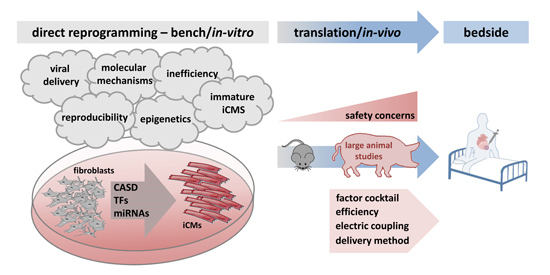
Direct Reprogramming—The Future of Cardiac Regeneration?
Abstract
:1. Introduction
2. Direct Lineage Reprogramming/Conversion of Fibroblasts into Cardiomyocytes in Vitro
2.1. The Starting Cell Population—Why Fibroblasts?
| Reference | Cell Source | Reprogramming Factors | Markers of iCMs, Percentages | Spontaneous Beating/When | Comments | |
|---|---|---|---|---|---|---|
| [15] | MCFs, TTFs | Starting with 14 F: GMT best | αMHC-GFP + TropT (FACS, day 7) | MCFs: yes, after 4–5 weeks (rare events) | No CPC stage | |
| MCFs: | GMT: ~4%–6% | |||||
| TTFs: | GMT: ~2.5% | |||||
| [22] | aMCFs, aTTFs | H2GMT | αMHC-GFP + TropT (FACS) | aMCFs: yes, after 5 weeks (rare events) aTTFs: yes, after 5 weeks (rare events) | – | |
| aTTFs (day 9): | GMT: ~2.9% | |||||
| aMCFs (day 7): | GMT: ~1.4% | |||||
| [23] | aMCFs, aTTFs | GMT | αMHC-GFP; Nkx2.5-GFP (FACS, day 21): no GFP+ cells | No beating | no CPC stage (Nkx2.5-GFP) | |
| [21] | MEFs (E13.5, w/o head, w/o visceral organs, p3–p5) MCFs (aMHC-tdTomato mice: Thy1+, tdTomato-) TTFs (3-day-old mice) | MTMc | MEFs: for initial qRT-PCR screening | MCFs: no, after 4 weeks (only rare events which were considered as cardiomyocyte contamination) | – | |
| αMHC-tdTomato (FACS, day 14) | ||||||
| MCFs: | GMT: 2.2% ± 0.2% | |||||
| TTFs: | GMT: 2.4% ± 0.2% | |||||
| TropT (FACS, day 14) | ||||||
| MCFs: | GMT: 12% ± 3.7% | |||||
| [24] | MCFs | miR-1, miR-133, miR-208, miR-499 + JI1 | αMHC-CFP (FACS, day 7) | MCFs: yes, after 10 days (rare events 1%–2% of total cell population) | short CPC stage: Mesp2 from day 1–5 (miR-1, -133, -208, -499); no pluripotency marker detected (Oct4, Nanog) | |
| negmiR: 0.1%–3.9% | ||||||
| [25] | MEFs (E14.5, w/o head, w/o visceral organs, w/o heart, p3–p5) aMCFs (8–10-week-old mice), isolation by culture, p3 | NH2GMT | TropT-GCaMP (Ca2+ oscillation, day 14) | MEFs: yes, after day 14 | iCMs not proliferative (Ki67) | |
| MEFs: | GMT: 0.03% ± 0.02% | |||||
| MCFs: | NH2GMT: 4.5% ± 0.3% | |||||
| [26] | MEFs (E13) | MpScMcSfNH1H2GTM | αMHC-GFP (FACS, day 7) | N.A. | – | |
| NegCtr: 0.03% ± 0.05% | ||||||
| [27] | MEFs (E14.5, w/o head, w/o visceral organs, w/o heart), p3–p5 aMCFs (8–10-week-old mice), isolation by culture, p3 | NH2GMT + small molecules (SB) | TropT-GCaMP (Ca2+ oscillation, day 14) | MEFs: robust beating, after day 11 aMCFs: yes, after day 16 | TGFβ signaling pathway plays a role in conversion | |
| MEFs: | NH2GMT + DMSO: 5.0% ± 1.8% | |||||
| aMCFs: | NH2GMT + DMSO: 1.5% ± 0.4% | |||||
| [9] | MEFs (E12.5, w/o head, w/o visceral organs) aMCFs (adult αMHC-GFP mice; Thy1+ GFP−) | GMT + miR133 | αMHC-GFP (FACS, day 7) | MEFs: GMT: yes, after 4 weeks; GMT + miR133: yes, after 10 days aMCFs: N.A. | iCMs not proliferative (EdU assay) Snai1/EMT mechanism no Mesp1+ CPCs (Mesp1-Cre x Stop-GFP mouse MEFs) mainly atrial-type myocytes | |
| MEFs: | GMT: ~19%; GMT + miR133: ~33% | |||||
| TropT (FACS, day 7) | ||||||
| MEFs: | GMT: ~1.9%; GMT + miR133: ~12% | |||||
| [28] | MEFs, TTFs, MCFs | 20 F H2GMT | Hcn4-GFP (FACS, day 7) | No (due to inadequate sarcomeric protein expression and organization, 12 weeks of culture) 0.0%–0.16% of H2GMT transduced fibroblasts show spontaneous beating (no further specification) | No Nkx2.5+ CPCs; well organized sarcomeric structures necessary for spontaneous beating, H2GMT: different types of CMs (atrial, pacemaker, and ventricular) | |
| TTFs: | 20F: 15%; | |||||
| [29] | MCFs (1.5 day old mice), TTFs | GMT (polycistronic vector, different order) | αMHC-GFP or TropT (FACS, day 10) | MCFs: MGT: yes, after 3 weeks | Stoichiometry is of critical importance (especially high Mef2c levels) | |
| MCFs: | G + M + T: ~5% (GFP), ~0.2% (TropT) | |||||
| [30] CASD approach | MEFs, TTFs | OSKMy + cytokines + small molecules | Nebulette-LacZ: initial screening | MEFs: yes, after day 11 TTFs: yes, after day 12 beating patches generated per 100,000 cells on day 18 MEFs: 145 ± 6 TTFs: 115 ± 7 | CPC stage: on day 9–10 (Flk1, Nkx2.5, Gata4) day 11: only atrial CMs only Mlc2a not Mlc2v | |
| MEFs: OSK + JI1 (day 1–day 9) + BMP4 (day 9–day 14): N.A. | ||||||
| TropT (FACS, day 18) | ||||||
| TTFs: OSKM + JI1 (day1–day9) + BMP4 (day 9–day 14), 39% ± 2% | ||||||
| [31] CASD approach | MEFs (E13.5, w/o head, w/o visceral organs, w/o heart), TTFs | Oct4 + small molecules (SCPF) + BMP4 | Beating cluster (day 30) | MEFs: yes, after day 20 TTFs: yes | CPC stage mostly ventricular iCMs (Mlc2v) | |
| MEFs: 99 ± 17 per 10,000 starting cells | ||||||
| TTFs: ~50 per 10,000 starting cells | ||||||
| Reference | Cell Source | Reprogramming Factors | Markers of iCMs, Percentages | Spontaneous Beating/When | Comments | ||
|---|---|---|---|---|---|---|---|
| [32] | HFFs, aHCFs, aHDFs | GMTH2Mc + miR-1, miR-133 | TropT (FACS, day 14) | Yes (only from HCFs), after 11 weeks | – | ||
| HFFs: | GMT: failed | ||||||
| Ca2+ transients (4 weeks) | |||||||
| aHCFs: | ~15% | ||||||
| [33] | HCFs (THY1+, CD31−) HFFs | GMTMpMc | TropT, αActinin (FACS, 4 weeks) | GMTMpMc-HCFs: no beating during longer periods of culture (no exact time designation) GMTMpMc-HCFs + ratCMs co-culture: beating after 7 days GMTMpMc-HCFs + conditioned medium: no beating | – | ||
| HCFs: | GMT: not sufficient | ||||||
| Ca2+ oscillation (4 weeks) | |||||||
| HCFs: | GMTMpMc: ~1% | ||||||
| [34] | H9Fs (THY1+), HDFs, HCFs | 7F (GMTMpMcEZ) 5F (GMTMpE) | αMHC-mCherry (FACS, day 14) | No beating events reported | H9Fs: well-organized sarcomeres after 10 weeks | ||
| H9Fs: | 7F (GMTMpMcEZ): 18.1% ± 11.2% | ||||||
| αMHC-mCherry + TropT (day 14) (FACS) | |||||||
| H9Fs: | 7F (GMTMpMcEZ): 13.0% ± 9.3% | ||||||
| [9] | HCFs (p1–p3) | GMTMpMc, miR-133 | TropT, αActinin (FACS, day 7) | N.A. | – | ||
| HCFs: | GMTMpMc: 2%–8% | ||||||
2.2. Reprogramming Factors—Transcription Factors and/or MicroRNA

2.3. Cell-Activation and Signaling-Directed (CASD) Lineage Conversion Method
2.4. Path of Conversion—Do Cells Pass through a Pluripotent or Progenitor State?
2.5. The Problem with Direct Reprogramming of Human Cells
2.6. Reliable Markers and Transdifferentiation of Induced Cardiomyocytes (iCMs)

3. Direct Lineage Reprogramming of Fibroblasts into Cardiomyocytes in Vivo
| Reference | Genetic Mouse Model | Application Form | Reprogramming Factors | iCMs Percentages/When | Functional Improvements |
|---|---|---|---|---|---|
| [50] | Periostin-Cre x R26LacZ Fsp1-Cre x R26LacZ αMHC-MerCreMer x R26eYFP | Retroviral delivery | GMT | Periostin-Cre x R26LacZ (4 weeks): ~35% iCMs (β-Gal+ and αActinin+) | Yes (MRI, serial echo), blinded study, 3 months after MI |
| [22] | Fsp1-Cre x R26LacZ Tcf21-iCre x R26tdTomato αMHC-MerCreMer x R26LacZ | Retroviral delivery | GMTH2 | Fsp1-Cre x R26LacZ (4 weeks): ~6.5% iCMs (β-Gal+) Tcf21-iCre x R26tdTomato (3–4 weeks): ~2.4% iCMs (tdTomato+ after Langendorf perfusion method) | Yes (MRI, echo), blinded study, 3 months after MI |
| [36] | Immunosuppressed mice (nude mice), no lineage tracing | Retroviral delivery | GMT; GMT polycistronic | GFP: no αActinin in GFP+ cells (2 weeks) GMT + GFP: ~1% of GFP+ cells express αActinin (2 weeks) GMT polycistronic + GFP: ~0.8% of GFP+ cells express αActinin (2 weeks) | N.A. |
| [24] | Fsp1-Cre x R26tdTomato x αMHC-CFP | Lentiviral delivery | miR-1, -133, -208, -499 | ~1% iCMs (CFP+ and tdTomato+) (6 weeks) | N.A. |
| [52] | Fisher 344 rats | Adenoviral (VEGF) & lentiviral (GMT) delivery | VEGF (directly after MI), GMT (3 weeks after MI) | N.A. | Yes (serial echo), 7 weeks after MI (best: GMT + VEGF) |
| [53] | Fsp1-Cre x R26tdTomato | Lentiviral delivery | miR-1, -133, -208, -499 | ~12% iCMs (tdTomato+ and TropT+) (7 weeks) (cave: NegmiR: 4%) | Yes (serial echo), 3 months after MI |
4. Underlying Mechanisms
5. Remaining Challenges of Direct Reprogramming

5.1. Inefficiency of the Reprogramming Process
5.2. Viral Delivery
5.3. Molecular Mechanisms Insufficiently Defined
5.4. Epigenetics
5.5. Induced Cardiomyocytes—An Immature and Heterogeneous Cell Population?
5.6. Reproducibility in Different Labs—Methodological Issues
6. Summary and Future Perspective
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Muraoka, N.; Ieda, M. Direct reprogramming of fibroblasts into myocytes to reverse fibrosis. Annu. Rev. Physiol. 2014, 76, 21–37. [Google Scholar] [CrossRef] [PubMed]
- Qian, L.; Srivastava, D. Direct cardiac reprogramming: From developmental biology to cardiac regeneration. Circ. Res. 2013, 113, 915–921. [Google Scholar] [CrossRef] [PubMed]
- Yi, B.A.; Mummery, C.L.; Chien, K.R. Direct cardiomyocyte reprogramming: A new direction for cardiovascular regenerative medicine. Cold Spring Harb. Perspect. Med. 2013, 3, a014050. [Google Scholar] [CrossRef] [PubMed]
- Doppler, S.A.; Deutsch, M.A.; Lange, R.; Krane, M. Cardiac regeneration: Current therapies-future concepts. J. Thorac. Dis. 2013, 5, 683–697. [Google Scholar] [PubMed]
- Chen, J.X.; Plonowska, K.; Wu, S.M. Somatic cell reprogramming into cardiovascular lineages. J. Cardiovasc. Pharmacol. Ther. 2014, 19, 340–349. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, K.; Yamanaka, S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell 2006, 126, 663–676. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gurdon, J.B.; Elsdale, T.R.; Fischberg, M. Sexually mature individuals of xenopus laevis from the transplantation of single somatic nuclei. Nature 1958, 182, 64–65. [Google Scholar] [CrossRef] [PubMed]
- Davis, R.L.; Weintraub, H.; Lassar, A.B. Expression of a single transfected cDNA converts fibroblasts to myoblasts. Cell 1987, 51, 987–1000. [Google Scholar] [CrossRef]
- Muraoka, N.; Yamakawa, H.; Miyamoto, K.; Sadahiro, T.; Umei, T.; Isomi, M.; Nakashima, H.; Akiyama, M.; Wada, R.; Inagawa, K.; et al. MiR-133 promotes cardiac reprogramming by directly repressing snai1 and silencing fibroblast signatures. EMBO J. 2014, 33, 1565–1581. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Q.; Brown, J.; Kanarek, A.; Rajagopal, J.; Melton, D.A. In vivo reprogramming of adult pancreatic exocrine cells to β-cells. Nature 2008, 455, 627–632. [Google Scholar] [CrossRef] [PubMed]
- Vierbuchen, T.; Ostermeier, A.; Pang, Z.P.; Kokubu, Y.; Sudhof, T.C.; Wernig, M. Direct conversion of fibroblasts to functional neurons by defined factors. Nature 2010, 463, 1035–1041. [Google Scholar] [CrossRef] [PubMed]
- Huang, P.; He, Z.; Ji, S.; Sun, H.; Xiang, D.; Liu, C.; Hu, Y.; Wang, X.; Hui, L. Induction of functional hepatocyte-like cells from mouse fibroblasts by defined factors. Nature 2011, 475, 386–389. [Google Scholar] [CrossRef] [PubMed]
- Sekiya, S.; Suzuki, A. Direct conversion of mouse fibroblasts to hepatocyte-like cells by defined factors. Nature 2011, 475, 390–393. [Google Scholar] [CrossRef] [PubMed]
- Batta, K.; Florkowska, M.; Kouskoff, V.; Lacaud, G. Direct reprogramming of murine fibroblasts to hematopoietic progenitor cells. Cell Rep. 2014, 9, 1871–1884. [Google Scholar] [CrossRef] [PubMed]
- Ieda, M.; Fu, J.D.; Delgado-Olguin, P.; Vedantham, V.; Hayashi, Y.; Bruneau, B.G.; Srivastava, D. Direct reprogramming of fibroblasts into functional cardiomyocytes by defined factors. Cell 2010, 142, 375–386. [Google Scholar] [CrossRef] [PubMed]
- Banerjee, I.; Fuseler, J.W.; Price, R.L.; Borg, T.K.; Baudino, T.A. Determination of cell types and numbers during cardiac development in the neonatal and adult rat and mouse. Am. J. Physiol. Heart Circ. Physiol. 2007, 293, H1883–H1891. [Google Scholar] [CrossRef] [PubMed]
- Ieda, M.; Tsuchihashi, T.; Ivey, K.N.; Ross, R.S.; Hong, T.T.; Shaw, R.M.; Srivastava, D. Cardiac fibroblasts regulate myocardial proliferation through β1 integrin signaling. Dev. Cell 2009, 16, 233–244. [Google Scholar] [CrossRef] [PubMed]
- Van Wijk, B.; van den Hoff, M. Epicardium and myocardium originate from a common cardiogenic precursor pool. Trends Cardiovasc. Med. 2010, 20, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Weintraub, H.; Tapscott, S.J.; Davis, R.L.; Thayer, M.J.; Adam, M.A.; Lassar, A.B.; Miller, A.D. Activation of muscle-specific genes in pigment, nerve, fat, liver, and fibroblast cell lines by forced expression of myod. Proc. Natl. Acad. Sci. USA 1989, 86, 5434–5438. [Google Scholar] [CrossRef] [PubMed]
- Chien, K.R.; Yi, B.A.; Xu, H.; Mummery, C.L. Cardiomyocyte reprogramming and the new age of cellular alchemy. J. Mol. Cell. Cardiol. 2012, 53, 311–313. [Google Scholar] [CrossRef] [PubMed]
- Protze, S.; Khattak, S.; Poulet, C.; Lindemann, D.; Tanaka, E.M.; Ravens, U. A new approach to transcription factor screening for reprogramming of fibroblasts to cardiomyocyte-like cells. J. Mol. Cell. Cardiol. 2012, 53, 323–332. [Google Scholar] [CrossRef] [PubMed]
- Song, K.; Nam, Y.J.; Luo, X.; Qi, X.; Tan, W.; Huang, G.N.; Acharya, A.; Smith, C.L.; Tallquist, M.D.; Neilson, E.G.; et al. Heart repair by reprogramming non-myocytes with cardiac transcription factors. Nature 2012, 485, 599–604. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.X.; Krane, M.; Deutsch, M.A.; Wang, L.; Rav-Acha, M.; Gregoire, S.; Engels, M.C.; Rajarajan, K.; Karra, R.; Abel, E.D.; et al. Inefficient reprogramming of fibroblasts into cardiomyocytes using Gata4, Mef2c, and Tbx5. Circ. Res. 2012, 111, 50–55. [Google Scholar] [CrossRef] [PubMed]
- Jayawardena, T.M.; Egemnazarov, B.; Finch, E.A.; Zhang, L.; Payne, J.A.; Pandya, K.; Zhang, Z.; Rosenberg, P.; Mirotsou, M.; Dzau, V.J. MicroRNA-mediated in vitro and in vivo direct reprogramming of cardiac fibroblasts to cardiomyocytes. Circ. Res. 2012, 110, 1465–1473. [Google Scholar] [CrossRef] [PubMed]
- Addis, R.C.; Ifkovits, J.L.; Pinto, F.; Kellam, L.D.; Esteso, P.; Rentschler, S.; Christoforou, N.; Epstein, J.A.; Gearhart, J.D. Optimization of direct fibroblast reprogramming to cardiomyocytes using calcium activity as a functional measure of success. J. Mol. Cell. Cardiol. 2013, 60, 97–106. [Google Scholar] [CrossRef] [PubMed]
- Christoforou, N.; Chellappan, M.; Adler, A.F.; Kirkton, R.D.; Wu, T.; Addis, R.C.; Bursac, N.; Leong, K.W. Transcription factors MYOCD, SRF, MESP1 and SMARCD3 enhance the cardio-inducing effect of GATA4, TBX5, and MEF2C during direct cellular reprogramming. PLoS ONE 2013, 8, e63577. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ifkovits, J.L.; Addis, R.C.; Epstein, J.A.; Gearhart, J.D. Inhibition of TGFβ signaling increases direct conversion of fibroblasts to induced cardiomyocytes. PLoS ONE 2014, 9, e89678. [Google Scholar] [CrossRef] [PubMed]
- Nam, Y.J.; Lubczyk, C.; Bhakta, M.; Zang, T.; Fernandez-Perez, A.; McAnally, J.; Bassel-Duby, R.; Olson, E.N.; Munshi, N.V. Induction of diverse cardiac cell types by reprogramming fibroblasts with cardiac transcription factors. Development 2014, 141, 4267–4278. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Liu, Z.; Yin, C.; Asfour, H.; Chen, O.; Li, Y.; Bursac, N.; Liu, J.; Qian, L. Stoichiometry of Gata4, Mef2c, and Tbx5 influences the efficiency and quality of induced cardiac myocyte reprogramming. Circ. Res. 2015, 116, 237–244. [Google Scholar] [CrossRef] [PubMed]
- Efe, J.A.; Hilcove, S.; Kim, J.; Zhou, H.; Ouyang, K.; Wang, G.; Chen, J.; Ding, S. Conversion of mouse fibroblasts into cardiomyocytes using a direct reprogramming strategy. Nat. Cell Biol. 2011, 13, 215–222. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Cao, N.; Spencer, C.I.; Nie, B.; Ma, T.; Xu, T.; Zhang, Y.; Wang, X.; Srivastava, D.; Ding, S. Small molecules enable cardiac reprogramming of mouse fibroblasts with a single factor, Oct4. Cell Rep. 2014, 6, 951–960. [Google Scholar] [CrossRef] [PubMed]
- Nam, Y.J.; Song, K.; Luo, X.; Daniel, E.; Lambeth, K.; West, K.; Hill, J.A.; DiMaio, J.M.; Baker, L.A.; Bassel-Duby, R.; et al. Reprogramming of human fibroblasts toward a cardiac fate. Proc. Natl. Acad. Sci. USA 2013, 110, 5588–5593. [Google Scholar] [CrossRef] [PubMed]
- Wada, R.; Muraoka, N.; Inagawa, K.; Yamakawa, H.; Miyamoto, K.; Sadahiro, T.; Umei, T.; Kaneda, R.; Suzuki, T.; Kamiya, K.; et al. Induction of human cardiomyocyte-like cells from fibroblasts by defined factors. Proc. Natl. Acad. Sci. USA 2013, 110, 12667–12672. [Google Scholar] [CrossRef] [PubMed]
- Fu, J.D.; Stone, N.R.; Liu, L.; Spencer, C.I.; Qian, L.; Hayashi, Y.; Delgado-Olguin, P.; Ding, S.; Bruneau, B.G.; Srivastava, D. Direct reprogramming of human fibroblasts toward a cardiomyocyte-like state. Stem Cell Rep. 2013, 1, 235–247. [Google Scholar] [CrossRef] [PubMed]
- Muraoka, N.; Ieda, M. Stoichiometry of transcription factors is critical for cardiac reprogramming. Circ. Res. 2015, 116, 216–218. [Google Scholar] [CrossRef] [PubMed]
- Inagawa, K.; Miyamoto, K.; Yamakawa, H.; Muraoka, N.; Sadahiro, T.; Umei, T.; Wada, R.; Katsumata, Y.; Kaneda, R.; Nakade, K.; et al. Induction of cardiomyocyte-like cells in infarct hearts by gene transfer of Gata4, Mef2c, and Tbx5. Circ. Res. 2012, 111, 1147–1156. [Google Scholar] [CrossRef] [PubMed]
- Mathison, M.; Singh, V.P.; Gersch, R.P.; Ramirez, M.O.; Cooney, A.; Kaminsky, S.M.; Chiuchiolo, M.J.; Nasser, A.; Yang, J.; Crystal, R.G.; et al. “Triplet” polycistronic vectors encoding Gata4, Mef2c, and Tbx5 enhances postinfarct ventricular functional improvement compared with singlet vectors. J. Thorac. Cardiovasc. Surg. 2014, 148, 1656–1664, e1652. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Gao, Y.; Gordon, A.; Wang, Z.Z.; Qian, Z.; Wu, W.S. Efficient generation of fully reprogrammed human ips cells via polycistronic retroviral vector and a new cocktail of chemical compounds. PLoS ONE 2011, 6, e26592. [Google Scholar] [CrossRef] [PubMed]
- Carey, B.W.; Markoulaki, S.; Hanna, J.; Saha, K.; Gao, Q.; Mitalipova, M.; Jaenisch, R. Reprogramming of murine and human somatic cells using a single polycistronic vector. Proc. Natl. Acad. Sci. USA 2009, 106, 157–162. [Google Scholar] [CrossRef] [PubMed]
- Carey, B.W.; Markoulaki, S.; Hanna, J.H.; Faddah, D.A.; Buganim, Y.; Kim, J.; Ganz, K.; Steine, E.J.; Cassady, J.P.; Creyghton, M.P.; et al. Reprogramming factor stoichiometry influences the epigenetic state and biological properties of induced pluripotent stem cells. Cell Stem Cell 2011, 9, 588–598. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Efe, J.A.; Zhu, S.; Talantova, M.; Yuan, X.; Wang, S.; Lipton, S.A.; Zhang, K.; Ding, S. Direct reprogramming of mouse fibroblasts to neural progenitors. Proc. Natl. Acad. Sci. USA 2011, 108, 7838–7843. [Google Scholar] [CrossRef] [PubMed]
- Thier, M.; Worsdorfer, P.; Lakes, Y.B.; Gorris, R.; Herms, S.; Opitz, T.; Seiferling, D.; Quandel, T.; Hoffmann, P.; Nothen, M.M.; et al. Direct conversion of fibroblasts into stably expandable neural stem cells. Cell Stem Cell 2012, 10, 473–479. [Google Scholar] [CrossRef] [PubMed]
- Zhu, S.; Ambasudhan, R.; Sun, W.; Kim, H.J.; Talantova, M.; Wang, X.; Zhang, M.; Zhang, Y.; Laurent, T.; Parker, J.; et al. Small molecules enable Oct4-mediated direct reprogramming into expandable human neural stem cells. Cell Res. 2014, 24, 126–129. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Huang, N.F.; Zou, J.; Laurent, T.J.; Lee, J.C.; Okogbaa, J.; Cooke, J.P.; Ding, S. Conversion of human fibroblasts to functional endothelial cells by defined factors. Arterioscler. Thromb. Vasc. Biol. 2013, 33, 1366–1375. [Google Scholar] [CrossRef] [PubMed]
- Li, K.; Zhu, S.; Russ, H.A.; Xu, S.; Xu, T.; Zhang, Y.; Ma, T.; Hebrok, M.; Ding, S. Small molecules facilitate the reprogramming of mouse fibroblasts into pancreatic lineages. Cell Stem Cell 2014, 14, 228–236. [Google Scholar] [CrossRef] [PubMed]
- Moretti, A.; Caron, L.; Nakano, A.; Lam, J.T.; Bernshausen, A.; Chen, Y.; Qyang, Y.; Bu, L.; Sasaki, M.; Martin-Puig, S.; et al. Multipotent embryonic isl1+ progenitor cells lead to cardiac, smooth muscle, and endothelial cell diversification. Cell 2006, 127, 1151–1165. [Google Scholar] [CrossRef] [PubMed]
- Maza, I.; Caspi, I.; Zviran, A.; Chomsky, E.; Rais, Y.; Viukov, S.; Geula, S.; Buenrostro, J.D.; Weinberger, L.; Krupalnik, V.; et al. Transient acquisition of pluripotency during somatic cell transdifferentiation with ipsc reprogramming factors. Nat. Biotechnol. 2015, 33, 769–774. [Google Scholar] [CrossRef] [PubMed]
- Bar-Nur, O.; Verheul, C.; Sommer, A.G.; Brumbaugh, J.; Schwarz, B.A.; Lipchina, I.; Huebner, A.J.; Mostoslavsky, G.; Hochedlinger, K. Lineage conversion induced by pluripotency factors involves transient passage through an iPSC stage. Nat. Biotechnol. 2015, 33, 761–768. [Google Scholar] [CrossRef] [PubMed]
- Pang, Z.P.; Yang, N.; Vierbuchen, T.; Ostermeier, A.; Fuentes, D.R.; Yang, T.Q.; Citri, A.; Sebastiano, V.; Marro, S.; Sudhof, T.C.; et al. Induction of human neuronal cells by defined transcription factors. Nature 2011, 476, 220–223. [Google Scholar] [PubMed]
- Qian, L.; Huang, Y.; Spencer, C.I.; Foley, A.; Vedantham, V.; Liu, L.; Conway, S.J.; Fu, J.D.; Srivastava, D. In vivo reprogramming of murine cardiac fibroblasts into induced cardiomyocytes. Nature 2012, 485, 593–598. [Google Scholar] [CrossRef] [PubMed]
- Zeisberg, E.M.; Kalluri, R. Origins of cardiac fibroblasts. Circ. Res. 2010, 107, 1304–1312. [Google Scholar] [CrossRef] [PubMed]
- Mathison, M.; Gersch, R.P.; Nasser, A.; Lilo, S.; Korman, M.; Fourman, M.; Hackett, N.; Shroyer, K.; Yang, J.; Ma, Y.; et al. In vivo cardiac cellular reprogramming efficacy is enhanced by angiogenic preconditioning of the infarcted myocardium with vascular endothelial growth factor. J. Am. Heart Assoc. 2012, 1, e005652. [Google Scholar] [CrossRef] [PubMed]
- Jayawardena, T.M.; Finch, E.A.; Zhang, L.; Zhang, H.; Hodgkinson, C.; Pratt, R.E.; Rosenberg, P.B.; Mirotsou, M.; Dzau, V.J. MicroRNA induced cardiac reprogramming in vivo: Evidence for mature cardiac myocytes and improved cardiac function. Circ. Res. 2015, 116, 418–424. [Google Scholar] [CrossRef] [PubMed]
- Torper, O.; Pfisterer, U.; Wolf, D.A.; Pereira, M.; Lau, S.; Jakobsson, J.; Bjorklund, A.; Grealish, S.; Parmar, M. Generation of induced neurons via direct conversion in vivo. Proc. Natl. Acad. Sci. USA 2013, 110, 7038–7043. [Google Scholar] [CrossRef] [PubMed]
- Pfisterer, U.; Kirkeby, A.; Torper, O.; Wood, J.; Nelander, J.; Dufour, A.; Bjorklund, A.; Lindvall, O.; Jakobsson, J.; Parmar, M. Direct conversion of human fibroblasts to dopaminergic neurons. Proc. Natl. Acad. Sci. USA 2011, 108, 10343–10348. [Google Scholar] [CrossRef] [PubMed]
- Caiazzo, M.; Dell'Anno, M.T.; Dvoretskova, E.; Lazarevic, D.; Taverna, S.; Leo, D.; Sotnikova, T.D.; Menegon, A.; Roncaglia, P.; Colciago, G.; et al. Direct generation of functional dopaminergic neurons from mouse and human fibroblasts. Nature 2011, 476, 224–227. [Google Scholar] [CrossRef] [PubMed]
- Srivastava, D.; Berry, E.C. Cardiac reprogramming: From mouse toward man. Curr. Opin. Genet. Dev. 2013, 23, 574–578. [Google Scholar] [CrossRef] [PubMed]
- Kormann, M.S.; Hasenpusch, G.; Aneja, M.K.; Nica, G.; Flemmer, A.W.; Herber-Jonat, S.; Huppmann, M.; Mays, L.E.; Illenyi, M.; Schams, A.; et al. Expression of therapeutic proteins after delivery of chemically modified mRNA in mice. Nat. Biotechnol. 2011, 29, 154–157. [Google Scholar] [CrossRef] [PubMed]
- Miki, K.; Yoshida, Y.; Yamanaka, S. Making steady progress on direct cardiac reprogramming toward clinical application. Circ. Res. 2013, 113, 13–15. [Google Scholar] [CrossRef] [PubMed]
- Kuppusamy, K.T.; Jones, D.C.; Sperber, H.; Madan, A.; Fischer, K.A.; Rodriguez, M.L.; Pabon, L.; Zhu, W.Z.; Tulloch, N.L.; Yang, X.; et al. Let-7 family of microrna is required for maturation and adult-like metabolism in stem cell-derived cardiomyocytes. Proc. Natl. Acad. Sci. USA 2015, 112, E2785–E2794. [Google Scholar] [CrossRef] [PubMed]
- Nahrendorf, M.; Pittet, M.J.; Swirski, F.K. Monocytes: Protagonists of infarct inflammation and repair after myocardial infarction. Circulation 2010, 121, 2437–2445. [Google Scholar] [CrossRef] [PubMed]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Doppler, S.A.; Deutsch, M.-A.; Lange, R.; Krane, M. Direct Reprogramming—The Future of Cardiac Regeneration? Int. J. Mol. Sci. 2015, 16, 17368-17393. https://doi.org/10.3390/ijms160817368
Doppler SA, Deutsch M-A, Lange R, Krane M. Direct Reprogramming—The Future of Cardiac Regeneration? International Journal of Molecular Sciences. 2015; 16(8):17368-17393. https://doi.org/10.3390/ijms160817368
Chicago/Turabian StyleDoppler, Stefanie A., Marcus-André Deutsch, Rüdiger Lange, and Markus Krane. 2015. "Direct Reprogramming—The Future of Cardiac Regeneration?" International Journal of Molecular Sciences 16, no. 8: 17368-17393. https://doi.org/10.3390/ijms160817368
APA StyleDoppler, S. A., Deutsch, M.-A., Lange, R., & Krane, M. (2015). Direct Reprogramming—The Future of Cardiac Regeneration? International Journal of Molecular Sciences, 16(8), 17368-17393. https://doi.org/10.3390/ijms160817368