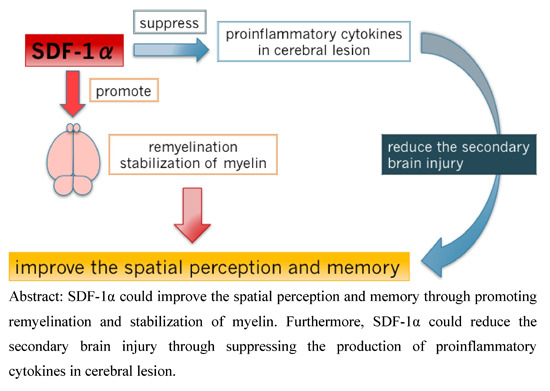
Stromal Cell-Derived Factor-1α Plays a Crucial Role Based on Neuroprotective Role in Neonatal Brain Injury in Rats
Abstract
:1. Introduction
2. Results
2.1. Magnetic Resonance Imaging (MRI)

2.2. Morris Water Maze (MWM) Test

2.3. Rotarod Test
2.4. Staining with 2% 2,3,5-Triphenyltetrasodium Chloride (TTC)


2.5. Immunohistochemistry

3. Discussion
4. Experimental Section
4.1. Animals
4.2. Neonatal HIE Animal Models
4.3. MRI
4.4. Behavioral Analyses
4.4.1. MWM Test
4.4.2. Rotarod Test
4.5. Assessment of Cerebral Injury
4.6. Immunohistochemical Staining
4.7. Statistical Analyses
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Hausenloy, D.J.; Yellon, D.M. The therapeutic potential of ischemic conditioning: An updated. Nat. Rev. Cardiol. 2011, 8, 619–629. [Google Scholar] [CrossRef] [PubMed]
- Hamrick, S.E.; Ferriero, D.M. The injury response in the term newborn brain: Can we neuroprotect? Curr. Opin. Neurol. 2003, 16, 147–154. [Google Scholar] [CrossRef] [PubMed]
- Vannucci, R.C.; Connor, J.R.; Mauger, D.T.; Palmer, C.; Smith, M.B.; Towfighi, J.; Vannucci, S.J. Rat model of perinatal hypoxic-ischemic brain damage. J. Neurosci. Res. 1999, 55, 158–163. [Google Scholar] [CrossRef]
- Gluckman, P.D.; Wyatt, J.S.; Azzopardi, D.; Ballard, R.; Edwards, A.D.; Ferriero, D.M.; Polin, R.A.; Robertson, C.M.; Thoresen, M.; Whitelaw, A.; et al. Selective head cooling with mild systemic hypothermia after neonatal encephalopathy: Multicentre randomized trial. Lancet 2005, 365, 663–670. [Google Scholar] [CrossRef]
- Zhao, F.; Qu, Y.; Liu, H.; Du, B.; Mu, D. Umbilical cord blood mesenchymal stem cells co-modified by TERT and BDNF: A novel neuroprotective therapy for neonatal hypoxic-ischemic brain damage. Int. J. Dev. Neurosci. 2014, 38, 147–154. [Google Scholar] [CrossRef] [PubMed]
- Lü, M.H.; Hu, C.J.; Chen, L.; Peng, X.; Chen, J.; Hu, J.Y.; Teng, M.; Liang, G.P. miR-27b represses migration of mouse MSCs to burned margins and prolongs wound repair through silencing SDF-1α. PLoS ONE 2013, 8, e68972. [Google Scholar] [CrossRef] [PubMed]
- Kucia, M.; Jankowski, K.; Reca, R.; Wysoczynski, M.; Bandura, L.; Allendorf, D.J.; Zhang, J.; Ratajczak, J.; Ratajczak, M.Z. CXCR4-SDF-1 signaling, locomotion, chemotaxis and adhesion. J. Mol. Histol. 2004, 35, 233–245. [Google Scholar] [CrossRef] [PubMed]
- Smart, N.; Riley, P.R. The stem cell movement. Circ. Res. 2008, 102, 1155–1168. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Duan, B.; Cheng, Z.; Jia, X.; Mao, L.; Fu, H.; Che, Y.; Ou, L.; Liu, L.; Kong, D. SDF-1/CXCR4 axis modulates bone marrow mesenchymal stem cell apoptosis, migration and cytokine secretion. Protein Cell 2011, 2, 845–854. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Zhang, Q.; Li, W.; Nie, D.; Chen, W.; Xu, C.; Yi, X.; Shi, J.; Tian, M.; Qin, J.; et al. Therapeutic effect of human umbilical cord mesenchymal stem cells on neonatal rat hypoxic-ischemic encephalopathy. J. Neurosci. Res. 2014, 92, 35–45. [Google Scholar] [CrossRef] [PubMed]
- Greggio, S.; de Paula, S.; Azevedo, P.N.; Venturin, G.T.; Dacosta, J.C. Intra-arterial transplantation of human umbilical cord blood mononuclear cells in neonatal hypoxic-ischemic rats. Life Sci. 2014, 96, 33–39. [Google Scholar] [CrossRef] [PubMed]
- Yasuhara, T.; Hara, K.; Maki, M.; Xu, L.; Yu, G.; Ali, M.M.; Masuda, T.; Yu, S.J.; Bae, E.K.; Hayashi, T.; et al. Mannitol facilitates neurotrophic factor up-regulation and behavioural recovery in neonatal hypoxic-ischaemic rats with human umbilical cord blood grafts. J. Cell Mol. Med. 2010, 14, 914–921. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.A.; Kim, B.I.; Jo, C.H.; Choi, C.W.; Kim, E.K.; Kim, H.S.; Yoon, K.S.; Choi, J.H. Mesenchymal stem-cell transplantation for hypoxic–ischemic brain injury in neonatal rat model. Pediatr. Res. 2010, 67, 42–46. [Google Scholar] [CrossRef] [PubMed]
- Robin, A.M.; Zhang, Z.G.; Wang, L.; Zhang, R.L.; Katakowski, M.; Zhang, L.; Wang, Y.; Zhang, C.; Chopp, M. Stromal cell-derived factor 1 α mediates neural progenitor cell motility after focal cerebral ischemia. J. Cereb. Blood Flow Metab. 2006, 26, 125–134. [Google Scholar] [CrossRef] [PubMed]
- Rosenkranz, K.; Kumbruch, S.; Lebermann, K.; Marschner, K.; Jensen, A.; Dermietzel, R.; Meier, C. The chemokine SDF-1/CXCL12 contributes to the fields “homing” of umbilical cord blood cells to a hypoxic-ischemic lesion in the rat brain. J. Neurosci. Res. 2010, 88, 1223–1233. [Google Scholar] [PubMed]
- Li, S.; Wei, M.; Zhou, Z.; Wang, B.; Zhao, X.; Zhang, J. SDF-1α induces angiogenesis after traumatic brain injury. Brain Res. 2012, 1444, 76–86. [Google Scholar] [CrossRef] [PubMed]
- Ziegler, M.; Elvers, M.; Baumer, Y.; Leder, C.; Ochmann, C.; Schönberger, T.; Jürgens, T.; Geisler, T.; Schlosshauer, B.; Lunov, O. The bispecific SDF1-GPVI fusion protein preserves myocardial function after transient ischemia in mice. Circulation 2012, 125, 685–696. [Google Scholar] [CrossRef] [PubMed]
- Shirozu, M.; Nakano, T.; Inazawa, J.; Tashiro, K.; Tada, H.; Shinohara, T.; Honjo, T. Structure and chromosomal localization of the human stromal cell-derived factor 1 (SDF1) gene. Genomics 1995, 28, 495–500. [Google Scholar] [CrossRef] [PubMed]
- Ohtani, Y.; Minami, M.; Kawaguchi, N.; Nishiyori, A.; Yamamoto, J.; Takami, S.; Satoh, M. Expression of stromal cell-derived factor-1 and CXCR4 chemokine receptor mRNAs in cultured rat glial and neuronal cells. Neurosci. Lett. 1998, 19, 163–166. [Google Scholar] [CrossRef]
- Stumm, R.K.; Rummel, J.; Junker, V.; Culmsee, C.; Pfeiffer, M.; Krieglstein, J.; Hollt, V.; Schulz, S. A dual role for the SDF-1/CXCR4 chemokine receptor system in adult brain: Isoform-selective regulation of SDF-1 expression modulates CXCR4-dependent neuronal plasticity and cerebral leukocyte recruitment after focal ischemia. J. Neurosci. 2002, 22, 5865–5878. [Google Scholar] [PubMed]
- Tran, P.B.; Miller, R.J. Chemokine receptors: Signposts to brain development and disease. Nat. Rev. Neurosci. 2003, 4, 444–455. [Google Scholar] [CrossRef] [PubMed]
- Stumm, R.K.; Zhou, C.; Ara, T.; Lazarini, F.; Dubois-Dalcq, M.; Nagasawa, T.; Höllt, V.; Schulz, S. CXCR4 regulates interneuron migration in the developing neocortex. J. Neurosci. 2003, 23, 5123–5130. [Google Scholar] [PubMed]
- Imitola, J.; Raddassi, K.; Park, K.I.; Mueller, F.J.; Nieto, M.; Teng, Y.D.; Frenkel, D.; Li, J.; Sidman, R.L.; Walsh, C.A.; et al. Directed migration of neural stem cells to sites of CNS injury by the stromal cell-derived factor 1α/CXC chemokine receptor 4 pathway. Proc. Natl. Acad. Sci. USA 2004, 52, 18117–18122. [Google Scholar] [CrossRef] [PubMed]
- Hillje, A.L.; Beckmann, E.; Pavlou, M.A.; Jaeger, C.; Pacheco, M.P.; Sauter, T.; Schwamborn, J.C.; Lewejohann, L. The neural stem cell fate determinant TRIM32 regulates complex behavioral traits. Front. Cell. Neurosci. 2015, 9, 75. [Google Scholar] [CrossRef] [PubMed]
- Ma, Q.; Jones, D.; Borghesani, P.R.; Segal, R.A.; Nagasawa, T.; Kishimoto, T.; Bronson, R.T.; Springer, T.A. Impaired B-lymphopoiesis, myelopoiesis, and derailed cerebellar neuron migration in CXCR4- and SDF-1-deficient mice. Proc. Natl. Acad. Sci. USA 1998, 95, 9448–9453. [Google Scholar] [CrossRef] [PubMed]
- Lu, M.; Grove, E.A.; Miller, R.J. Abnormal development of the hippocampal dentate gyrus in mice lacking the CXCR4 chemokine receptor. Proc. Natl. Acad. Sci. USA 2002, 99, 7090–7095. [Google Scholar] [CrossRef] [PubMed]
- Meucci, O.; Fatatis, A.; Simen, A.A.; Bushell, T.J.; Gray, P.W.; Miller, R.J. Chemokines regulate hippocampal neuronal signaling and gp-120 neurotoxicity. Proc. Natl. Acad. Sci. USA 1998, 95, 14500–14505. [Google Scholar] [CrossRef] [PubMed]
- Gillard, S.E.; Lu, M.; Mastracci, R.M.; Miller, R.J. Expression of functional chemokine receptors by rat cerebellar neurons. J. Neuroimmunol. 2002, 124, 16–28. [Google Scholar] [CrossRef]
- Jaerve, A.; Schira, J.; Müller, H.W. Concise review: The potential of stromal cell-derived factor 1 and its receptors to promote stem cell functions in spinal cord repair. Stem Cells Transl. Med. 2012, 1, 732–739. [Google Scholar] [CrossRef] [PubMed]
- Sun, W.; Liu, J.; Huan, Y.; Zhang, C. Intracranial injection of recombinant stromal-derived factor-1 alpha (SDF-1α) attenuates traumatic brain injury in rats. Inflamm. Res. 2014, 63, 287–297. [Google Scholar] [CrossRef] [PubMed]
- Balduini, W.; de Angelis, V.; Mazzoni, E.; Cimino, M. Long-lasting behavioral alterations following a hypoxic/ischemic brain injury in neonatal rats. Brain Res. 2000, 859, 318–325. [Google Scholar] [CrossRef]
- Hilton, G.D.; Stoica, B.A.; Byrnes, K.R.; Faden, A.I. Roscovitine reduces neuronal loss, glial activation, and neurologic deficits after brain trauma. J. Cereb. Blood Flow Metab. 2008, 28, 1845–1859. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Dore, S. Inflammation after intracerebral hemorrhage. J. Cereb. Blood Flow Metab. 2007, 27, 894–908. [Google Scholar] [CrossRef] [PubMed]
- Kroncke, K.D.; Fehsel, K.; Kolb-Bachofen, V. Nitric oxide: Cytotoxicity versus cytoprotection—How, why, when, and where? Nitric Oxide 1997, 1, 107–120. [Google Scholar] [CrossRef] [PubMed]
- Fawcett, J.W.; Asher, R.A. The glial scar and central nervous system repair. Brain Res. Bull. 1999, 49, 377–391. [Google Scholar] [CrossRef]
- Sizonenko, S.V.; Camm, E.J.; Dayer, A.; Kiss, J.Z. Glial responses to neonatal hypoxic-ischemic injury in the rat cerebral cortex. Int. J. Dev. Neurosci. 2008, 26, 37–45. [Google Scholar] [CrossRef] [PubMed]
- McGraw, J.; Hiebert, G.W.; Steeves, J.D. Modulating astrogliosis after neurotrauma. J. Neurosci. Res. 2001, 63, 109–115. [Google Scholar] [CrossRef]
- He, L.F.; Chen, H.J.; Qian, L.H.; Chen, G.Y.; Buzby, J.S. Curcumin protects pre-oligodendrocytes from activated microglia in vitro and in vivo. Brain Res. 2010, 1339, 60–69. [Google Scholar] [CrossRef] [PubMed]
- Cai, Z.; Lin, S.; Pang, Y.; Rhodes, P.G. Brain injury induced by intracerebral injection of interleukin-1β and tumor necrosis factor-α in the neonatal rat. Pediatr. Res. 2004, 56, 377–384. [Google Scholar] [CrossRef] [PubMed]
- Zaheer, A.; Zaheer, S.; Thangavel, R.; Wu, Y.; Sahu, S.K.; Yang, B. Glia maturation factor modulates β-amyloid-induced glial activation, inflammatory cytokine/chemokine production and neuronal damage. Brain Res. 2008, 1208, 192–203. [Google Scholar] [CrossRef] [PubMed]
- Deguchi, K.; Oguchi, K.; Takashima, S. Characteristic neuropathology of leukomalacia in extremely low birth weight infants. Pediatr. Neurol. 1997, 16, 296–300. [Google Scholar] [CrossRef]
- Kim, K.; Shin, M.S.; Cho, H.S.; Kim, Y.P. Effects of endurance exercise on expressions of glial fibrillary acidic protein and myelin basic protein in developing rats with maternal infection-induced cerebral palsy. J. Exerc. Rehabil. 2014, 10, 9–14. [Google Scholar] [CrossRef] [PubMed]
- Cai, Z.W.; Pang, Y.; Xiao, F.; Rhodes, P.G. Chronic ischemia preferentially causes white matter injury in the neonatal rat brain. Brain Res. 2001, 898, 126–135. [Google Scholar] [CrossRef]
- Follett, P.L.; Rosenberg, P.A.; Volpe, J.J.; Jensen, F.E. NBQX attenuates excitotoxic injury in developing white matter. J. Neurosci. 2000, 20, 9235–9241. [Google Scholar] [PubMed]
- Dwork, A.J.; Mancevski, B.; Rosoklija, G. White matter and cognitive function in schizophrenia. Int. J. Neuropsychopharmacol. 2007, 10, 513–536. [Google Scholar] [CrossRef] [PubMed]
- Lin, S.; Rhodes, P.G.; Lei, M.; Zhang, F.; Cai, Z. α-Phenyl-n-tert-butyl-nitrone attenuates hypoxic-ischemic white matter injury in the neonatal rat brain. Brain Res. 2004, 1007, 132–141. [Google Scholar] [CrossRef] [PubMed]
- Huang, Z.; Liu, J.; Cheung, P.Y.; Chen, C. Long-term cognitive impairment and myelination deficiency in a rat model of perinatal hypoxic-ischemic brain injury. Brain Res. 2009, 1301, 100–109. [Google Scholar] [CrossRef] [PubMed]
- Dineen, R.A.; Vilisaar, J.; Hlinka, J.; Bradshaw, C.M.; Morgan, P.S.; Constantinescu, C.S.; Auer, D.P. Disconnection as a mechanism for cognitive dysfunction in multiple sclerosis. Brain 2009, 132, 239–249. [Google Scholar] [CrossRef] [PubMed]
- D’Hooge, R.; de Deyn, P.P. Applications of the Morris water maze in the study of learning and memory. Brain Res. Rev. 2001, 36, 60–90. [Google Scholar] [CrossRef]
- Armstrong, R.C.; Mierzwa, A.J.; Sullivan, G.M.; Sanchez, M.A. Myelin and oligodendrocyte lineage cells in white matter pathology and plasticity after traumatic brain injury. Neuropharmacology 2015, in press. [Google Scholar] [CrossRef] [PubMed]
- Rice, J.E.; Vannucci, R.C.; Brierley, J.B. The influence of immaturity on hypoxic-ischemic brain damage in the rat. Ann. Neurol. 1981, 9, 131–141. [Google Scholar] [CrossRef] [PubMed]
- Morris, R. Developments of a water-maze procedure for studying spatial learning in the rat. J. Neurosci. Methods 1984, 11, 47–60. [Google Scholar] [CrossRef]
- Sakata, A.; Mogi, M.; Iwanami, J.; Tsukuda, K.; Min, L.J.; Jing, F.; Iwai, M.; Ito, M.; Horiuchi, M. Female exhibited severe cognitive impairment in type 2 diabetes mellitus mice. Life Sci. 2010, 86, 638–645. [Google Scholar] [CrossRef] [PubMed]
- Letourneur, A.; Freret, T.; Roussel, S.; Boulouard, M.; Divoux, D.; Toutain, J.; Bernaudin, M.; Schumann-Bard, P.; Bouet, V.; Touzani, O. Maternal hypertension during pregnancy modifies the response of the immature brain to hypoxia-ischemia: Sequential MRI and behavioral investigations. Exp. Neurol. 2012, 233, 264–272. [Google Scholar] [CrossRef] [PubMed]
- Bochelen, D.; Rudin, M.; Sauter, A. Calcineurin inhibitors FK506 and SDZ ASM 981 alleviate the outcome of focal cerebral ischemic/reperfusion injury. J. Pharmacol. Exp. Ther. 1999, 288, 653–659. [Google Scholar] [PubMed]
- Yu, Q.; Liu, L.; Lin, J.; Wang, Y.; Xuan, X.; Guo, Y.; Hu, S. SDF-1α/CXCR4 axis mediates the migration of mesenchymal stem cells to the hypoxic-ischemic brain lesion in a rat model. Cell J. 2015, 16, 440–447. [Google Scholar] [PubMed]
- Shyu, W.C.; Lin, S.Z.; Yen, P.S.; Su, C.Y.; Chen, D.C.; Wang, H.J.; Li, H. Stromal cell-derived factor-1 α promotes neuroprotection, angiogenesis, and mobilization/homing of bone marrow-derived cells in stroke rats. J. Pharmacol. Exp. Ther. 2008, 324, 834–849. [Google Scholar] [CrossRef] [PubMed]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mori, M.; Matsubara, K.; Matsubara, Y.; Uchikura, Y.; Hashimoto, H.; Fujioka, T.; Matsumoto, T. Stromal Cell-Derived Factor-1α Plays a Crucial Role Based on Neuroprotective Role in Neonatal Brain Injury in Rats. Int. J. Mol. Sci. 2015, 16, 18018-18032. https://doi.org/10.3390/ijms160818018
Mori M, Matsubara K, Matsubara Y, Uchikura Y, Hashimoto H, Fujioka T, Matsumoto T. Stromal Cell-Derived Factor-1α Plays a Crucial Role Based on Neuroprotective Role in Neonatal Brain Injury in Rats. International Journal of Molecular Sciences. 2015; 16(8):18018-18032. https://doi.org/10.3390/ijms160818018
Chicago/Turabian StyleMori, Miki, Keiichi Matsubara, Yuko Matsubara, Yuka Uchikura, Hisashi Hashimoto, Toru Fujioka, and Takashi Matsumoto. 2015. "Stromal Cell-Derived Factor-1α Plays a Crucial Role Based on Neuroprotective Role in Neonatal Brain Injury in Rats" International Journal of Molecular Sciences 16, no. 8: 18018-18032. https://doi.org/10.3390/ijms160818018