Combined Enzymatic and Mechanical Cell Disruption and Lipid Extraction of Green Alga Neochloris oleoabundans
Abstract
:1. Introduction
2. Results and Discussion
2.1. Cell Disruption by Ultrasonication

2.2. Cell Disruption by High Pressure Homogenization

2.3. Cell Disruption by Enzymatic Lyses

2.4. Cell Disruption by Combined Processes

2.5. Cell Morphology Observation
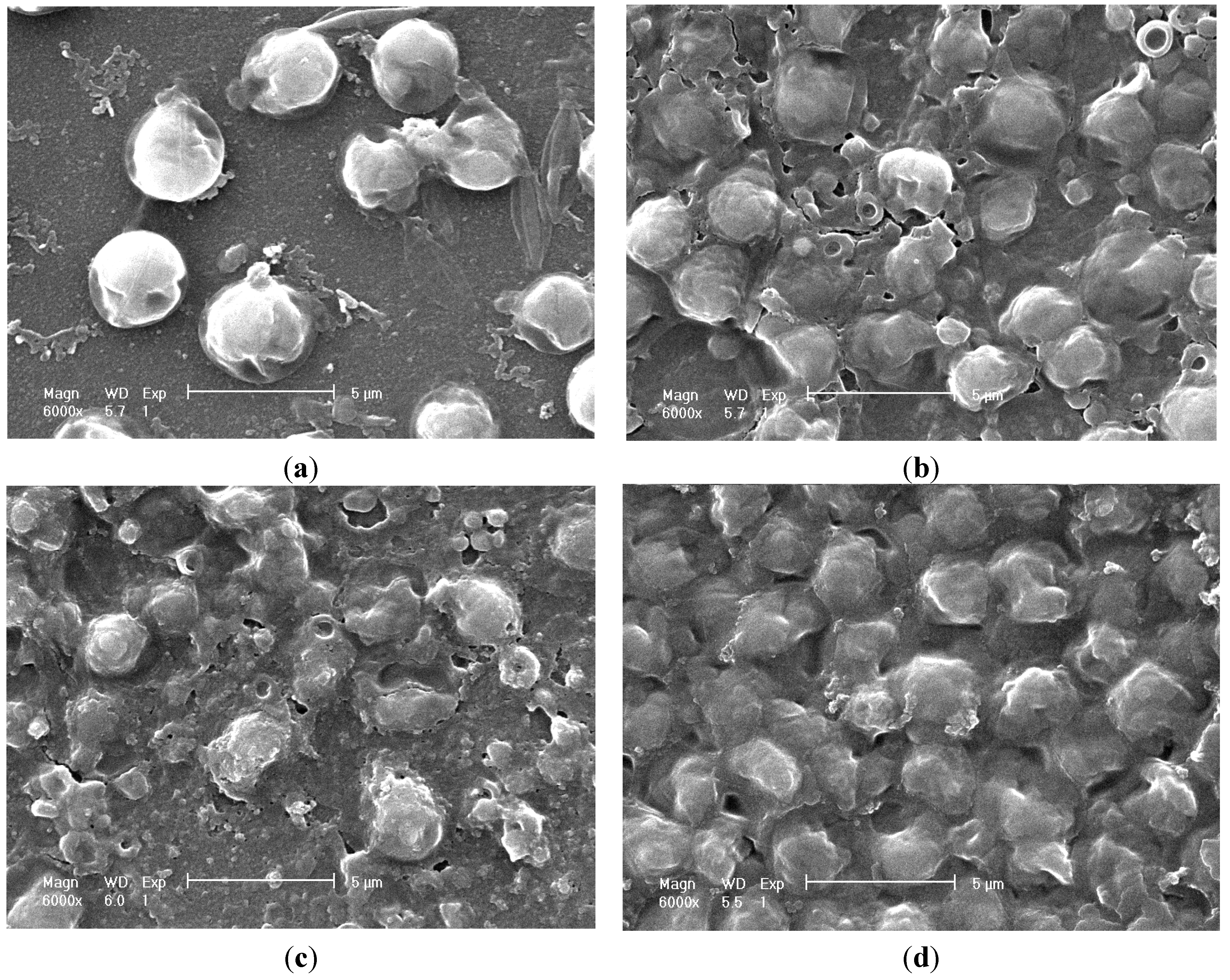

2.6. Discussion
| Disruption Processing | E | US | E + US | HPH | E + HPH |
|---|---|---|---|---|---|
| Oil recovery | |||||
| Oil yield (g/L) | 2.35 | 3.84 | 5.55 | 4.96 | 6.67 |
| Oil loss (g/L) | 4.85 | 3.36 | 1.70 | 2.24 | 0.53 |
| Count for oil loss (USD) | 0.0024 | 0.0017 | 0.00065 | 0.0011 | 0.00027 |
| Enzyme | |||||
| Papain (g/L) | 0.4 | 0.2 | 0.2 | ||
| Cellulase (g/L) | 0.6 | 0.2 | 0.2 | ||
| Cost for enzymes (USD) | 0.0066 | 0.0023 | 0.0023 | ||
| Electricity (for mechanical disruption) | |||||
| Electricity currency (A, 220 V) | 3.5 | 2.5 | 13.5 | 10.5 | |
| Operation time (h) | 0.5 | 0.5 | 0.07 | 0.035 | |
| Energy (kJ/L) | 1386 | 990 | 712 | 277 | |
| Cost for electricity (USD) | 0.0347 | 0.0243 | 0.0178 | 0.00693 | |
| Total disruption processing cost (USD) | 0.009 | 0.0364 | 0.0273 | 0.0189 | 0.00953 |
| Disruption cost to yielded oil (USD/kg) | 3.83 | 9.81 | 5.41 | 3.81 | 1.43 |
3. Experimental Section
3.1. Strain and Culture Conditions
3.2. Measurements of Cell Density
3.3. Biomass Harvest
3.4. Cell Disruption by Ultrasonic Wave
3.5. Cell Disruption by Homogenization
3.6. Cell Disruption by Enzymatic Lyses
| Patterns | Reaction Schedule |
|---|---|
| Pattern 1 | (cellulase + papain), reacting for 3 h |
| Pattern 2 | cellulase, reacting for 3 h → papain, reacting for 2 h |
| Pattern 3 | papain, reacting for 2 h → cellulase, reacting for 3 h |
3.7. Cell Disruption by Combined Processes of Enzymatic Lyses and Mechanical Methods
3.8. Determination of Cell Disruption Degree
3.9. Lipid Extraction
3.10. Morphological Observation
3.11. Data Statistic Analysis
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Chisti, Y. Biodiesel from microalgae. Biotechnol. Adv. 2007, 25, 294–306. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Horsman, M.; Wang, B.; Wu, N.; Lan, C.Q. Effects of nitrogen sources on cell growth and lipid accumulation of green alga Neochloris oleoabundans. Appl. Microbiol. Biotechnol. 2008, 81, 629–636. [Google Scholar] [CrossRef] [PubMed]
- Irina, G.A.; Harwood, J.L. Lipids and lipid metabolism in eukaryotic algae. Prog. Lipid Res. 2006, 45, 160–186. [Google Scholar] [CrossRef] [PubMed]
- Selvakumar, P.; Umadevi, K. Enhanced lipid and fatty acid content under photoheterotrophic condition in the mass cultures of Tetraselmis gracilis and Platymonas convolutae. Algal Res. 2014, 6, 180–185. [Google Scholar] [CrossRef]
- Sarma, S.J.; Das, R.K.; Brar, S.K.; Le Bihan, Y.; Buelna, G.; Verma, M.; Soccol, C.R. Application of magnesium sulfate and its nanoparticles for enhanced lipid production by mixotrophic cultivation of algae using biodiesel waste. Energy 2014, 78, 16–22. [Google Scholar] [CrossRef]
- Nakanishi, A.; Aikawa, S.; Ho, S.-H.; Chen, C.-Y.; Chang, J.-S.; Hasunuma, T.; Kondo, A. Development of lipid productivities under different CO2 conditions of marine microalgae Chlamydomonas sp. JSC4. Bioresour. Technol. 2014, 152, 247–252. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Han, F.; Xu, H.; Mu, J.; Chen, D.; Feng, B.; Zeng, H. Potential lipid accumulation and growth characteristic of the green alga Chlorella with combination cultivation mode of nitrogen (N) and phosphorus (P). Bioresour. Technol. 2014, 174, 24–32. [Google Scholar] [CrossRef] [PubMed]
- Feng, P.; Yang, K.; Xu, Z.; Wang, Z.; Fan, L.; Qin, L.; Zhu, S.; Shang, C.; Chai, P.; Yuan, Z.; et al. Growth and lipid accumulation characteristics of Scenedesmus obliquus in semi-continuous cultivation outdoors for biodiesel feedstock production. Bioresour. Technol. 2014, 173, 406–414. [Google Scholar] [CrossRef] [PubMed]
- Bilad, M.R.; Discart, V.; Vandamme, D.; Foubert, I.; Muylaert, K.; Vankelecom, I.F.J. Harvesting microalgal biomass using a magnetically induced membrane vibration (MMV) system: Filtration performance and energy consumption. Bioresour. Technol. 2013, 138, 329–338. [Google Scholar] [CrossRef] [PubMed]
- Chen, F.; Liu, Z.; Li, D.; Liu, C.; Zheng, P.; Chen, S. Using ammonia for algae harvesting and as nutrient in subsequent cultures. Bioresour. Technol. 2012, 121, 298–303. [Google Scholar] [CrossRef] [PubMed]
- Yap, B.H.J.; Crawford, S.A.; Dumsday, G.J.; Scales, P.J.; Martin, G.J.O. A mechanistic study of algal cell disruption and its effect on lipid recovery by solvent extraction. Algal Res. 2014, 5, 112–120. [Google Scholar] [CrossRef]
- Shin, H.-Y.; Ryu, J.-H.; Bae, S.-Y.; Crofcheck, C.; Crocker, M. Lipid extraction from Scenedesmus sp. microalgae for biodiesel production using hot compressed hexane. Fuel 2014, 130, 66–69. [Google Scholar] [CrossRef]
- Reddy, H.K.; Muppaneni, T.; Sun, Y.; Li, Y.; Ponnusamy, S.; Patil, P.D.; Dailey, P.; Schaub, T.; Holguin, F.O.; Dungan, B.; et al. Subcritical water extraction of lipids from wet algae for biodiesel production. Fuel 2014, 133, 73–81. [Google Scholar] [CrossRef]
- Bai, X.; Ghasemi Naghdi, F.; Ye, L.; Lant, P.; Pratt, S. Enhanced lipid extraction from algae using free nitrous acid pretreatment. Bioresour. Technol. 2014, 159, 36–40. [Google Scholar] [CrossRef] [PubMed]
- Patil, P.D.; Gude, V.G.; Mannarswamy, A.; Cooke, P.; Nirmalakhandan, N.; Lammers, P.; Deng, S. Comparison of direct transesterification of algal biomass under supercritical methanol and microwave irradiation conditions. Fuel 2012, 97, 822–831. [Google Scholar] [CrossRef]
- Show, K.-Y.; Lee, D.-J.; Tay, J.-H.; Lee, T.-M.; Chang, J.-S. Microalgal drying and cell disruption—Recent advances. Bioresour. Technol. 2014, 184, 258–266. [Google Scholar] [CrossRef] [PubMed]
- Johansen, M.N. Microalgae: Biotechnology, Microbiology, and Energy; Nova Science Publishers: New York, NY, USA, 2012. [Google Scholar]
- Lee, A.K.; Lewis, D.M.; Ashman, P.J. Disruption of microalgal cells for the extraction of lipids for biofuels: Processes and specific energy requirements. Biomass Bioenergy 2012, 46, 89–101. [Google Scholar] [CrossRef]
- Halim, R.; Rupasinghe, T.W.T.; Tull, D.L.; Webley, P.A. Mechanical cell disruption for lipid extraction from microalgal biomass. Bioresour. Technol. 2013, 140, 53–63. [Google Scholar] [CrossRef] [PubMed]
- Samarasinghe, N.; Fernando, S.; Lacey, R.; Faulkner, W.B. Algal cell rupture using high pressure homogenization as a prelude to oil extraction. Renew. Energy 2012, 48, 300–308. [Google Scholar] [CrossRef]
- Choi, S.-A.; Jung, J.-Y.; Kim, K.; Lee, J.-S.; Kwon, J.-H.; Kim, S.W.; Yang, J.-W.; Park, J.-Y. Acid-catalyzed hot-water extraction of docosahexaenoic acid (DHA)-rich lipids from Aurantiochytrium sp. KRS101. Bioresour. Technol. 2014, 161, 469–472. [Google Scholar] [CrossRef] [PubMed]
- Park, J.-Y.; Oh, Y.-K.; Lee, J.-S.; Lee, K.; Jeong, M.-J.; Choi, S.-A. Acid-catalyzed hot-water extraction of lipids from Chlorella vulgaris. Bioresour. Technol. 2014, 153, 408–412. [Google Scholar] [CrossRef] [PubMed]
- McMillan, J.R.; Watson, I.A.; Ali, M.; Jaafar, W. Evaluation and comparison of algal cell disruption methods: Microwave, waterbath, blender, ultrasonic and laser treatment. Appl. Energy 2013, 103, 128–134. [Google Scholar] [CrossRef]
- Chen, C.-Y.; Bai, M.-D.; Chang, J.-S. Improving microalgal oil collecting efficiency by pretreating the microalgal cell wall with destructive bacteria. Biochem. Eng. J. 2013, 81, 170–176. [Google Scholar] [CrossRef]
- Lee, J.-Y.; Yoo, C.; Jun, S.-Y.; Ahn, C.-Y.; Oh, H.-M. Comparison of several methods for effective lipid extraction from microalgae. Bioresour. Technol. 2010, 101 (Suppl. S1), S75–S77. [Google Scholar] [CrossRef]
- Araujo, G.S.; Matos, L.J.B.L.; Fernandes, J.O.; Cartaxo, S.J.M.; Gonçalves, L.R.B.; Fernandes, F.A.N.; Farias, W.R.L. Extraction of lipids from microalgae by ultrasound application: Prospection of the optimal extraction method. Ultrason. Sonochem. 2013, 20, 95–98. [Google Scholar] [CrossRef] [PubMed]
- Gerde, J.A.; Montalbo-Lomboy, M.; Yao, L.; Grewell, D.; Wang, T. Evaluation of microalgae cell disruption by ultrasonic treatment. Bioresour. Technol. 2012, 125, 175–181. [Google Scholar] [CrossRef] [PubMed]
- Keris-Sen, U.D.; Sen, U.; Soydemir, G.; Gurol, M.D. An investigation of ultrasound effect on microalgal cell integrity and lipid extraction efficiency. Bioresour. Technol. 2014, 152, 407–413. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Yuan, W.; Jiang, X.; Jing, Y.; Wang, Z. Disruption of microalgal cells using high-frequency focused ultrasound. Bioresour. Technol. 2014, 153, 315–321. [Google Scholar] [CrossRef] [PubMed]
- Günerken, E.; D’Hondt, E.; Eppink, M.H.M.; Garcia-Gonzalez, L.; Elst, K.; Wijffels, R.H. Cell disruption for microalgae biorefineries. Biotechnol. Adv. 2015, 33, 243–260. [Google Scholar] [CrossRef] [PubMed]
- Spiden, E.M.; Yap, B.H.J.; Hill, D.R.A.; Kentish, S.E.; Scales, P.J.; Martin, G.J.O. Quantitative evaluation of the ease of rupture of industrially promising microalgae by high pressure homogenization. Bioresour. Technol. 2013, 140, 165–171. [Google Scholar] [CrossRef] [PubMed]
- Imam, S.H.; Buchanan, M.J.; Shin, H.-C.; Snell, W.J. The chlamydomonas cell wall: Characterization of the wall framework. J. Cell Biol. 1985, 101, 1599–1607. [Google Scholar] [CrossRef] [PubMed]
- Taher, H.; Al-Zuhair, S.; Al-Marzouqi, A.H.; Haik, Y.; Farid, M. Effective extraction of microalgae lipids from wet biomass for biodiesel production. Biomass Bioenergy 2014, 66, 159–167. [Google Scholar] [CrossRef]
- Jin, G.; Yang, F.; Hu, C.; Shen, H.; Zhao, Z.K. Enzyme-assisted extraction of lipids directly from the culture of the oleaginous yeast Rhodosporidium toruloides. Bioresour. Technol. 2012, 111, 378–382. [Google Scholar] [CrossRef] [PubMed]
- Chantanachat, S.; Bold, H.C. Phycological Studies. II. Some Algae from Arid Soils; University of Texas Publications: Austin, TX, USA, 1962; pp. 1–74. [Google Scholar]
- Tornabene, T.; Holzer, G.; Lien, S.; Burris, N. Lipid composition of the nitrogen starved green alga Neochloris oleoabundans. Enzyme Microb. Technol. 1983, 5, 435–440. [Google Scholar] [CrossRef]
- Yap, B.H.J.; Dumsday, G.J.; Scales, P.J.; Martin, G.J.O. Energy evaluation of algal cell disruption by high pressure homogenisation. Bioresour. Technol. 2014, 184, 280–285. [Google Scholar] [CrossRef] [PubMed]
- Halim, R.; Harun, R.; Danquah, M.K.; Webley, P.A. Microalgal cell disruption for biofuel development. Appl. Energy 2012, 91, 116–121. [Google Scholar] [CrossRef]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, D.; Li, Y.; Hu, X.; Su, W.; Zhong, M. Combined Enzymatic and Mechanical Cell Disruption and Lipid Extraction of Green Alga Neochloris oleoabundans. Int. J. Mol. Sci. 2015, 16, 7707-7722. https://doi.org/10.3390/ijms16047707
Wang D, Li Y, Hu X, Su W, Zhong M. Combined Enzymatic and Mechanical Cell Disruption and Lipid Extraction of Green Alga Neochloris oleoabundans. International Journal of Molecular Sciences. 2015; 16(4):7707-7722. https://doi.org/10.3390/ijms16047707
Chicago/Turabian StyleWang, Dongqin, Yanqun Li, Xueqiong Hu, Weimin Su, and Min Zhong. 2015. "Combined Enzymatic and Mechanical Cell Disruption and Lipid Extraction of Green Alga Neochloris oleoabundans" International Journal of Molecular Sciences 16, no. 4: 7707-7722. https://doi.org/10.3390/ijms16047707
APA StyleWang, D., Li, Y., Hu, X., Su, W., & Zhong, M. (2015). Combined Enzymatic and Mechanical Cell Disruption and Lipid Extraction of Green Alga Neochloris oleoabundans. International Journal of Molecular Sciences, 16(4), 7707-7722. https://doi.org/10.3390/ijms16047707





