. Control. Release 2005, 107, 502–512. [Google Scholar] [CrossRef] [PubMed]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wei, W.-H.; Dong, X.-M.; Liu, C.-G. In Vitro Investigation of Self-Assembled Nanoparticles Based on Hyaluronic Acid-Deoxycholic Acid Conjugates for Controlled Release Doxorubicin: Effect of Degree of Substitution of Deoxycholic Acid. Int. J. Mol. Sci. 2015, 16, 7195-7209. https://doi.org/10.3390/ijms16047195
Wei W-H, Dong X-M, Liu C-G. In Vitro Investigation of Self-Assembled Nanoparticles Based on Hyaluronic Acid-Deoxycholic Acid Conjugates for Controlled Release Doxorubicin: Effect of Degree of Substitution of Deoxycholic Acid. International Journal of Molecular Sciences. 2015; 16(4):7195-7209. https://doi.org/10.3390/ijms16047195
Chicago/Turabian StyleWei, Wen-Hao, Xue-Meng Dong, and Chen-Guang Liu. 2015. "In Vitro Investigation of Self-Assembled Nanoparticles Based on Hyaluronic Acid-Deoxycholic Acid Conjugates for Controlled Release Doxorubicin: Effect of Degree of Substitution of Deoxycholic Acid" International Journal of Molecular Sciences 16, no. 4: 7195-7209. https://doi.org/10.3390/ijms16047195
APA StyleWei, W.-H., Dong, X.-M., & Liu, C.-G. (2015). In Vitro Investigation of Self-Assembled Nanoparticles Based on Hyaluronic Acid-Deoxycholic Acid Conjugates for Controlled Release Doxorubicin: Effect of Degree of Substitution of Deoxycholic Acid. International Journal of Molecular Sciences, 16(4), 7195-7209. https://doi.org/10.3390/ijms16047195





