SLM Produced Porous Titanium Implant Improvements for Enhanced Vascularization and Osteoblast Seeding
Abstract
:1. Introduction
2. Results
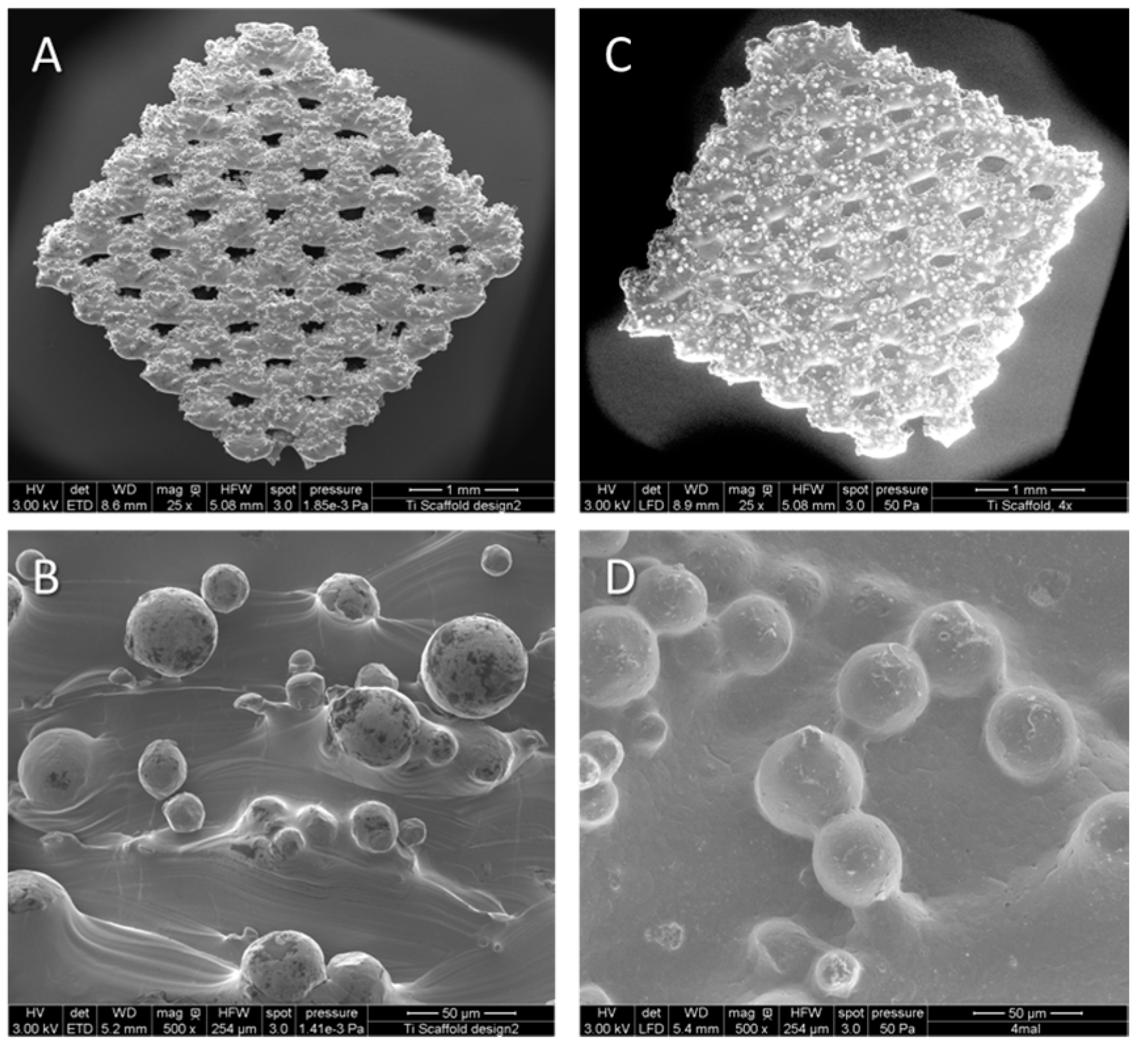
2.1. Manufacturing of Titanium Implants and Titanium Polycaprolactone (PCL) Implants
2.2. Characterization of PCL Coating on Porous Titanium Implants
| Scaffold Modification (At-%) | Titanium Implant | Titanium PCL Implant |
|---|---|---|
| Ti | 70.09 | 3.04 |
| C | 4.68 | 74.87 |
| O | 0.87 | 21.41 |
2.3. Cross Sections Established of Titanium Implants



2.4. Migration Assays of GM7373 on Vascular Endothelial Growth Factor (VEGF), High Mobility Group Box 1 (HMGB1), Chemokine (C-X-C Motif) Ligand 12 (CXCL12)
2.5. Live Cell Imaging (LCI)

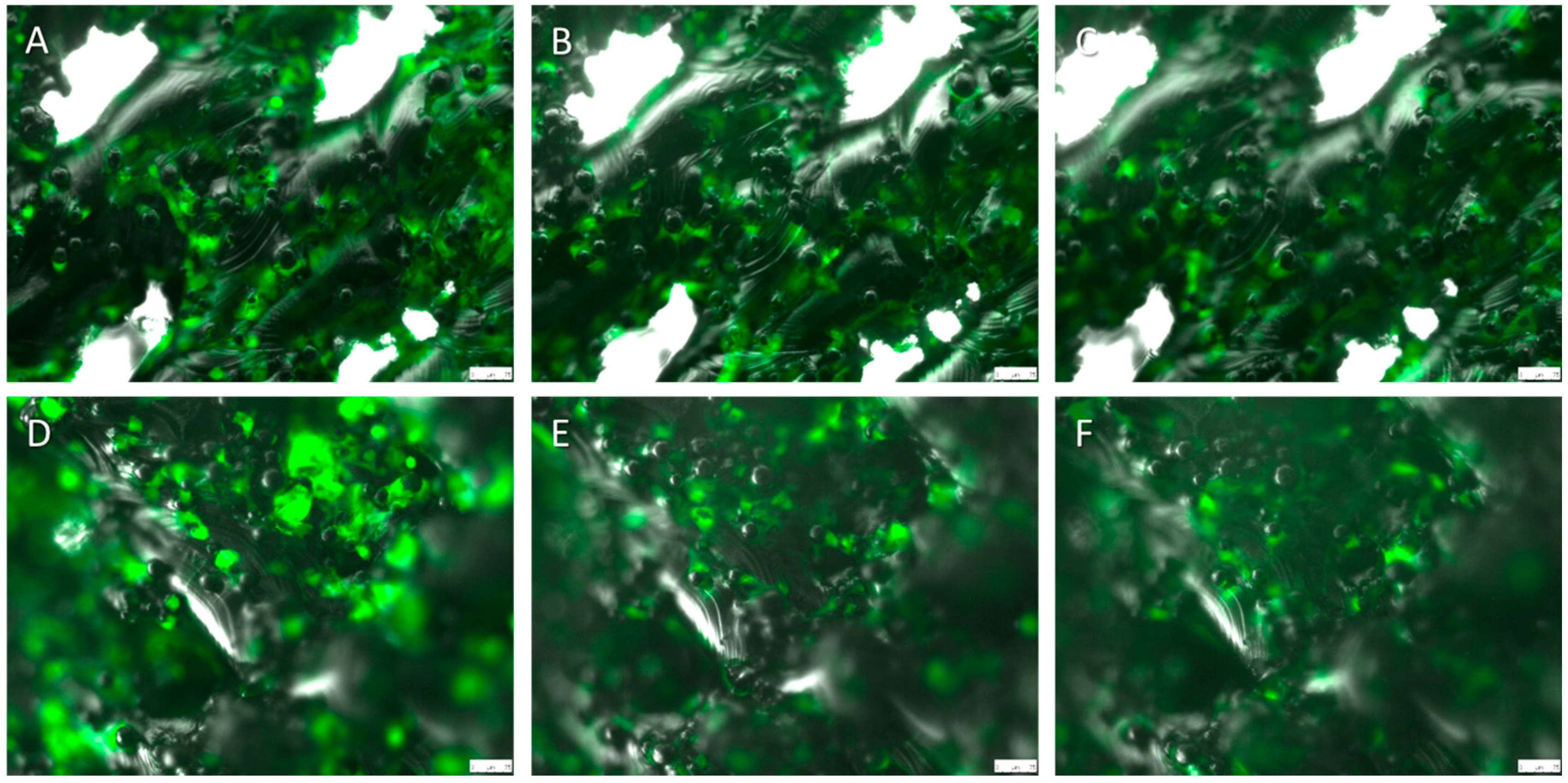

3. Discussion
4. Experimental Section
4.1. Manufacturing Titanium Implant
4.2. Dip-Coating Process for Application of Polymeric Coatings to Porous Titanium Scaffolds
4.3. Scanning Electron Microscopy and EDX Measurements
4.4. Cell Culture
4.4.1. Greenfluorescent Protein (GFP)–Osteoblast Isolation
4.4.2. GM7373
4.5. Cross Sections Established of Titanium Implants
4.6. Migration Assays of GM7373 on HMGB1, VEGF, CXCL12
4.7. Live Cell Imaging
4.8. Statistical Analysis
5. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Thijs, L.; Verhaeghe, F.; Craeghs, T.; Humbeeck, J.V.; Kruth, J.-P. A study of the microstructural evolution during selective laser melting of Ti–6Al–4V. Acta Mater. 2010, 58, 3303–3312. [Google Scholar] [CrossRef]
- Kruth, J.P.; Froyen, L.; van Vaerenbergh, J.; Mercelis, P.; Rombouts, M.; Lauwers, B. Selective laser melting of iron-based powder. J. Mater. Proc. Technol. 2004, 149, 616–622. [Google Scholar] [CrossRef]
- Murray, J.L.; Wriedt, H.A. The O–Ti (oxygen–titanium) system. JPE 1987, 8, 148–165. [Google Scholar] [CrossRef]
- Louvis, E.; Fox, P.; Sutcliffe, C.J. Selective laser melting of aluminium components. J. Mater. Proc. Technol. 2011, 211, 275–284. [Google Scholar] [CrossRef]
- Vandenbroucke, B.; Kruth, J.P. Selective laser melting of biocompatible metals for rapid manufacturing of medical parts. Rapid Prototyp. J. 2007, 13, 196–203. [Google Scholar] [CrossRef]
- Van Bael, S.; Chai, Y.C.; Truscello, S.; Moesen, M.; Kerckhofs, G.; van Oosterwyck, H.; Kruth, J.P.; Schrooten, J. The effect of pore geometry on the in vitro biological behavior of human periosteum-derived cells seeded on selective laser-melted Ti6Al4V bone scaffolds. Acta Biomater. 2012, 8, 2824–2834. [Google Scholar]
- Fukuda, A.; Takemoto, M.; Saito, T.; Fujibayashi, S.; Neo, M.; Pattanayak, D.K.; Matsushita, T.; Sasaki, K.; Nishida, N.; Kokubo, T.; et al. Osteoinduction of porous ti implants with a channel structure fabricated by selective laser melting. Acta Biomater. 2011, 7, 2327–2336. [Google Scholar] [CrossRef] [PubMed]
- Van der Stok, J.; van der Jagt, O.P.; Yavari, S.A.; de Haas, M.F.; Waarsing, J.H.; Jahr, H.; van Lieshout, E.M.; Patka, P.; Verhaar, J.A.; Zadpoor, A.A.; et al. Selective laser melting-produced porous titanium scaffolds regenerate bone in critical size cortical bone defects. J. Orthop. Res. 2013, 31, 792–799. [Google Scholar]
- Loh, Q.L.; Choong, C. Three-dimensional scaffolds for tissue engineering applications: Role of porosity and pore size. Tissue Eng. 2013, 19, 485–502. [Google Scholar] [CrossRef]
- Muller, D.; Chim, H.; Bader, A.; Whiteman, M.; Schantz, J.T. Vascular guidance: Microstructural scaffold patterning for inductive neovascularization. Stem Cells Int. 2010, 2011, 547247. [Google Scholar] [PubMed]
- Saran, U.; Gemini Piperni, S.; Chatterjee, S. Role of angiogenesis in bone repair. Arch. Biochem. Biophys. 2014, 561, 109–117. [Google Scholar] [CrossRef] [PubMed]
- Artel, A.; Mehdizadeh, H.; Chiu, Y.C.; Brey, E.M.; Cinar, A. An agent-based model for the investigation of neovascularization within porous scaffolds. Tissue Eng. 2011, 17, 2133–2141. [Google Scholar] [CrossRef]
- Lindhorst, D.; Tavassol, F.; von See, C.; Schumann, P.; Laschke, M.W.; Harder, Y.; Bormann, K.H.; Essig, H.; Kokemuller, H.; Kampmann, A.; et al. Effects of vegf loading on scaffold-confined vascularization. J. Biomed. Mater. Res. 2010, 95, 783–792. [Google Scholar] [CrossRef]
- Ring, A.; Langer, S.; Homann, H.H.; Kuhnen, C.; Schmitz, I.; Steinau, H.U.; Drucke, D. Analysis of neovascularization of PEGT/PBT-copolymer dermis substitutes in balb/c-mice. Burns 2006, 32, 35–41. [Google Scholar] [CrossRef] [PubMed]
- Ferrara, N. Vascular endothelial growth factor: Basic science and clinical progress. Endocr. Rev. 2004, 25, 581–611. [Google Scholar] [CrossRef] [PubMed]
- Wake, H.; Mori, S.; Liu, K.; Takahashi, H.K.; Nishibori, M. High mobility group box 1 complexed with heparin induced angiogenesis in a matrigel plug assay. Acta Med. Okayama 2009, 63, 249–262. [Google Scholar] [PubMed]
- Kew, R.R.; Penzo, M.; Habiel, D.M.; Marcu, K.B. The IKKα-dependent Nf-κB p52/RelB noncanonical pathway is essential to sustain a CXCL12 autocrine loop in cells migrating in response to HMGB1. J. Immunol. 2012, 188, 2380–2386. [Google Scholar] [CrossRef] [PubMed]
- De Giglio, E.; Cometa, S.; Ricci, M.A.; Zizzi, A.; Cafagna, D.; Manzotti, S.; Sabbatini, L.; Mattioli-Belmonte, M. Development and characterization of rhVEGF-loaded poly(HEMA-MOEP) coatings electrosynthesized on titanium to enhance bone mineralization and angiogenesis. Acta Biomater. 2010, 6, 282–290. [Google Scholar] [CrossRef] [PubMed]
- Fu, Y.; Zhang, Q.; Sun, Y.; Liao, W.; Bai, X.; Zhang, L.; Du, L.; Jin, Y.; Wang, Q.; Li, Z.; et al. Controlled-release of bone morphogenetic protein-2 from a microsphere coating applied to acid-etched Ti6Al4V implants increases biological bone growth in vivo. J. Orthop. Res. 2014, 32, 744–751. [Google Scholar] [CrossRef] [PubMed]
- Wulf, K.; Teske, M.; Lobler, M.; Luderer, F.; Schmitz, K.P.; Sternberg, K. Surface functionalization of poly(epsilon-caprolactone) improves its biocompatibility as scaffold material for bioartificial vessel prostheses. J. Biomed. Mater. Res. 2011, 98, 89–100. [Google Scholar] [CrossRef]
- Cipitria, A.; Reichert, J.C.; Epari, D.R.; Saifzadeh, S.; Berner, A.; Schell, H.; Mehta, M.; Schuetz, M.A.; Duda, G.N.; Hutmacher, D.W. Polycaprolactone scaffold and reduced rhBMP-7 dose for the regeneration of critical-sized defects in sheep tibiae. Biomaterials 2013, 34, 9960–9968. [Google Scholar] [CrossRef] [PubMed]
- Dash, T.K.; Konkimalla, V.B. Poly-ε-caprolactone based formulations for drug delivery and tissue engineering: A review. J. Control. Release 2012, 158, 15–33. [Google Scholar] [CrossRef] [PubMed]
- El-Hajje, A.; Kolos, E.C.; Wang, J.K.; Maleksaeedi, S.; He, Z.; Wiria, F.E.; Choong, C.; Ruys, A.J. Physical and mechanical characterisation of 3D-printed porous titanium for biomedical applications. J. Mater. Sci. Mater. Med. 2014, 25, 2471–2480. [Google Scholar] [CrossRef] [PubMed]
- Udroiu, R. Powder bed additive manufacturing systems and its applications. Acad. J. Manuf. Eng. 2012, 10, 122–129. [Google Scholar]
- Murr, L.E.; Quinones, S.A.; Gaytan, S.M.; Lopez, M.I.; Rodela, A.; Martinez, E.Y.; Hernandez, D.H.; Martinez, E.; Medina, F.; Wicker, R.B. Microstructure and mechanical behavior of Ti-6Al-4V produced by rapid-layer manufacturing, for biomedical applications. J. Mech. Behav. Biomed. Mater. 2009, 2, 20–32. [Google Scholar] [CrossRef] [PubMed]
- Kumara, S.; Pityanab, S. Laser-based additive manufacturing of metals. Adv. Mater. Res. 2011, 227, 4. [Google Scholar]
- Lewis, G. Properties of open-cell porous metals and alloys for orthopaedic applications. J. Mater. Sci. Mater. Med. 2013, 24, 2293–2325. [Google Scholar] [CrossRef] [PubMed]
- Yavari, S.A.; van der Stok, J.; Chai, Y.C.; Wauthle, R.; Tahmasebi Birgani, Z.; Habibovic, P.; Mulier, M.; Schrooten, J.; Weinans, H.; Zadpoor, A.A. Bone regeneration performance of surface-treated porous titanium. Biomaterials 2014, 35, 6172–6181. [Google Scholar] [CrossRef] [PubMed]
- Muller, U.; Imwinkelried, T.; Horst, M.; Sievers, M.; Graf-Hausner, U. Do human osteoblasts grow into open-porous titanium? Eur. Cells Mater. 2006, 11, 8–15. [Google Scholar]
- Van der Stok, J.; Wang, H.; Amin Yavari, S.; Siebelt, M.; Sandker, M.; Waarsing, J.H.; Verhaar, J.A.; Jahr, H.; Zadpoor, A.A.; Leeuwenburgh, S.C.; et al. Enhanced bone regeneration of cortical segmental bone defects using porous titanium scaffolds incorporated with colloidal gelatin gels for time- and dose-controlled delivery of dual growth factors. Tissue Eng. 2013, 19, 2605–2614. [Google Scholar] [CrossRef]
- Laschke, M.W.; Rucker, M.; Jensen, G.; Carvalho, C.; Mulhaupt, R.; Gellrich, N.C.; Menger, M.D. Improvement of vascularization of PLGA scaffolds by inosculation of in situ-preformed functional blood vessels with the host microvasculature. Ann. Surg. 2008, 248, 939–948. [Google Scholar] [CrossRef] [PubMed]
- Mitola, S.; Belleri, M.; Urbinati, C.; Coltrini, D.; Sparatore, B.; Pedrazzi, M.; Melloni, E.; Presta, M. Cutting edge: Extracellular high mobility group box-1 protein is a proangiogenic cytokine. J. Immunol. 2006, 176, 12–15. [Google Scholar] [CrossRef] [PubMed]
- Palumbo, R.; Sampaolesi, M.; de Marchis, F.; Tonlorenzi, R.; Colombetti, S.; Mondino, A.; Cossu, G.; Bianchi, M.E. Extracellular HMGB1, a signal of tissue damage, induces mesoangioblast migration and proliferation. J. Cell Biol. 2004, 164, 441–449. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zachary, I. Vegf signalling: Integration and multi-tasking in endothelial cell biology. Biochem. Soc. Trans. 2003, 31, 1171–1177. [Google Scholar] [CrossRef] [PubMed]
- Poh, C.K.; Shi, Z.; Lim, T.Y.; Neoh, K.G.; Wang, W. The effect of VEGF functionalization of titanium on endothelial cells in vitro. Biomaterials 2010, 31, 1578–1585. [Google Scholar] [CrossRef] [PubMed]
- Palumbo, R.; Galvez, B.G.; Pusterla, T.; De Marchis, F.; Cossu, G.; Marcu, K.B.; Bianchi, M.E. Cells migrating to sites of tissue damage in response to the danger signal HMGB1 require NF-κB activation. J. Cell Biol. 2007, 179, 33–40. [Google Scholar] [CrossRef] [PubMed]
- Schiraldi, M.; Raucci, A.; Munoz, L.M.; Livoti, E.; Celona, B.; Venereau, E.; Apuzzo, T.; de Marchis, F.; Pedotti, M.; Bachi, A.; et al. HMGB1 promotes recruitment of inflammatory cells to damaged tissues by forming a complex with CXCL12 and signaling via CXCR4. J. Exp. Med. 2012, 209, 551–563. [Google Scholar] [CrossRef] [PubMed]
- Xiao, Y.; Yuan, M.; Zhang, J.; Yan, J.; Lang, M. Functional poly(epsilon-caprolactone) based materials: Preparation, self-assembly and application in drug delivery. Curr. Top. Med. Chem. 2014, 14, 781–818. [Google Scholar] [CrossRef] [PubMed]
- Niu, X.; Yang, C.; Chen, D.; He, S.; Yan, D.; Yao, Y. Impact of drug-eluting stents with different coating strategies on stent thrombosis: A meta-analysis of 19 randomized trials. Cardiol. J. 2014, 21, 557–568. [Google Scholar] [CrossRef] [PubMed]
- Galli, C.; Piergianni, M.; Piemontese, M.; Lumetti, S.; Ravanetti, F.; Cacchioli, A.; Macaluso, G.M.; Passeri, G. Periostin improves cell adhesion to implantable biomaterials and osteoblastic differentiation on implant titanium surfaces in a topography-dependent fashion. J. Biomed. Mater. Res. 2014, 102, 3855–3861. [Google Scholar] [CrossRef]
- Sola-Ruiz, M.F.; Perez-Martinez, C.; Martin-Del-Llano, J.J.; Carda-Batalla, C.; Labaig-Rueda, C. In vitro preliminary study of osteoblast response to surface roughness of titanium discs and topical application of melatonin. Med. Oral Patol. Oral Cir. Bucal 2015, 20, e88–e93. [Google Scholar] [CrossRef] [PubMed]
- Sheikh, F.; Barakat, N.M.; Kanjwal, M.; Aryal, S.; Khil, M.; Kim, H.-Y. Novel self-assembled amphiphilic poly(ε-caprolactone)-grafted-poly(vinyl alcohol) nanoparticles: Hydrophobic and hydrophilic drugs carrier nanoparticles. J. Mater. Sci: Mater. Med. 2009, 20, 821–831. [Google Scholar] [CrossRef]
- Brynda, E.; Pachernik, J.; Houska, M.; Pientka, Z.; Dvorak, P. Surface immobilized protein multilayers for cell seeding. Langmuir 2005, 21, 7877–7883. [Google Scholar] [CrossRef] [PubMed]
- Williams, J.M.; Adewunmi, A.; Schek, R.M.; Flanagan, C.L.; Krebsbach, P.H.; Feinberg, S.E.; Hollister, S.J.; Das, S. Bone tissue engineering using polycaprolactone scaffolds fabricated via selective laser sintering. Biomaterials 2005, 26, 4817–4827. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.D.; Qian, H.Y.; Neff, L.; Satomura, K.; Horowitz, M.C. Thy-1 antigen expression by cells in the osteoblast lineage. J. Bone Miner. Res. 1999, 14, 362–375. [Google Scholar] [CrossRef] [PubMed]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Matena, J.; Petersen, S.; Gieseke, M.; Kampmann, A.; Teske, M.; Beyerbach, M.; Escobar, H.M.; Haferkamp, H.; Gellrich, N.-C.; Nolte, I. SLM Produced Porous Titanium Implant Improvements for Enhanced Vascularization and Osteoblast Seeding. Int. J. Mol. Sci. 2015, 16, 7478-7492. https://doi.org/10.3390/ijms16047478
Matena J, Petersen S, Gieseke M, Kampmann A, Teske M, Beyerbach M, Escobar HM, Haferkamp H, Gellrich N-C, Nolte I. SLM Produced Porous Titanium Implant Improvements for Enhanced Vascularization and Osteoblast Seeding. International Journal of Molecular Sciences. 2015; 16(4):7478-7492. https://doi.org/10.3390/ijms16047478
Chicago/Turabian StyleMatena, Julia, Svea Petersen, Matthias Gieseke, Andreas Kampmann, Michael Teske, Martin Beyerbach, Hugo Murua Escobar, Heinz Haferkamp, Nils-Claudius Gellrich, and Ingo Nolte. 2015. "SLM Produced Porous Titanium Implant Improvements for Enhanced Vascularization and Osteoblast Seeding" International Journal of Molecular Sciences 16, no. 4: 7478-7492. https://doi.org/10.3390/ijms16047478
APA StyleMatena, J., Petersen, S., Gieseke, M., Kampmann, A., Teske, M., Beyerbach, M., Escobar, H. M., Haferkamp, H., Gellrich, N.-C., & Nolte, I. (2015). SLM Produced Porous Titanium Implant Improvements for Enhanced Vascularization and Osteoblast Seeding. International Journal of Molecular Sciences, 16(4), 7478-7492. https://doi.org/10.3390/ijms16047478





