New Biofuel Alternatives: Integrating Waste Management and Single Cell Oil Production
Abstract
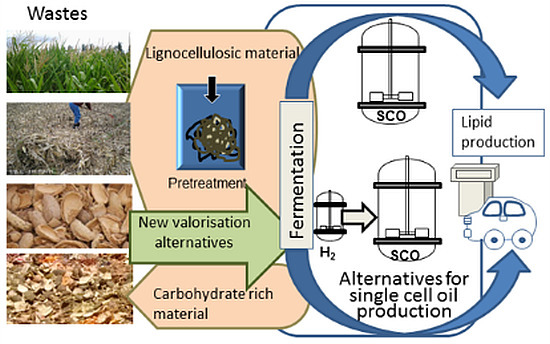
:1. Introduction
2. Lipid-Accumulating Organisms
| Bacteria | Carbon Sources | Reference |
|---|---|---|
| Rhodococcus opacus PD630 | Carob waste | [22] |
| Gordonia sp. DG | Orange waste | [22] |
| Bacillus subtilis | Glucose | [23] |
| Yeast | ||
| Lipomyces starkeyi | Glucose/xylose | [24] |
| Cryptococcus curvatus | Acetic acid Glycerol | [25,26] |
| Rhodotorula glutinis | Distillery wastewater | [27] |
| Rhodotorula mucilaginosa | Hydrolysate of cassava starch | [28] |
| Rhodosporidium toruloides | Glucose | [29] |
| Yarrowia lipolytica | Glucose wastes | [30] |
| Trichosporon fermentans | Glucose | [31] |
| Mould | ||
| Mortierella isabellina | Xylose | [32,33] |
| Cunninghamella echinulata | Xylose | [32] |
| Thamnidium elegans | Glucose, fructose and sucrose | [34] |
| Mucor sp. | Cheese whey | [35] |
2.1. Triacylglycerol (TAG) Biosynthesis in Microorganisms
2.1.1. De Novo Lipid Accumulation

2.1.2. Ex Novo Lipid Accumulation
3. Reducing Fermentation Costs with the Use of Wastes and Lignocellulosic Biomass
| Substrate | Microorganism | Lipid Yields (g Lipid/g Biomass) | Reference |
|---|---|---|---|
| High carbohydrate content | |||
| Glucose derived from maize starch hydrolysate | Mortierella alpina | 0.33–0.36 | [73] |
| Molasses | Candida lipolytica, Candida tropicalis, Rhodotorula mucilaginosa | 0.16–0.60 | [74] |
| 0.12–0.46 | |||
| 0.39–0.69 | |||
| Glycerol | Mortierella alpina | 0.05–0.33 | [42] |
| Crude glycerol | Cryptococcus curvatus | 0.44–0.52 | [41] |
| Lignocellulosic material | |||
| Rice hull hydrolysate | Mortierella isabellina | 0.64 | [75] |
| Cassava starch hydrolysate | Rhodosporidium toruloides | 0.63 | [76] |
| Corncobs | Trichosporon dermatis | 0.17 | [77] |
| Corn stover | Cryptococcus curvatus | 0.16 | [78] |
| Rice straw hydrolysate | Trichosporon fermentans | 0.4 | [79] |
| Wheat straw | Cryptococcus curvatus | 0.05 | [80] |
| Complex substrates | |||
| Distillery wastewater | Rhodotorula glutinis, Cryptococcus curvatus | 0.25 | [27] |
| 0.27 | |||
| Pre–treated sewage sludge (ultrasounds) | Lipomyces starkeyi | 0.32–0.35 | [81] |
| Waste cooking oil | Yarrowia lipolytica | 0.17–0.55 | [30] |
| Waste motor oil | |||
| Palm oil mill effluent | Rhodotorula glutinis | 0.21–0.38 | [82] |
4. Improving Lipid Production from Wastes
5. Conclusions
Acknowledgements
Author Contributions
Conflicts of Interest
References
- Balan, V.; Chiaramonti, D.; Kumar, S. Review of US and EU initiatives toward development, demonstration, and commercialization of lignocellulosic biofuels. Biofuels Bioprod. Bioref. 2013, 7, 732–759. [Google Scholar] [CrossRef]
- Helwani, Z.; Othman, M.R.; Aziz, N.; Fernando, W.J.N.; Kim, J. Technologies for production of biodiesel focusing on green catalytic techniques: A review. Fuel Process. Technol. 2009, 90, 1502–1514. [Google Scholar] [CrossRef]
- Moser, B.R. Biodiesel production, properties, and feedstocks. In Biofuels; Springer New York: New York, NY, USA, 2011; pp. 285–347. [Google Scholar]
- Leung, D.Y.C.; Wu, X.; Leung, M.K.H. A review on biodiesel production using catalyzed transesterification. Appl. Energy 2010, 87, 1083–1095. [Google Scholar] [CrossRef]
- Olkiewicz, M.; Fortuny, A.; Stüber, F.; Fabregat, A.; Font, J.; Bengoa, C. Evaluation of different sludges from WWTP as a potential source for biodiesel production. Procedia Eng. 2012, 42, 634–643. [Google Scholar] [CrossRef]
- Lin, Y.C.; Hsu, K.; Chen, C.B. Experimental investigation of the performance and emissions of a heavy–duty diesel engine fueled with waste cooking oil biodiesel/ultra-low sulfur diesel blends. Energy 2011, 36, 241–248. [Google Scholar] [CrossRef]
- Fierro, J.; Martínez, E.J.; Morán, A.; Gómez, X. Valorisation of used cooking oil sludge by codigestion with swine manure. Waste Manag 2014, 34, 1537–1545. [Google Scholar]
- Li, Q.; Wang, M.Y. Use food industry waste to produce microbial oil. Sci. Technol. Food Ind. 1997, 6, 65–69. [Google Scholar]
- Liang, M.H.; Jiang, J.G. Advancing oleaginous microorganisms to produce lipid via metabolic engineering technology. Prog. Lipid Res. 2013, 52, 395–408. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Du, W.; Liu, D. Perspectives of microbial oils for biodiesel production. Appl. Microbiol. Biotechnol. 2008, 80, 749–756. [Google Scholar] [CrossRef] [PubMed]
- Vicente, G.; Bautista, L.F.; Rodríguez, R.; Gutiérrez, F.J.; Sádaba, I.; Ruiz-Vázquez, R.M.; Torres-Martínez, S.; Garre, V. Biodiesel production from biomass of an oleaginous fungus. Biochem. Eng. J. 2009, 48, 22–27. [Google Scholar] [CrossRef]
- Wu. H.; Li, Y.; Chen, L.; Zong, M. Production of microbial oil with high oleic acid content by Trichosporon capitatum. Appl. Energy 2011, 88, 138–142. [Google Scholar] [CrossRef]
- Gouveia, L.; Oliveira, A.C. Microalgae as a raw material for biofuels production. J. Ind. Microbiol. Biotechnol. 2009, 36, 269–274. [Google Scholar] [CrossRef] [PubMed]
- Sing, S.F.; Isdepsky, A.; Borowitzka, M.A.; Moheimani, N.R. Production of biofuels from microalgae. Mitig. Adapt. Strateg. Glob. Chang. 2013, 18, 47–72. [Google Scholar] [CrossRef]
- Ratledge, C. Single cell oils—Have they a biotechnological future? Trends Biotechnol. 1993, 11, 278–284. [Google Scholar] [CrossRef] [PubMed]
- Zhang, F.; Rodriguez, S.; Keasling, J.D. Metabolic engineering of microbial pathways for advanced biofuels production. Curr. Opin. Biotechnol. 2011, 22, 775–783. [Google Scholar] [CrossRef] [PubMed]
- Papanikolaou, S.; Aggelis, G. Lipids of oleaginous yeasts. Part I: Biochemistry of single cell oil production. Eur. J. Lipid Sci. Technol. 2011, 113, 1031–1051. [Google Scholar] [CrossRef]
- Richardson, A.J.; Burton, J.R.; Sewell, R.P.; Spreckelsen, T.F.; Montgomery, P. Docosahexaenoic acid for reading, cognition and behavior in children aged 7–9 years: A randomized, controlled trial (the DOLAB study). PLoS ONE 2012, 7, e43909. [Google Scholar] [CrossRef] [PubMed]
- Subramaniam, R.; Dufreche, S.; Zappi, M.; Bajpai, R. Microbial lipids from renewable resources: Production and characterization. J. Ind. Microbiol. Biotechnol. 2010, 37, 1271–1287. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.; Chen, X.F.; Xiong, L.; Chen, X.D.; Ma, L.L.; Chen, Y. Single cell oil production from low-cost substrates: The possibility and potential of its industrialization. Biotechnol. Adv. 2013, 31, 129–139. [Google Scholar] [CrossRef] [PubMed]
- Spiekermann, P.; Rehm, B.H.A.; Kalscheuer, R.; Baumeister, D.; Steinbüchel, A. A sensitive, viable-colony staining method using Nile red for direct screening of bacteria that accumulate polyhydroxyalkanoic acids and other lipid storage compounds. Arch. Microbiol. 1999, 171, 73–80. [Google Scholar] [CrossRef] [PubMed]
- Gouda, M.K.; Omar, S.H.; Aouad, L.M. Single cell oil production by Gordonia sp. DG using agro-industrial wastes. World J. Microb. Biotechnol. 2008, 24, 1703–1711. [Google Scholar] [CrossRef]
- Patnayak, S.; Sree, A. Screening of bacterial associates of marine sponges for single cell oil and PUFA. Lett. Appl. Microbiol. 2005, 40, 358–363. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Kong, X.; Hua, Y.; Feng, B.; Zhao, Z. Medium optimization for lipid production through co–fermentation of glucose and xylose by the oleaginous yeast Lipomyces starkeyi. Eur. J. Lipid Sci. Technol. 2008, 110, 405–412. [Google Scholar] [CrossRef]
- Christophe, G.; Deo, J.L.; Kumar, V.; Nouaille, R.; Fontanille, P.; Larroche, C. Production of oils from acetic acid by the oleaginous yeast Cryptococcus curvatus. Appl. Biochem. Biotechnol. 2012, 167, 1270–1279. [Google Scholar] [CrossRef] [PubMed]
- Meesters, P.A.E.P.; Huijberts, G.N.M.; Eggink, G. High–cell–density cultivation of the lipid accumulating yeast Cryptococcus curvatus using glycerol as a carbon source. J. Appl. Microbiol. Biotechnol. 1996, 45, 575–579. [Google Scholar] [CrossRef]
- Gonzalez-Garcia, Y.; Hernandez, R.; Zhang, G.; Escalante, F.M.; Holmes, W.; French, W.T. Lipids accumulation in Rhodotorula glutinis and Cryptococcus curvatus growing on distillery wastewater as culture medium. Environ. Prog. Sustain. Energy 2013, 32, 69–74. [Google Scholar] [CrossRef]
- Li, M.; Liu, G.L.; Chi, Z.; Chi, Z.M. Single cell oil production from hydrolysate of cassava starch by marine–derived yeast Rhodotorula mucilaginosa TJY15a. Biomass Bioenergy 2010, 34, 101–107. [Google Scholar] [CrossRef]
- Li, Y.H.; Zhao, Z.B.; Bai, F.W. High–density cultivation of oleaginous yeast Rhodosporidium toruloides Y4 in fed-batch culture. Enzym. Microb. Technol. 2007, 41, 312–317. [Google Scholar] [CrossRef]
- Katre, G.; Joshi, C.; Khot, M.; Zinjarde, S.; RaviKumar, A. Evaluation of single cell oil (SCO) from a tropical marine yeast Yarrowia lipolytica NCIM 3589 as a potential feedstock for biodiesel. AMB Express 2012, 2, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Zhu, L.Y.; Zong, M.H.; Wu, H. Efficient lipid production with Trichosporon fermentans and its use for biodiesel preparation. Bioresour. Technol. 2008, 99, 7881–7885. [Google Scholar] [CrossRef] [PubMed]
- Fakas, S.; Papanikolaou, S.; Batsos, A.; Galiotou-Panayotou, M.; Mallouchos, A.; Aggelis, G. Evaluating renewable carbon sources as substrates for single cell oil production by Cunninghamella echinulata and Mortierella isabellina. Biomass Bioenergy 2009, 33, 573–580. [Google Scholar] [CrossRef]
- Gao, D.; Zeng, J.; Zheng, Y.; Yu, X.; Chen, S. Microbial lipid production from xylose by Mortierella isabellina. Bioresour. Technol. 2013, 133, 315–321. [Google Scholar] [CrossRef] [PubMed]
- Papanikolaou, S.; Diamantopoulou, P.; Chatzifragkou, A.; Philippoussis, A.; Aggelis, G. Suitability of low-cost sugars as substrates for lipid production by the fungus Thamnidium elegans. Energy Fuels 2010, 24, 4078–4086. [Google Scholar] [CrossRef]
- Vamvakaki, A.N.; Kandarakis, I.; Kaminarides, S.; Komaitis, M.; Papanikolaou, S. Cheese whey as a renewable substrate for microbial lipid and biomass production by Zygomycetes. Eng. Life Sci. 2010, 10, 348–360. [Google Scholar] [CrossRef]
- Meng, X.; Yang, J.; Xu, X.; Zhang, L.; Nie, Q.; Xian, M. Biodiesel production from oleaginous microorganisms. Renew. Energy 2009, 34, 1–5. [Google Scholar] [CrossRef]
- Amaretti, A.; Raimondi, S.; Sala, M.; Roncaglia, L.; de Lucia, M.; Leonardi, A.; Rossi, M. Single cell oils of the cold-adapted oleaginous yeast Rhodotorula glacialis. DBVPG 4785. Microb. Cell Factories 2010, 9, 73–73. [Google Scholar]
- Papanikolaou, S.; Galiotou-Panayotou, M.; Fakas, S.; Komaitis, M.; Aggelis, G. Lipid production by oleaginous Mucorales cultivated on renewable carbon sources. Eur. J. Lipid Sci. Technol. 2007, 109, 1060–1070. [Google Scholar] [CrossRef]
- Koga, Y.; Morii, H. Recent advances in structural research on ether lipids from archaea including comparative and physiological aspects. Biosci. Biotechnol. Biochem. 2005, 69, 2019–2034. [Google Scholar] [CrossRef] [PubMed]
- Matsumi, R.; Atomi, H.; Driessen, A.J.M.; An der Oost, J. Isoprenoid biosynthesis in Archaea-biochemical and evolutionary implications. Res. Microbiol. 2011, 162, 39–52. [Google Scholar] [CrossRef] [PubMed]
- Liang, Y.; Cui, Y.; Trushenski, J.; Blackburn, J.W. Converting crude glycerol derived from yellow grease to lipids through yeast fermentation. Bioresour. Technol. 2010, 101, 7581–7586. [Google Scholar] [CrossRef] [PubMed]
- Dedyukhina, E.G.; Chistyakova, T.I.; Kamzolova, S.V.; Vinter, M.V.; Vainshtein, M.B. Arachidonic acid synthesis by glycerol grown Mortierella alpina. Eur. J. Lipid Sci. Technol. 2012, 114, 833–841. [Google Scholar] [CrossRef]
- Chatzifragkou, A.; Fakas, S.; Galiotou-Panayotou, M.; Komaitis, M.; Aggelis, G.; Papanikolaou, S. Commercial sugars as substrates for lipid accumulation in Cunninghamella echinulata and Mortierella isabellina fungi. Eur. J. Lipid Sci. Technol. 2010, 112, 1048–1057. [Google Scholar] [CrossRef]
- Ratledge, C. Fatty acid biosynthesis in microorganisms being used for single cell oil production. Biochimie 2004, 86, 807–815. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Du, W.; Zhao, X.; Zhang, G.; Liu, D. Microbial oil production from various carbon sources and its use for biodiesel preparation. Biofuels Bioprod. Biorefin. 2013, 7, 65–77. [Google Scholar] [CrossRef]
- Wu, S.; Hu, C.; Jin, G.; Zhao, X.; Zhao, Z.K. Phosphate–limitation mediated lipid production by Rhodosporidium toruloides. Bioresour. Technol. 2010, 101, 6124–6129. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.; Zhao, X.; Shen, H.; Wang, Q.; Zhao, Z.K. Microbial lipid production by Rhodosporidium toruloides under sulphate-limited conditions. Bioresour. Technol. 2011, 102, 1803–1807. [Google Scholar] [CrossRef] [PubMed]
- Davis, M.S.; Solbiati, J.; Cronan, J.E. Overproduction of acetyl–CoA carboxylase activity increases the rate of fatty acid biosynthesis in Escherichia coli. J. Biol. Chem. 2000, 275, 28593–28598. [Google Scholar] [CrossRef] [PubMed]
- Steen, E.J.; Kang, Y.; Bokinsky, G.; Hu, Z.; Schirmer, A.; McClure, A.; del Cardayre, S.B.; Keasling, J.D. Microbial production of fatty–acid–derived fuels and chemicals from plant biomass. Nature 2010, 463, 559–562. [Google Scholar] [CrossRef] [PubMed]
- Peralta–Yahya, P.P.; Zhang, F.; del Cardayre, S.B.; Keasling, J.D. Microbial engineering for the production of advanced biofuels. Nature 2012, 488, 320–328. [Google Scholar] [CrossRef] [PubMed]
- Liu, T.; Vora, H.; Khosla, C. Quantitative analysis and engineering of fatty acid biosynthesis in E. coli. Metab. Eng. 2010, 12, 378–386. [Google Scholar] [CrossRef] [PubMed]
- Huffer, S.; Roche, C.M.; Blanch, H.W.; Clark, D.S. Escherichia coli for biofuel production: Bridging the gap from promise to practice. Trends Biotechnol. 2012, 30, 538–545. [Google Scholar] [CrossRef] [PubMed]
- Valle-Rodríguez, J.O.; Shi, S.; Siewers, V.; Nielsen, J. Metabolic engineering of Saccharomyces cerevisiae for production of fatty acid ethyl esters, an advanced biofuel, by eliminating non-essential fatty acid utilization pathways. Appl. Energy 2014, 115, 226–232. [Google Scholar] [CrossRef]
- Runguphan, W.; Keasling, J.D. Metabolic engineering of Saccharomyces cerevisiae for production of fatty acid–derived biofuels and chemicals. Metab. Eng. 2014, 21, 103–113. [Google Scholar] [CrossRef] [PubMed]
- Guo, X.; Ota, Y. Incorporation of eicosapentaenoic and docosahexaenoic acids by a yeast (FO726A). J. Appl. Microbiol. 2000, 89, 107–115. [Google Scholar] [CrossRef] [PubMed]
- Papanikolaou, S.; Chevalot, I.; Komaitis, M.; Marc, I.; Aggelis, G. Single cell oil production by Yarrowia lipolytica growing on an industrial derivative of animal fat in batch cultures. Appl. Microbiol. Biotechnol. 2002, 58, 308–312. [Google Scholar] [CrossRef] [PubMed]
- Papanikolaou, S.; Aggelis, G. Modeling lipid accumulation and degradation in Yarrowia lipolytica cultivated on industrial fats. Curr. Microbiol. 2003, 46, 398–402. [Google Scholar] [CrossRef] [PubMed]
- Kohlwein, S.; Paltauf, F. Uptake of fatty acids by yeasts, Saccharomyces uvarum and Saccharomycopsis lipolytica. Biochim. Biophys. Acta 1984, 792, 310–317. [Google Scholar] [CrossRef] [PubMed]
- Fickers, P.; Benetti, P.H.; Waché, Y.; Marty, A.; Mauersberger, S.; Smit, M.S.; Nicaud, J.M. Hydrophobic substrate utilisation by the yeast Yarrowia lipolytica, and its potential applications. FEMS Yeast Res. 2005, 5, 527–543. [Google Scholar] [CrossRef] [PubMed]
- Aggelis, G.; Papadiotis, G.; Komaitis, M. Microbial fatty acid specificity. Folia Microbiol. 1997, 42, 117–120. [Google Scholar] [CrossRef]
- Aoki, H.; Miyamoto, N.; Furuya, Y.; Mankura, M.; Yasushi, E.; Kenshiro, F. Incorporation and accumulation of docosahexaenoic acid from the medium by Pichia methanolica HA-32. Biosci. Biotechnol. Biochem. 2002, 66, 2632–2638. [Google Scholar] [CrossRef] [PubMed]
- Beopoulos, A.; Chardot, T.; Nicaud, J.M. Yarrowia lipolytica: A model and a tool to understand the mechanisms implicated in lipid accumulation. Biochimie 2009, 91, 692–696. [Google Scholar] [CrossRef] [PubMed]
- Ageitos, J.M.; Vallejo, J.A.; Veiga-Crespo, P.; Villa, T.G. Oily yeasts as oleaginous cell factories. Appl. Microbiol. Biotechnol. 2011, 90, 1219–1227. [Google Scholar] [CrossRef] [PubMed]
- Buijs, N.A.; Siewers, V.; Nielsen, J. Advanced biofuel production by the yeast Saccharomyces cerevisiae. Curr. Opin. Chem. Biol. 2013, 17, 480–488. [Google Scholar] [CrossRef] [PubMed]
- Santamauro, F.; Whiffin, F.M.; Scott, R.J.; Chuck, C.J. Low-cost lipid production by an oleaginous yeast cultured in non–sterile conditions using model waste resources. Biotechnol. Biofuels 2014, 7, 34. [Google Scholar] [CrossRef] [PubMed]
- Alvarez, R.M.; Rodriguez, B.; Romano, J.M.; Diaz, A.O.; Gomez, E.; Miro, D.; Navarro, L.; Saura, G.; Garcia, J.L. Lipid accumulation in Rhodotorula glutinis on sugar cane molasses in single-stage continuous culture. World J. Microb. Biotechnol. 1992, 8, 214–215. [Google Scholar] [CrossRef]
- Kalogiannis, S.; Iakovidou, G.; Liakopoulou-Kyriakides, M.; Kyriakidis, D.A.; Skaracis, G.N. Optimization of xanthan gum production by Xanthomonas campestris grown in molasses. Process Biochem. 2003, 39, 249–256. [Google Scholar] [CrossRef]
- Jin, M.; Slininger, P.J.; Dien, B.S.; Waghmode, S.; Moser, B.R.; Orjuela, A.; da Costa-Sousa, L.; Balan, V. Microbial lipid-based lignocellulosic biorefinery: Feasibility and challenges. Trends Biotechnol. 2015, 33, 43–54. [Google Scholar] [CrossRef] [PubMed]
- Veana, F.; Martínez-Hernández, J.L.; Aguilar, C.N.; Rodríguez-Herrera, R.; Michelena, G. Utilization of molasses and sugar cane bagasse for production of fungal invertase in solid state fermentation using Aspergillus niger GH1. Braz. J. Microbiol. 2014, 45, 373–377. [Google Scholar] [CrossRef] [PubMed]
- Yen, H.W.; Yang, Y.C.; Yu, Y.H. Using crude glycerol and thin stillage for the production of microbial lipids through the cultivation of Rhodotorula glutinis. J. Biosci. Bioeng. 2012, 114, 453–456. [Google Scholar] [CrossRef] [PubMed]
- Saenge, C.; Cheirsilp, B.; Suksaroge, T.T.; Bourtoom, T. Potential use of oleaginous red yeast Rhodotorula glutinis for the bioconversion of crude glycerol from biodiesel plant to lipids and carotenoids. Process Biochem. 2011, 46, 210–218. [Google Scholar] [CrossRef]
- Thiru, M.; Sankh, S.; Rangaswamy, V. Process for biodiesel production from Cryptococcus curvatus. Bioresour. Technol. 2011, 102, 10436–10440. [Google Scholar] [CrossRef] [PubMed]
- Zhu, M.; Yu, L.J.; Wu, Y.X. An inexpensive medium for production of arachidonic acid by Mortierella alpina. J. Ind. Microbiol. Biotechnol. 2003, 30, 75–79. [Google Scholar] [CrossRef] [PubMed]
- Karatay, S.E.; Dönmez, G. Improving the lipid accumulation properties of the yeast cells for biodiesel production using molasses. Bioresour. Technol. 2010, 101, 7988–7990. [Google Scholar] [CrossRef] [PubMed]
- Economou, C.N.; Aggelis, G.; Pavlou, S.; Vayenas, D.V. Single cell oil production from rice hulls hydrolysate. Bioresour. Technol. 2011, 102, 9737–9742. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Guo, F.J.; Rong, Y.J.; Chi, Z.M. Lipid production from hydrolysate of cassava starch by Rhodosporidium toruloides 21167 for biodiesel making. Renew. Energy 2012, 46, 164–168. [Google Scholar] [CrossRef]
- Huang, C.; Chen, X.F.; Xiong, L.; Ma, L.L. Oil production by the yeast Trichosporon dermatis cultured in enzymatic hydrolysates of corncobs. Bioresour. Technol. 2012, 110, 711–714. [Google Scholar] [CrossRef] [PubMed]
- Gong, Z.; Shen, H.; Yang, X.; Wang, Q.; Xie, H.; Zhao, Z.K. Lipid production from corn stover by the oleaginous yeast Cryptococcus curvatus. Biotechnol. Biofuels 2014, 7, 158. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.; Zong, M.H.; Wu, H.; Liu, Q.P. Microbial oil production from rice straw hydrolysate by Trichosporon fermentans. Bioresour. Technol. 2009, 100, 4535–4538. [Google Scholar] [CrossRef] [PubMed]
- Yu, X.C.; Zheng, Y.B.; Dorgan, K.M.; Chen, S.L. Oil production by oleaginous yeasts using the hydrolysate from pretreatment of wheat straw with dilute sulfuric acid. Bioresour. Technol. 2011, 102, 6134–6140. [Google Scholar] [CrossRef] [PubMed]
- Angerbauer, C.; Siebenhofer, M.; Mittelbach, M.; Guebitz, G.M. Conversion of sewage sludge into lipids by Lipomyces starkeyi for biodiesel production. Bioresour. Technol. 2008, 99, 3051–3056. [Google Scholar] [CrossRef] [PubMed]
- Saenge, C.; Cheirsilp, B.; Suksaroge, T.T.; Bourtoom, T. Efficient concomitant production of lipids and carotenoids by oleaginous red yeast Rhodotorula glutinis cultured in palm oil mill effluent and application of lipids for biodiesel production. Biotechnol. Bioprocess Eng. 2011, 16, 23–33. [Google Scholar] [CrossRef]
- Lam, M.K.; Lee, K.T.; Mohammed, A.R. Homogeneous, heterogeneous and enzymatic catalysis for transesterification of high free fatty acid oil (waste cooking oil) to biodiesel: A review. Biotechnol. Adv. 2010, 28, 500–518. [Google Scholar] [CrossRef] [PubMed]
- Papanikolaou, S.; Muniglia, L.; Chevalot, I.; Aggelis, G.; Marc, I. Accumulation of a cocoa-butter-like lipid by Yarrowia lipolytica cultivated on agro-industrial residues. Curr. Microbiol. 2003, 46, 0124–0130. [Google Scholar] [CrossRef]
- El Bialy, H.; Gomaa, O.M.; Azab, K.S. Conversion of oil waste to valuable fatty acids using oleaginous yeast. World J. Microb. Biotechnol. 2011, 27, 2791–2798. [Google Scholar] [CrossRef]
- Grammelis, P.; Malliopoulou, A.; Basinas, P.; Danalatos, N.G. Cultivation and characterization of Cynara cardunculus for solid biofuels production in the Mediterranean region. Int. J. Mol. Sci. 2008, 9, 1241–1258. [Google Scholar] [CrossRef] [PubMed]
- Taherzadeh, M.J.; Karimi, K. Pretreatment of lignocellulosic wastes to improve ethanol and biogas production: A review. Int. J. Mol. Sci. 2008, 9, 1621–1651. [Google Scholar] [CrossRef] [PubMed]
- Zheng, J.; Rehmann, L. Extrusion pretreatment of lignocellulosic biomass: A review. Int. J. Mol. Sci. 2014, 15, 18967–18984. [Google Scholar] [CrossRef] [PubMed]
- Bai, Y.Y.; Xiao, L.P.; Shi, Z.J.; Sun, R.C. Structural variation of bamboo lignin before and after ethanol organosolv pretreatment. Int. J. Mol. Sci. 2013, 14, 21394–21413. [Google Scholar] [CrossRef] [PubMed]
- Granda, C.B.; Zhu, L.; Holtzapple, M.T. Sustainable liquid biofuels and their environmental impact. Environ. Prog. 2007, 26, 233–250. [Google Scholar] [CrossRef]
- Alvira, E.; Tomás-Pejó, M.; Ballesteros, M.; Negro, J. Pretreatment technologies for an efficient bioethanol production process based on enzymatic hydrolysis: A review. Bioresour. Technol. 2010, 101, 4851–4861. [Google Scholar] [CrossRef] [PubMed]
- Tsigie, Y.A.; Wang, C.Y.; Truong, C.T.; Ju, Y.H. Lipid production from Yarrowia lipolytica Po1g grown in sugarcane bagasse hydrolysate. Bioresour. Technol. 2011, 102, 9216–9222. [Google Scholar] [CrossRef] [PubMed]
- Ratledge, C. Biochemistry, stoichiometry, substrates and economics. In Single Cell Oil; Moreton, R.S., Ed.; Longman Scientific & Technical: Harlow, UK, 1988; pp. 33–70. [Google Scholar]
- Papanikolaou, S.; Sarantou, S.; Komaitis, M.; Aggelis, G. Repression of reserve lipid turnover in Cunninghamella echinulata and Mortierella isabellina cultivated in multiplelimited media. J. Appl. Microbiol. 2004, 97, 867–874. [Google Scholar] [CrossRef] [PubMed]
- Dey, P.; Maiti, M.K. Molecular characterization of a novel isolate of Candida tropicalis for enhanced lipid production. J. Appl. Microbiol. 2013, 114, 1357–1368. [Google Scholar] [CrossRef] [PubMed]
- Mondala, A.; Hernandez, R.; Holmes, W.; French, T.; McFarland, L.; Sparks, D.; Haque, M. Enhanced microbial oil production by activated sludge microorganisms via co-fermentation of glucose and xylose. AIChE J. 2013, 59, 4036–4044. [Google Scholar] [CrossRef]
- Mondala, A.; Hernandez, R.; French, T.; Green, M.; McFarland, L.; Ingram, L. Enhanced microbial oil production by activated sludge microorganisms from sugarcane bagasse hydrolyzate. Renew. Energy 2015, 78, 114–118. [Google Scholar] [CrossRef]
- Kosa, M.; Ragauskas, A.J. Bioconversion of lignin model compounds with oleaginous Rhodococci. Appl. Microbiol. Biotechnol. 2012, 93, 891–900. [Google Scholar] [CrossRef] [PubMed]
- Kosa, M.; Ragauskas, A.J. Lignin to lipid bioconversion by oleaginous Rhodococci. Green Chem. 2013, 15, 2070–2074. [Google Scholar] [CrossRef]
- Ganuza, E.; Anderson, A.J.; Ratledge, C. High-cell-density cultivation of Schizochytrium sp. in an ammonium/pH-auxostat fed-batch system. Biotechnol. Lett. 2008, 30, 1559–1564. [Google Scholar] [CrossRef] [PubMed]
- Matsakas, L.; Bonturi, N.; Miranda, E.A.; Rova, U.; Christakopoulos, P. High concentrations of dried sorghum stalks as a biomass feedstock for single cell oil production by Rhodosporidium toruloides. Biotechnol. Biofuels 2015, 8, 6. [Google Scholar] [CrossRef] [PubMed]
- Čertík, M.; Adamechová, Z.; Laoteng, K. Microbial production of γ-linolenic acid: Submerged vs. solid-state fermentations. Food Sci. Biotechnol. 2012, 21, 921–926. [Google Scholar] [CrossRef]
- Hölker, U.; Lenz, J. Solid-state fermentation—Are there any biotechnological advantages? Curr. Opin. Microbiol. 2005, 8, 301–306. [Google Scholar] [CrossRef] [PubMed]
- Stenberg, K.; Bollók, M.; Réczey, K.; Galbe, M.; Zacchi, G. Effect of substrate and cellulase concentration on simultaneous saccharification and fermentation of steam-pretreated softwood for ethanol production. Biotechnol. Bioeng. 2000, 68, 204–210. [Google Scholar] [CrossRef] [PubMed]
- Öhgren, K.; Bura, R.; Lesnicki, G.; Saddler, J.; Zacchi, G. A comparison between simultaneous saccharification and fermentation and separate hydrolysis and fermentation using steam-pretreated corn stover. Process Biochem. 2007, 42, 834–839. [Google Scholar] [CrossRef]
- Zheng, Y.; Yu, X.; Zeng, J.; Chen, S. Feasibility of filamentous fungi for biofuel production using hydrolysate from dilute sulfuric acid pretreatment of wheat straw. Biotechnol. Biofuels 2012, 5, 50. [Google Scholar] [CrossRef] [PubMed]
- Bagy, M.M.K.; Abd-Alla, M.H.; Morsy, F.M.; Hassan, E.A. Two stage biodiesel and hydrogen production from molasses by oleaginous fungi and Clostridium acetobutylicum ATCC 824. Int. J. Hydrog. Energy 2014, 39, 3185–3197. [Google Scholar] [CrossRef]
- Gómez, X.; Fernández, C.; Fierro, J.; Sánchez, M.E.; Escapa, A.; Morán, A. Hydrogen production: Two stage processes for waste degradation. Bioresour. Technol. 2011, 102, 8621–8627. [Google Scholar] [CrossRef] [PubMed]
- Bartacek, J.; Zabranska, J.; Lens, P.N.L. Developments and constraints in fermentative hydrogen production. Biofuels Bioprod. Biorefin. 2007, 1, 201–214. [Google Scholar] [CrossRef]
- Chi, Z.; Zheng, Y.; Ma, J.; Chen, S. Oleaginous yeast Cryptococcus curvatus culture with dark fermentation hydrogen production effluent as feedstock for microbial lipid production. Int. J. Hydrog. Energy 2011, 36, 9542–9550. [Google Scholar] [CrossRef]
- Carere, C.R.; Kalia, V.; Sparling, R.; Cicek, N.; Levin, D.B. Pyruvate catabolism and hydrogen synthesis pathway genes of Clostridium thermocellum ATCC 27405. Indian J. Microbiol. 2008, 48, 252–266. [Google Scholar] [CrossRef] [PubMed]
- Geng, A.; He, Y.; Qian, C.; Yan, X.; Zhou, Z. Effect of key factors on hydrogen production from cellulose in a co-culture of Clostridium thermocellum and Clostridium thermopalmarium. Bioresour. Technol. 2010, 101, 4029–4033. [Google Scholar] [CrossRef] [PubMed]
- Cao, G.L.; Guo, W.Q.; Wang, A.J.; Zhao, L.; Xu, C.J.; Zhao, Q.L.; Ren, N.Q. Enhanced cellulosic hydrogen production from lime-treated cornstalk wastes using thermophilic anaerobic microflora. Int. J. Hydrog. Energy 2012, 37, 13161–13166. [Google Scholar] [CrossRef]
- Magnusson, L.; Islam, R.; Sparling, R.; Levin, D.; Cicek, N. Direct hydrogen production from cellulosic waste materials with a single-step dark fermentation process. Int. J. Hydrog. Energy 2008, 33, 5398–5403. [Google Scholar] [CrossRef]
- Chen, C.C.; Chuang, Y.S.; Lin, C.Y.; Lay, C.H.; Sen, B. Thermophilic dark fermentation of untreated rice straw using mixed cultures for hydrogen production. Int. J. Hydrog. Energy 2012, 37, 15540–15546. [Google Scholar] [CrossRef]
- Cao, G.L.; Zhao, L.; Wang, A.J.; Wang, Z.Y.; Ren, N.Q. Single-step bioconversion of lignocellulose to hydrogen using novel moderately thermophilic bacteria. Biotechnol. Biofuels 2014, 7, 82. [Google Scholar] [CrossRef] [PubMed]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Martínez, E.J.; Raghavan, V.; González-Andrés, F.; Gómez, X. New Biofuel Alternatives: Integrating Waste Management and Single Cell Oil Production. Int. J. Mol. Sci. 2015, 16, 9385-9405. https://doi.org/10.3390/ijms16059385
Martínez EJ, Raghavan V, González-Andrés F, Gómez X. New Biofuel Alternatives: Integrating Waste Management and Single Cell Oil Production. International Journal of Molecular Sciences. 2015; 16(5):9385-9405. https://doi.org/10.3390/ijms16059385
Chicago/Turabian StyleMartínez, Elia Judith, Vijaya Raghavan, Fernando González-Andrés, and Xiomar Gómez. 2015. "New Biofuel Alternatives: Integrating Waste Management and Single Cell Oil Production" International Journal of Molecular Sciences 16, no. 5: 9385-9405. https://doi.org/10.3390/ijms16059385
APA StyleMartínez, E. J., Raghavan, V., González-Andrés, F., & Gómez, X. (2015). New Biofuel Alternatives: Integrating Waste Management and Single Cell Oil Production. International Journal of Molecular Sciences, 16(5), 9385-9405. https://doi.org/10.3390/ijms16059385