Advances in the in Vivo Raman Spectroscopy of Malignant Skin Tumors Using Portable Instrumentation
Abstract
:1. Introduction
2. Instrumentation and Experimental Considerations
2.1. Portable Raman Acquisition Systems
| Cancer Type | Technique | Raman Excitation Wavelength (nm) | Spot Size (mm) | Power (mW) | Signal Integration Time (s) | Number of Skin Lesions Studied and/or Patients | Reference |
|---|---|---|---|---|---|---|---|
| MM, BCC, SCC, actinic keratosis (AK), atypical nevi, melanocytic nevi, blue nevi, and seborrheic keratoses | Raman | 785 | 3.5 | 300 | 1 | 518 (453 patients) | [36] |
| BCC, inflammatory scar tissues | Raman + OCT a | 785 | 0.044 | 40 | 30 | 1 patient | [15] |
| MM, BCC, SCC, pigmented nevi | Raman | 785 | 1 | 150 | 30 | 50 | [37] |
| MM, BCC, SCC, pigmented nevi | Raman + OCT | 785 | 1 | 150 | 30 | 23, 50 | [38,39] |
| MM, BCC, SCC, pigmented nevi | Raman | 785 | 0.1 | 17 | 10 | 137 | [40,41] |
| BCC, SCC, inflammatory scar tissues | Raman | 825 | 0.005 b | 40 | 30 | 21 (19 patients) | [42] |
| BCC | Raman | 830 | 1.6 | 110 | 30 | 10 patients | [43] |
| BCC, SCC | Raman | 830 | - | 200 | 20 (2 s × 10 spectra) | 31 (17 patients) | [44] |
| BCC, SCC, AK | Raman | 830 | 0.17 | 200 | 20 (2 s × 10 spectra) | 49 (25 patients) | [45] |
| MM, BCC, SCC, AK, and non-melanoma pigmented lesions | Raman | 830 | 0.2 | 100 | 1 | 137 (76 patients) | [46,47] |
| BCC | Multi modal c | 830 | 0.2 | 56 | 4 | 1 (healthy) d | [48] |
| MM, eczema, psoriatic skin, malignant Kaposi sarcomas | Raman | 1064 | 10 | - | - | 1 (healthy) d | [31] |
| MM, BCC, pigmented nevi | Raman | 1064 | 0.1 | 120 | 480 | 81 (72 patients) | [49] |
| Carotenoid concentration in BCC and actinic keratoses | Raman | 488 | 2 | 10 | 20 | 14 patients | [50] |
| MM | Multi modal e | 1064 | 0.08 | - | 35 | Mice injected with human MM cells | [51] |
2.2. Comparison between Portable and Benchtop Systems
3. Results and Discussion
3.1. Bands Assignment
| Cancer Type | Peak Position (cm−1) a | Assignment | Reference |
|---|---|---|---|
| BCC | 500–600 | S-S disulfide stretching | [56] |
| BCC | 727 | v(CN)Adenine, Lipids | [56] |
| BCC | 746 | Thymine | [56] |
| BCC | 786–788 | Nucleic acid backbone (PO2 symmetric stretching) | [56,57] |
| BCC, SCC, MM | 832 | Proline, hydroxyproline, tyrosine, stretch of nucleic acids, DNA (PO2 symmetric stretching) | [44,46,56] |
| BCC, SCC | 920–943 | v(CC) skeletal of collagen backbone Proline, hydroxyproline | [31,42,44,58] |
| BCC, SCC | 1000–1010 | Phenylalanine (ring breathing); keratin | [42,44,56] |
| BCC, SCC | 1085–1098 | v(CC) lipids Nucleic acid backbone v(PO2) symmetric stretching | [15,31,56,57,59] |
| BCC, SCC | 1127–1130 | Lipids v(CC) symmetric stretching of acyl-backbone, trans conformation | [44,56] |
| BCC, SCC | 1207–1209 | Tyrosine, phenylalanine | [44,56] |
| MM, BCC | 1220–1280 | Amide III (δ(NH) bending and ν(CΝ) stretching vibrations) (protein band), tropocollagen (proline-rich), v(CH) ethylene (triolein and phospholipids) | [15,31,42,43,45,46,49,56,58–61] |
| MM, BCC | 1300 | δ(CH2) twist, lipids | [46,60,62] |
| BCC | 1336 | CH deformations, adenine, phenylalanine | [37,38,56] |
| MM, BCC | 1440–1460 | δ(CH2) scissoring in lipids and δ(CH2) scissoring vibration in proteins | [15,42,46,56,59,62] |
| MM, BCC | 1520–1570 | Nucleic acids | [37] |
| MM, BCC | 1640–1685 | Amide I (C=O stretching), collagen, elastin | [37,38,42,43,46,59,60] |
| BCC | 1651 | Lipids (C=C stretching), phenylalanine | [56] |
| MM, BCC | 3250 | H2O | [49] |
3.2. Studies of NMSC and MM with Portable Raman Instrumentation
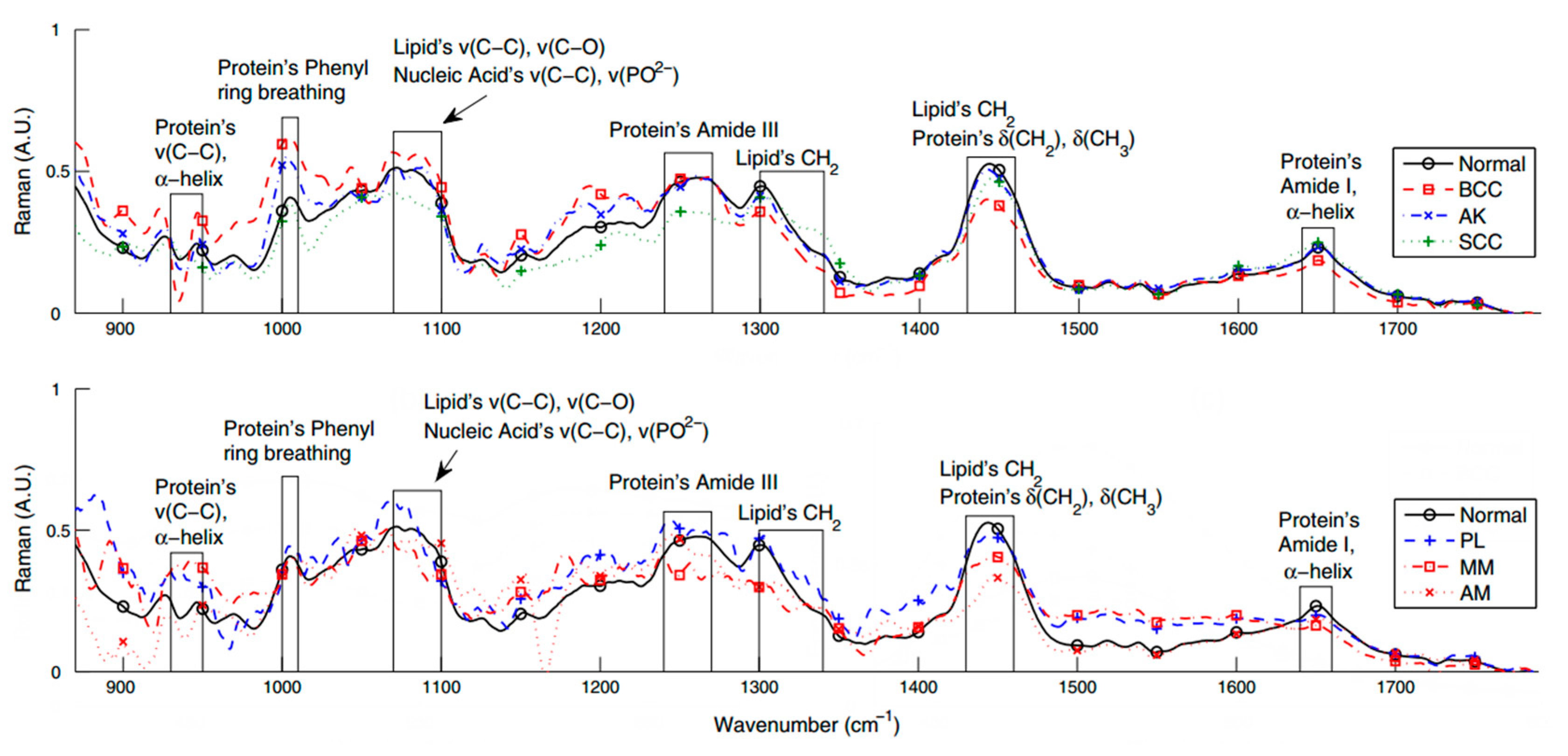

4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Housman, T.S.; Feldman, S.R.; Williford, P.M.; Fleischer, A.B.; Goldman, N.D.; Acostamadiedo, J.M.; Chen, G.J. Skin cancer is among the most costly of all cancers to treat for the medicare population. J. Am. Acad. Dermatol. 2003, 48, 425–429. [Google Scholar] [CrossRef] [PubMed]
- Staples, M.P.; Elwood, M.; Burton, R.C.; Williams, J.L.; Marks, R.; Giles, G.G. Non-melanoma skin cancer in Australia: The 2002 national survey and trends since 1985. Med. J. Aust. 2006, 184, 6–10. [Google Scholar] [PubMed]
- Rogers, H.W.; Weinstock, M.A.; Harris, A.R.; Hinckley, M.R.; Feldman, S.R.; Fleischer, A.B.; Coldiron, B.M. Incidence estimate of nonmelanoma skin cancer in the united states, 2006. Arch. Dermatol. 2010, 146, 283–287. [Google Scholar] [CrossRef] [PubMed]
- Brewster, D.H.; Bhatti, L.A.; Inglis, J.H.C.; Nairn, E.R.; Doherty, V.R. Recent trends in incidence of nonmelanoma skin cancers in the east of Scotland, 1992–2003. Brit. J. Dermatol. 2007, 156, 1295–1300. [Google Scholar] [CrossRef] [PubMed]
- Hoey, S.E.H.; Devereux, C.E.J.; Murray, L.; Catney, D.; Gavin, A.; Kumar, S.; Donnelly, D.; Dolan, O.M. Skin cancer trends in northern Ireland and consequences for provision of dermatology services. Br. J. Dermatol. 2007, 156, 1301–1307. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Donaldson, M.R.; Coldiron, B.M. No end in sight: The skin cancer epidemic continues. Semin. Cutan. Med. Surg. 2011, 30, 3–5. [Google Scholar] [CrossRef] [PubMed]
- Sng, J.; Koh, D.; Siong, W.C.; Choo, T.B. Skin cancer trends among Asians living in Singapore from 1968 to 2006. J. Am. Acad. Dermatol. 2009, 61, 426–432. [Google Scholar] [CrossRef] [PubMed]
- Gandini, S.; Sera, F.; Cattaruzza, M.S.; Pasquini, P.; Abeni, D.; Boyle, P.; Melchi, C.F. Meta-analysis of risk factors for cutaneous melanoma: I. Common and atypical naevi. Eur. J. Cancer 2005, 41, 28–44. [Google Scholar] [CrossRef] [PubMed]
- Forchetti, G.; Suppa, M.; del Marmol, V. Overview on non-melanoma skin cancers in solid organ transplant recipients. G. Ital. Dermatol. V 2014, 149, 383–387. [Google Scholar]
- Simoes, M.C.F.; Sousa, J.J.S.; Pais, A.A.C.C. Skin cancer and new treatment perspectives: A review. Cancer Lett. 2015, 357, 8–42. [Google Scholar] [CrossRef] [PubMed]
- Jensen, A.O.; Bautz, A.; Olesen, A.B.; Karagas, M.R.; Sorensen, H.T.; Friis, S. Mortality in Danish patients with nonmelanoma skin cancer, 1978–2001. Br. J. Dermatol. 2008, 159, 419–425. [Google Scholar] [PubMed]
- Lewis, K.G.; Weinstock, M.A. Nonmelanoma skin cancer mortality (1988–2000): The Rhode Island follow-back study. Arch. Dermatol. 2004, 140, 837–842. [Google Scholar] [CrossRef] [PubMed]
- Hannuksela-Svahn, A.; Pukkala, E.; Karvonen, J. Basal cell skin carcinoma and other nonmelanoma skin cancers in Finland from 1956 through 1995. Arch. Dermatol. 1999, 135, 781–786. [Google Scholar] [CrossRef] [PubMed]
- Bassukas, I.D.; Lippold, A.; Hundeiker, M. Parametric estimation of the “risk of melanoma-related death” with the recursion formula of the gompertz survival function. In Skin Cancer and UV Radiation; Altmeyer, P., Hoffmann, K., Stücker, M., Eds.; Springer: Berlin, Germany; Heidelberg, Germany, 1997; pp. 527–533. [Google Scholar]
- Patil, C.A.; Kirshnamoorthi, H.; Ellis, D.L.; van Leeuwen, T.G.; Mahadevan-Jansen, A. A clinical instrument for combined Raman spectroscopy-optical coherence tomography of skin cancers. Laser Surg. Med. 2011, 43, 143–151. [Google Scholar] [CrossRef] [PubMed]
- Mordechai, S.; Sahu, R.K.; Hammody, Z.; Mark, S.; Kantarovich, K.; Guterman, H.; Podshyvalov, A.; Goldstein, J.; Argov, S. Possible common biomarkers from FTIR microspectroscopy of cervical cancer and melanoma. J. Microsc. 2004, 215, 86–91. [Google Scholar] [CrossRef] [PubMed]
- Baranska, M.; Byrne, H.J. Optical diagnostics-spectropathology for the next generation. Analyst 2015, 140, 2064–2065. [Google Scholar] [PubMed]
- Kourkoumelis, N. Spectroscopy for biosciences. Contemp. Phys. 2015. [Google Scholar] [CrossRef]
- Bigio, I.J.; Mourant, J.R. Ultraviolet and visible spectroscopies for tissue diagnostics: Fluorescence spectroscopy and elastic-scattering spectroscopy. Phys. Med. Biol. 1997, 42, 803–814. [Google Scholar] [CrossRef] [PubMed]
- Seddon, A.B. Mid-infrared (IR)—A hot topic: The potential for using mid-IR light for non-invasive early detection of skin cancer in vivo. Phys. Status Solidi 2013, 250, 1020–1027. [Google Scholar] [CrossRef]
- Kendall, C.; Isabelle, M.; Bazant-Hegemark, F.; Hutchings, J.; Orr, L.; Babrah, J.; Baker, R.; Stone, N. Vibrational spectroscopy: A clinical tool for cancer diagnostics. Analyst 2009, 134, 1029–1045. [Google Scholar] [CrossRef] [PubMed]
- Chan, J.W.; Lieu, D.K. Label-free biochemical characterization of stem cells using vibrational spectroscopy. J. Biophotonics 2009, 2, 656–668. [Google Scholar] [CrossRef] [PubMed]
- Kong, K.; Kendall, C.; Stone, N.; Notingher, I. Raman spectroscopy for medical diagnostics—From in-vitro biofluid assays to in-vivo cancer detection. Adv. Drug Deliv. Rev. 2015, in press. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kourkoumelis, N.; Polymeros, A.; Tzaphlidou, M. Background estimation of biomedical Raman spectra using a geometric approach. J. Spectrosc. 2012, 27, 441–447. [Google Scholar] [CrossRef]
- Devpura, S.; Barton, K.N.; Brown, S.L.; Palyvoda, O.; Kalkanis, S.; Naik, V.M.; Siddiqui, F.; Naik, R.; Chetty, I.J. Vision 20/20: The role of Raman spectroscopy in early stage cancer detection and feasibility for application in radiation therapy response assessment. Med. Phys. 2014, 41. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Zhao, J.; Short, M.; Zeng, H. Real-time in vivo cancer diagnosis using Raman spectroscopy. J. Biophotonics 2014. [Google Scholar] [CrossRef] [PubMed]
- Vandenabeele, P.; Jehlicka, J.; Vitek, P.; Edwards, H.G.M. On the definition of Raman spectroscopic detection limits for the analysis of biomarkers in solid matrices. Planet. Space Sci. 2012, 62, 48–54. [Google Scholar] [CrossRef]
- Tfaili, S.; Josse, G.; Gobinet, C.; Angiboust, J.F.; Manfait, M.; Piot, O. Shedding light on the laser wavelength effect in Raman analysis of skin epidermises. Analyst 2012, 137, 4241–4246. [Google Scholar] [CrossRef] [PubMed]
- Naito, S.; Min, Y.K.; Sugata, K.; Osanai, O.; Kitahara, T.; Hiruma, H.; Hamaguchi, H. In vivo measurement of human dermis by 1064 nm-excited fiber Raman spectroscopy. Skin Res. Technol. 2008, 14, 18–25. [Google Scholar] [CrossRef] [PubMed]
- Patil, C.A.; Pence, I.J.; Lieber, C.A.; Mahadevan-Jansen, A. 1064 nm dispersive Raman spectroscopy of tissues with strong near-infrared autofluorescence. Opt. Lett. 2014, 39, 303–306. [Google Scholar] [CrossRef] [PubMed]
- Fendel, S.; Schrader, B. Investigation of skin and skin lesions by NIR-FT-Raman spectroscopy. Fresenius J. Anal. Chem. 1998, 360, 609–613. [Google Scholar] [CrossRef]
- Franzen, L.; Selzer, D.; Fluhr, J.W.; Schaefer, U.F.; Windbergs, M. Towards drug quantification in human skin with confocal Raman microscopy. Eur. J. Pharm. Biopharm. 2013, 84, 437–444. [Google Scholar] [CrossRef] [PubMed]
- Tfaili, S.; Gobinet, C.; Josse, G.; Angiboust, J.F.; Baillet, A.; Manfait, M.; Piot, O. Vibrational spectroscopies for the analysis of cutaneous permeation: Experimental limiting factors identified in the case of caffeine penetration. Anal. Bioanal. Chem. 2013, 405, 1325–1332. [Google Scholar] [CrossRef] [PubMed]
- Tfayli, A.; Piot, O.; Pitre, F.; Manfait, M. Follow-up of drug permeation through excised human skin with confocal Raman microspectroscopy. Eur. Biophys. J. Biophys. 2007, 36, 1049–1058. [Google Scholar] [CrossRef] [PubMed]
- Franzen, L.; Anderski, J.; Planz, V.; Kostka, K.H.; Windbergs, M. Combining confocal Raman microscopy and freeze-drying for quantification of substance penetration into human skin. Exp. Dermatol. 2014, 23, 942–944. [Google Scholar] [CrossRef] [PubMed]
- Lui, H.; Zhao, J.H.; McLean, D.; Zeng, H.S. Real-time Raman spectroscopy for in vivo skin cancer diagnosis. Cancer Res. 2012, 72, 2491–2500. [Google Scholar] [CrossRef] [PubMed]
- Zakharov, V.P.; Bratchenko, I.A.; Kozlov, S.V.; Moryatov, A.A.; Myakinin, O.O.; Artemyev, D.N. Two-step Raman spectroscopy method for tumor diagnosis. In Proceedings of the SPIE 9129,Biophotonics: Photonic Solutions for Better Health Care IV, Brussels, Belgium, 14–17 April 2014.
- Zakharov, V.P.; Bratchenko, I.A.; Kozlov, S.V.; Moryatov, A.A.; Kornilin, D.V.; Myakinin, O.O.; Artemyev, D.N. Advances in tumor diagnosis using OCT and Raman spectroscopy. In Proceedings of the SPIE 9129,Biophotonics: Photonic Solutions for Better Health Care IV, Brussels, Belgium, 14–17 April 2014.
- Zakharov, V.P.; Bratchenko, I.A.; Artemyev, D.N.; Myakinin, O.O.; Kornilin, D.V.; Kozlov, S.V.; Moryatov, A.A. Comparative analysis of combined spectral and optical tomography methods for detection of skin and lung cancers. J. Biomed. Opt. 2015, 20. [Google Scholar] [CrossRef] [PubMed]
- Schleusener, J.; Reble, C.; Helfmann, J.; Gersonde, I.; Cappius, H.J.; Glanert, M.; Fluhr, J.W.; Meinke, M.C. Design and technical evaluation of fibre-coupled Raman probes for the image-guided discrimination of cancerous skin. Meas. Sci. Technol. 2014, 25. [Google Scholar] [CrossRef]
- Schleusener, J.; Gluszczynska, P.; Reble, C.; Gersonde, I.; Helfmann, J.; Fluhr, J.W.; Lademann, J.; Röwert-Huber, J.; Meinke, M.C. In vivo study for the discrimination of cancerous and normal skin using fiber probe based Raman spectroscopy. Exp. Dermatol. 2015, in press. [Google Scholar] [CrossRef] [PubMed]
- Lieber, C.A.; Majumder, S.K.; Ellis, D.L.; Billheimer, D.D.; Mahadevan-Jansen, A. In vivo nonmelanoma skin cancer diagnosis using Raman microspectroscopy. Laser Surg. Med. 2008, 40, 461–467. [Google Scholar] [CrossRef] [PubMed]
- Tfayli, A.; Piot, O.; Derancourt, S.; Cadiot, G.; Diebold, M.D.; Bernard, P.; Manfait, M. In vivo analysis of tissue by Raman microprobe: Examination of human skin lesions and esophagus Barrett’s mucosa on an animal model. In Proceedings of the SPIE 6093,Biomedical Vibrational Spectroscopy III: Advances in Research and Industry, San Jose, CA, USA, 27 February 2006.
- Silveira, F.L.; Pacheco, M.T.T.; Bodanese, B.; Zângaro, R.A.; Silveira, L. Discriminating model for skin cancer diagnosis in vivo through Raman spectroscopy. In Proceedings of the SPIE 8565, Photonic Therapeutics and Diagnostics IX, San Francisco, CA, USA, 2 February 2013.
- Silveira, F.L.; Pacheco, M.T.; Bodanese, B.; Pasqualucci, C.A.; Zangaro, R.A.; Silveira, L., Jr. Discrimination of non-melanoma skin lesions from non-tumor human skin tissues in vivo using Raman spectroscopy and multivariate statistics. Lasers Surg. Med. 2015, 47, 6–16. [Google Scholar] [CrossRef] [PubMed]
- Lim, L.; Nichols, B.; Migden, M.R.; Rajaram, N.; Reichenberg, J.S.; Markey, M.K.; Ross, M.I.; Tunnell, J.W. Clinical study of noninvasive in vivo melanoma and nonmelanoma skin cancers using multimodal spectral diagnosis. J. Biomed. Opt. 2014, 19. [Google Scholar] [CrossRef] [PubMed]
- Motz, J.T.; Gandhi, S.J.; Scepanovic, O.R.; Haka, A.S.; Kramer, J.R.; Dasari, R.R.; Feld, M.S. Real-time raman system for in vivo disease diagnosis. J. Biomed. Opt. 2005, 10. [Google Scholar] [CrossRef] [PubMed]
- Sharma, M.; Marple, E.; Reichenberg, J.; Tunnell, J.W. Design and characterization of a novel multimodal fiber-optic probe and spectroscopy system for skin cancer applications. Rev. Sci. Instrum. 2014, 85. [Google Scholar] [CrossRef] [PubMed]
- Philipsen, P.A.; Knudsen, L.; Gniadecka, M.; Ravnbak, M.H.; Wulf, H.C. Diagnosis of malignant melanoma and basal cell carcinoma by in vivo NIR-FT Raman spectroscopy is independent of skin pigmentation. Photoch. Photobiol. Sci. 2013, 12, 770–776. [Google Scholar] [CrossRef] [PubMed]
- Hata, T.R.; Scholz, T.A.; Ermakov, I.V.; McClane, R.W.; Khachik, F.; Gellermann, W.; Pershing, L.K. Non-invasive Raman spectroscopic detection of carotenoids in human skin. J. Investig. Dermatol. 2000, 115, 441–448. [Google Scholar] [CrossRef] [PubMed]
- Karagiannis, G.T.; Grivas, I.; Tsingotjidou, A.; Apostolidis, G.K.; Grigoriadou, I.; Dori, I.; Poulatsidou, K.-N.; Doumas, A.; Wesarg, S.; Georgoulias, P. Early detection of melanoma with the combined use of acoustic microscopy, infrared reflectance and Raman spectroscopy. In Proceedings of the SPIE 9323, Photons Plus Ultrasound: Imaging and Sensing, San Francisco, CA, USA, 13–18 February 2015.
- Lieber, C.A.; Majumder, S.K.; Billheimer, D.; Ellis, D.L.; Mahadevan-Jansen, A. Raman microspectroscopy for skin cancer detection in vitro. J. Biomed. Opt. 2008, 13. [Google Scholar] [CrossRef] [PubMed]
- Zheng, J.K.; Pang, S.; Labuza, T.P.; He, L.L. Evaluation of surface-enhanced raman scattering detection using a handheld and a bench-top Raman spectrometer: A comparative study. Talanta 2014, 129, 79–85. [Google Scholar] [CrossRef] [PubMed]
- Mogensen, M.; Jemec, G.B.E. Diagnosis of nonmelanoma skin cancer/keratinocyte carcinoma: A review of diagnostic accuracy of nonmelanoma skin cancer diagnostic tests and technologies. Dermatol. Surg. 2007, 33, 1158–1174. [Google Scholar] [CrossRef] [PubMed]
- Hanlon, E.B.; Manoharan, R.; Koo, T.W.; Shafer, K.E.; Motz, J.T.; Fitzmaurice, M.; Kramer, J.R.; Itzkan, I.; Dasari, R.R.; Feld, M.S. Prospects for in vivo Raman spectroscopy. Phys. Med. Biol. 2000, 45, R1–R59. [Google Scholar] [CrossRef] [PubMed]
- Nijssen, A.; Schut, T.C.B.; Heule, F.; Caspers, P.J.; Hayes, D.P.; Neumann, M.H.A.; Puppels, G.J. Discriminating basal cell carcinoma from its surrounding tissue by Raman spectroscopy. J. Investig. Dermatol. 2002, 119, 64–69. [Google Scholar] [CrossRef] [PubMed]
- Kong, K.; Rowlands, C.J.; Varma, S.; Perkins, W.; Leach, I.H.; Koloydenko, A.A.; Pitiot, A.; Williams, H.C.; Notingher, I. Increasing the speed of tumour diagnosis during surgery with selective scanning Raman microscopy. J. Mol. Struct. 2014, 1073, 58–65. [Google Scholar] [CrossRef]
- Ly, E.; Piot, O.; Durlach, A.; Bernard, P.; Manfait, M. Polarized Raman microspectroscopy can reveal structural changes of peritumoral dermis in basal cell carcinoma. Appl. Spectrosc. 2008, 62, 1088–1094. [Google Scholar] [CrossRef] [PubMed]
- Choi, J.; Choo, J.; Chung, H.; Gweon, D.G.; Park, J.; Kim, H.J.; Park, S.; Oh, C.H. Direct observation of spectral differences between normal and basal cell carcinoma (BCC) tissues using confocal Raman microscopy. Biopolymers 2005, 77, 264–272. [Google Scholar] [CrossRef] [PubMed]
- Gniadecka, M.; Philipsen, P.A.; Sigurdsson, S.; Wessel, S.; Nielsen, O.F.; Christensen, D.H.; Hercogova, J.; Rossen, K.; Thomsen, H.K.; Gniadecki, R.; et al. Melanoma diagnosis by Raman spectroscopy and neural networks: Structure alterations in proteins and lipids in intact cancer tissue. J. Investig. Dermatol. 2004, 122, 443–449. [Google Scholar] [CrossRef] [PubMed]
- Larraona-Puy, M.; Ghita, A.; Zoladek, A.; Perkins, W.; Varma, S.; Leach, I.H.; Koloydenko, A.A.; Williams, H.; Notingher, I. Development of Raman microspectroscopy for automated detection and imaging of basal cell carcinoma. J. Biomed. Opt. 2009, 14. [Google Scholar] [CrossRef] [PubMed]
- Gniadecka, M.; Wulf, H.C.; Mortensen, N.N.; Nielsen, O.F.; Christensen, D.H. Diagnosis of basal cell carcinoma by Raman spectroscopy. J. Raman Spectrosc. 1997, 28, 125–129. [Google Scholar] [CrossRef]
- Dukor, R.K. Vibrational spectroscopy in the detection of cancer. In Handbook of Vibrational Spectroscopy; Chalmers, J., Griffiths, P.R., Eds.; John Wiley and Sons Ltd.: Hoboken, NJ, USA, 2002; Volume 5, pp. 3335–3361. [Google Scholar]
- Cartaxo, S.B.; Santos, I.D.D.O.; Bitar, R.; Oliveira, A.F.; Ferreira, L.M.; Martinho, H.S.; Martin, A.A. FT-Raman spectroscopy for the differentiation between cutaneous melanoma and pigmented nevus. Acta Cir. Bras. 2010, 25, 351–356. [Google Scholar] [CrossRef] [PubMed]
- Darvin, M.E.; Sterry, W.; Lademann, J.; Vergou, T. The role of carotenoids in human skin. Molecules 2011, 16, 10491–10506. [Google Scholar] [CrossRef]
- Lademann, J.; Kocher, W.; Yu, R.; Meinke, M.C.; Na Lee, B.; Jung, S.; Sterry, W.; Darvin, M.E. Cutaneous carotenoids: The mirror of lifestyle? Skin Pharmacol. Physiol. 2014, 27. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Wang, Y.; Liu, N.; Lin, D.; Weng, C.; Zhang, J.; Zhu, L.; Chen, W.; Chen, R.; Feng, S. Near-infrared confocal micro-Raman spectroscopy combined with PCA-LDA multivariate analysis for detection of esophageal cancer. Laser Phys. 2013, 23. [Google Scholar] [CrossRef]
- Zhao, J.; Huang, Z.; Zeng, H.; McLean, D.I.; Lui, H. Quantitative analysis of skin chemicals using rapid near-infrared Raman spectroscopy. In Proceedings of the SPIE 6842, Photonic Therapeutics and Diagnostics IV, San Jose, CA, USA, 19 January 2008.
- Salomatina, E.; Jiang, B.; Novak, J.; Yaroslavsky, A.N. Optical properties of normal and cancerous human skin in the visible and near-infrared spectral range. J. Biomed. Opt. 2006, 11. [Google Scholar] [CrossRef] [PubMed]
- Bodanese, B.; Silveira, L.; Albertini, R.; Zangaro, R.A.; Pacheco, M.T.T. Differentiating normal and basal cell carcinoma human skin tissues in vitro using dispersive Raman spectroscopy: A comparison between principal components analysis and simplified biochemical models. Photomed. Laser Surg. 2010, 28, S119–S127. [Google Scholar] [CrossRef] [PubMed]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kourkoumelis, N.; Balatsoukas, I.; Moulia, V.; Elka, A.; Gaitanis, G.; Bassukas, I.D. Advances in the in Vivo Raman Spectroscopy of Malignant Skin Tumors Using Portable Instrumentation. Int. J. Mol. Sci. 2015, 16, 14554-14570. https://doi.org/10.3390/ijms160714554
Kourkoumelis N, Balatsoukas I, Moulia V, Elka A, Gaitanis G, Bassukas ID. Advances in the in Vivo Raman Spectroscopy of Malignant Skin Tumors Using Portable Instrumentation. International Journal of Molecular Sciences. 2015; 16(7):14554-14570. https://doi.org/10.3390/ijms160714554
Chicago/Turabian StyleKourkoumelis, Nikolaos, Ioannis Balatsoukas, Violetta Moulia, Aspasia Elka, Georgios Gaitanis, and Ioannis D. Bassukas. 2015. "Advances in the in Vivo Raman Spectroscopy of Malignant Skin Tumors Using Portable Instrumentation" International Journal of Molecular Sciences 16, no. 7: 14554-14570. https://doi.org/10.3390/ijms160714554
APA StyleKourkoumelis, N., Balatsoukas, I., Moulia, V., Elka, A., Gaitanis, G., & Bassukas, I. D. (2015). Advances in the in Vivo Raman Spectroscopy of Malignant Skin Tumors Using Portable Instrumentation. International Journal of Molecular Sciences, 16(7), 14554-14570. https://doi.org/10.3390/ijms160714554







