Extraction of Peptidoglycan from L. paracasei subp. Paracasei X12 and Its Preliminary Mechanisms of Inducing Immunogenic Cell Death in HT-29 Cells
Abstract
:1. Introduction
2. Results
2.1. Extraction and Qualitative Analysis of X12-PG


| Amino Acids | Concentration (μg/mL) | Contents (mmol/g) | |
|---|---|---|---|
| 1 | Glu | 56.44 | 0.381 |
| 2 | Arg | 6.80 | 0.039 |
| 3 | Thr | 12.74 | 0.106 |
| 4 | Tyr | 11.11 | 0.060 |
| 5 | Val | 8.52 | 0.072 |
| 6 | Met | 11.35 | 0.076 |
| 7 | Cys | 7.27 | 0.060 |
| 8 | Ile | 8.19 | 0.062 |
| 9 | Phe | 9.55 | 0.057 |
| 10 | Asp | 48.54 | 0.361 |
| 11 | Ser | 9.75 | 0.093 |
| 12 | Gly | 7.88 | 0.105 |
| 13 | His | 12.26 | 0.078 |
| 14 | Ala | 57.83 | 0.649 |
| 15 | Pro | 1.02 | 0.009 |
| 16 | Leu | 16.85 | 0.128 |
| 17 | Lys | 39.56 | 0.268 |
2.2. X12-PG Induced Apoptosis in HT-29 Cells

| Treatment | Apoptotic Cells (%) |
|---|---|
| OXP (15 μmol/L) | 74.21 ± 0.38 |
| X12-PG (400 μg/mL) | 50.42 ± 0.46 |
| X12-PG (800 μg/mL) | 55.65 ± 0.40 |
| X12-PG (1600 μg/mL) | 61.03 ± 0.35 |
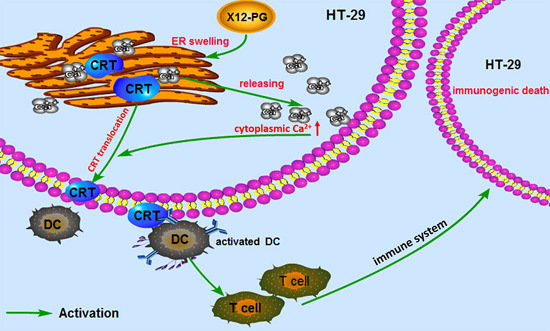
2.3. Damaged to Endoplasmic Reticulum Induced by X12-PG in HT-29 Cells

2.4. X12-PG Induced the Translocation of Calreticulin (CRT) and Release of HMGB1

| X12-PG Treatment (μg/mL) | HMGB1 Concentration (ng/mL) |
|---|---|
| 0 | 7.56 ± 0.35 |
| 400 | 9.52 ± 0.35 |
| 800 | 15.86 ± 0.32 * |
| 1600 | 21.58 ± 0.36 ** |

2.5. X12-PG Promoted the Release of Ca2+ from the ER into the Cytoplasm

3. Discussion

4. Experimental Section
4.1. Reagents
4.2. Cell Culture
4.3. Isolation and Purification of X12-PG
4.4. Analytical Methods of X12-PG
4.5. Transmission Electron Microscope (TEM)
4.6. MTT Assay
4.7. Immunofluoroscence Assay
4.8. Flow Cytometry Assay
4.9. Detection of HMGB1 Release
4.10. Statistical Analysis
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Siegel, R.L.; Miller, K.D.; Jemal, A. Cancer statistics, 2015. CA: Cancer J. Clin. 2015, 65, 5–29. [Google Scholar] [CrossRef] [PubMed]
- Kasdagly, M.; Radhakrishnan, S.; Reddivari, L.; Veeramachaneni, D.R.; Vanamala, J. Colon carcinogenesis: Influence of western diet-induced obesity and targeting stem cells using dietary bioactive compounds. Nutrition 2014, 30, 1242–1256. [Google Scholar] [CrossRef] [PubMed]
- Terzić, J.; Grivennikov, S.; Karin, E.; Karin, M. Inflammation and colon cancer. Gastroenterology 2010, 138, 2101–2114. [Google Scholar] [CrossRef] [PubMed]
- McCoy, W.; Mason, J.M., 3rd. Enterococcal endocarditis associated with carcinoma of the sigmoid; report of a case. J. Med. Assoc. State Ala. 1951, 21, 162–166. [Google Scholar] [PubMed]
- Arthur, J.C.; Perez-Chanona, E.; Mühlbauer, M.; Tomkovich, S.; Uronis, J.M.; Fan, T.-J.; Campbell, B.J.; Abujamel, T.; Dogan, B.; Rogers, A.B. Intestinal inflammation targets cancer-inducing activity of the microbiota. Science 2012, 338, 120–123. [Google Scholar] [CrossRef] [PubMed]
- Keku, T.O.; Dulal, S.; Deveaux, A.; Jovov, B.; Han, X. The gastrointestinal microbiota and colorectal cancer. Am. J. Physiol. Gastrointest. Liver Physiol. 2014, 308, G351–G363. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.; Cai, G.; Qiu, Y.; Fei, N.; Zhang, M.; Pang, X.; Jia, W.; Cai, S.; Zhao, L. Structural segregation of gut microbiota between colorectal cancer patients and healthy volunteers. ISME J. 2012, 6, 320–329. [Google Scholar] [CrossRef] [PubMed]
- Klaenhammer, T.R.; Kleerebezem, M.; Kopp, M.V.; Rescigno, M. The impact of probiotics and prebiotics on the immune system. Nat. Rev. Immunol. 2012, 12, 728–734. [Google Scholar] [CrossRef] [PubMed]
- Gourbeyre, P.; Denery, S.; Bodinier, M. Probiotics, prebiotics, and synbiotics: Impact on the gut immune system and allergic reactions. J. Leukoc. Biol. 2011, 89, 685–695. [Google Scholar] [CrossRef] [PubMed]
- Nova, E.; Abad, C.; Gómez-Martínez, S.; Pozo-Rubio, T.; Marcos, A. Probiotics and Prebiotics in Immune System Protection. In Probiotics and Prebiotics in Food, Nutrition and Health; Otles, S., Ed.; CRC Press: Boca Raton, FL, USA, 2013; Volume 13, p. 269. [Google Scholar]
- Urbanska, A.M.; Bhathena, J.; Martoni, C.; Prakash, S. Estimation of the potential antitumor activity of microencapsulated Lactobacillus acidophilus yogurt formulation in the attenuation of tumorigenesis in Apc (Min/+) mice. Dig. Dis. Sci. 2009, 54, 264–273. [Google Scholar] [CrossRef] [PubMed]
- Ishikawa, H.; Akedo, I.; Otani, T.; Suzuki, T.; Nakamura, T.; Takeyama, I.; Ishiguro, S.; Miyaoka, E.; Sobue, T.; Kakizoe, T. Randomized trial of dietary fiber and Lactobacillus casei administration for prevention of colorectal tumors. Int. J. Cancer 2005, 116, 762–767. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Qin, H.; Yang, Z.; Xia, Y.; Liu, W.; Yang, J.; Jiang, Y.; Zhang, H.; Wang, Y.; Zheng, Q. Randomised clinical trial: The effects of perioperative probiotic treatment on barrier function and post—Operative infectious complications in colorectal cancer surgery—A double-blind study. Aliment. Pharm. Ther. 2011, 33, 50–63. [Google Scholar] [CrossRef] [PubMed]
- Kumar, M.; Nagpal, R.; Verma, V.; Kumar, A.; Kaur, N.; Hemalatha, R.; Gautam, S.K.; Singh, B. Probiotic metabolites as epigenetic targets in the prevention of colon cancer. Nutr. Rev. 2013, 71, 23–34. [Google Scholar] [CrossRef] [PubMed]
- Amrouche, T.; Boutin, Y.; Prioult, G.; Fliss, I. Effects of bifidobacterial cytoplasm, cell wall and exopolysaccharide on mouse lymphocyte proliferation and cytokine production. Int. Dairy J. 2006, 16, 70–80. [Google Scholar] [CrossRef]
- Clinical Outcomes of Surgical Therapy Study Group. A comparison of laparoscopically assisted and open colectomy for colon cancer. N. Engl. J. Med. 2004, 350, 2050–2059. [Google Scholar]
- Wiwanitkit, V. Plateletcrit, mean platelet volume, platelet distribution width: Its expected values and correlation with parallel red blood cell parameters. Clin. Appl. Thromb. Hemost. 2004, 10, 175–178. [Google Scholar] [CrossRef] [PubMed]
- Aliustaoglu, M.; Bilici, A.; Seker, M.; Dane, F.; Gocun, M.; Konya, V.; Ustaalioglu, B.B.; Gumus, M. The association of pre-treatment peripheral blood markers with survival in patients with pancreatic cancer. Hepato-Gastroenterology 2010, 57, 640–645. [Google Scholar] [PubMed]
- Kahouli, I.; Tomaro-Duchesneau, C.; Prakash, S. Probiotics in colorectal cancer (CRC) with emphasis on mechanisms of action and current perspectives. J. Med. Microbiol. 2013, 62, 1107–1123. [Google Scholar] [CrossRef] [PubMed]
- Sorbara, M.T.; Philpott, D.J. Peptidoglycan: A critical activator of the mammalian immune system during infection and homeostasis. Immunol. Rev. 2011, 243, 40–60. [Google Scholar] [CrossRef] [PubMed]
- Fichera, G.A.; Giese, G. Non-immunologically-mediated cytotoxicity of Lactobacillus casei and its derivative peptidoglycan against tumor cell lines. Cancer Lett. 1994, 85, 93–103. [Google Scholar] [CrossRef]
- Wang, S.-M.; Zhang, L.-W.; Fan, R.-B.; Han, X.; Yi, H.-X.; Zhang, L.-L.; Xue, C.-H.; Li, H.-B.; Zhang, Y.-H.; Shigwedha, N. Induction of HT-29 cells apoptosis by lactobacilli isolated from fermented products. Res. Microbiol. 2014, 165, 202–214. [Google Scholar] [CrossRef] [PubMed]
- Tesniere, A.; Panaretakis, T.; Kepp, O.; Apetoh, L.; Ghiringhelli, F.; Zitvogel, L.; Kroemer, G. Molecular characteristics of immunogenic cancer cell death. Cell Death Differ. 2007, 15, 3–12. [Google Scholar] [CrossRef] [PubMed]
- Obeid, M.; Tesniere, A.; Ghiringhelli, F.; Fimia, G.M.; Apetoh, L.; Perfettini, J.-L.; Castedo, M.; Mignot, G.; Panaretakis, T.; Casares, N. Calreticulin exposure dictates the immunogenicity of cancer cell death. Nat. Med. 2007, 13, 54–61. [Google Scholar] [CrossRef] [PubMed]
- Ardelt, P.U.; Kneitz, B.; Adam, P.; Reiss, C.; Kocot, A.; Fensterle, J.; Chen, L.; Pasqualini, R.; Arap, W.; Gerharz, E.W. Reactive antibodies against bacillus Calmette-Guerin heat-shock protein-65 potentially predict the outcome of immunotherapy for high-grade transitional cell carcinoma of the bladder. Cancer 2010, 116, 600–609. [Google Scholar] [CrossRef] [PubMed]
- Apetoh, L.; Ghiringhelli, F.; Tesniere, A.; Obeid, M.; Ortiz, C.; Criollo, A.; Mignot, G.; Maiuri, M.C.; Ullrich, E.; Saulnier, P. Toll-like receptor 4-dependent contribution of the immune system to anticancer chemotherapy and radiotherapy. Nat. Med. 2007, 13, 1050–1059. [Google Scholar] [CrossRef] [PubMed]
- Elliott, M.R.; Chekeni, F.B.; Trampont, P.C.; Lazarowski, E.R.; Kadl, A.; Walk, S.F.; Park, D.; Woodson, R.I.; Ostankovich, M.; Sharma, P. Nucleotides released by apoptotic cells act as a find-me signal to promote phagocytic clearance. Nature 2009, 461, 282–286. [Google Scholar] [CrossRef] [PubMed]
- Menger, L.; Vacchelli, E.; Adjemian, S.; Martins, I.; Ma, Y.; Shen, S.; Yamazaki, T.; Sukkurwala, A.Q.; Michaud, M.; Mignot, G. Cardiac glycosides exert anticancer effects by inducing immunogenic cell death. Sci. Transl. Med. 2012, 4, 143ra99. [Google Scholar] [CrossRef] [PubMed]
- Kroemer, G.; Galluzzi, L.; Kepp, O.; Zitvogel, L. Immunogenic cell death in cancer therapy. Annu. Rev. Immunol. 2013, 31, 51–72. [Google Scholar] [CrossRef] [PubMed]
- Schumann, P. 5 Peptidoglycan Structure. Methods Microbiol. 2011, 38, 101–129. [Google Scholar]
- Ko, A.; Kanehisa, A.; Martins, I.; Senovilla, L.; Chargari, C.; Dugue, D.; Marino, G.; Kepp, O.; Michaud, M.; Perfettini, J. Autophagy inhibition radiosensitizes in vitro, yet reduces radioresponses in vivo due to deficient immunogenic signalling. Cell Death Differ. 2014, 21, 92–99. [Google Scholar] [CrossRef] [PubMed]
- Sekine, K.; Toida, T.; Saito, M.; Kuboyama, M.; Kawashima, T.; Hashimoto, Y. A new morphologically characterized cell wall preparation (whole peptidoglycan) from Bifidobacterium infantis with a higher efficacy on the regression of an established tumor in mice. Cancer Res. 1985, 45, 1300–1307. [Google Scholar] [PubMed]
- Jun, Y.; Hong, H. Isolation and purification of whole peptidoglycan from Bifidobacterium Bifidum. Chin. J. Microecol. 1997, 5, 12–15. [Google Scholar]
- Butler, C.A.; Hoffman, P.S. Characterization of a major 31-kilodalton peptidoglycan-bound protein of Legionella pneumophila. J. Bacteriol. 1990, 172, 2401–2407. [Google Scholar] [PubMed]
- Schleifer, K.H.; Kandler, O. Peptidoglycan types of bacterial cell walls and their taxonomic implications. Bacteriol. Rev. 1972, 36, 407–477. [Google Scholar] [PubMed]
- Vacchelli, E.; Aranda, F.; Eggermont, A.; Galon, J.; Sautès-Fridman, C.; Cremer, I.; Zitvogel, L.; Kroemer, G.; Galluzzi, L. Trial watch: Chemotherapy with immunogenic cell death inducers. Oncoimmunology 2014, 3, e27878. [Google Scholar] [CrossRef] [PubMed]
- Prisciandaro, L.D.; Geier, M.S.; Butler, R.N.; Cummins, A.G.; Howarth, G.S. Evidence supporting the use of probiotics for the prevention and treatment of chemotherapy-induced intestinal mucositis. Crit. Rev. Food Sci. Nutr. 2011, 51, 239–247. [Google Scholar] [CrossRef] [PubMed]
- Russo, F.; Orlando, A.; Linsalata, M.; Cavallini, A.; Messa, C. Effects of Lactobacillus rhamnosus GG on the cell growth and polyamine metabolism in HGC-27 human gastric cancer cells. Nutr. Cancer 2007, 59, 106–114. [Google Scholar] [CrossRef] [PubMed]
- Thirabunyanon, M.; Boonprasom, P.; Niamsup, P. Probiotic potential of lactic acid bacteria isolated from fermented dairy milks on antiproliferation of colon cancer cells. Biotechnol. Lett. 2009, 31, 571–576. [Google Scholar] [CrossRef] [PubMed]
- Choi, S.; Kim, Y.; Han, K.; You, S.; Oh, S.; Kim, S. Effects of Lactobacillus strains on cancer cell proliferation and oxidative stress in vitro. Lett. Appl. Microbiol. 2006, 42, 452–458. [Google Scholar] [CrossRef] [PubMed]
- Zitvogel, L.; Galluzzi, L.; Smyth, M.J.; Kroemer, G. Mechanism of action of conventional and targeted anticancer therapies: Reinstating immunosurveillance. Immunity 2013, 39, 74–88. [Google Scholar] [CrossRef] [PubMed]
- Görlach, A.; Klappa, P.; Kietzmann, D.T. The endoplasmic reticulum: Folding, calcium homeostasis, signaling, and redox control. Antioxid. Redox Sign. 2006, 8, 139–1418. [Google Scholar] [CrossRef] [PubMed]
- Tufi, R.; Panaretakis, T.; Bianchi, K.; Criollo, A.; Fazi, B.; di Sano, F.; Tesniere, A.; Kepp, O.; Paterlini-Brechot, P.; Zitvogel, L. Reduction of endoplasmic reticulum Ca2+; levels favors plasma membrane surface exposure of calreticulin. Cell Death Differ. 2008, 15, 274–282. [Google Scholar] [CrossRef] [PubMed]
- Dubois, M.; Gilles, K.A.; Hamilton, J.K.; Rebers, P.A.; Smith, F. Colorimetric method for determination of sugars and related substances. Anal. Chem. 1956, 28, 350–356. [Google Scholar] [CrossRef]
- Kumar, S.; Ciraolo, G.; Hinge, A.; Filippi, M.-D. An efficient and reproducible process for transmission electron microscopy (TEM) of rare cell populations. J. Immunol. Methods 2014, 404, 87–90. [Google Scholar] [CrossRef] [PubMed]
- Borhani Dizaji, N.; Basseri, H.R.; Naddaf, S.R.; Heidari, M. Molecular characterization of calreticulin from Anopheles stephensi midgut cells and functional assay of the recombinant calreticulin with Plasmodium berghei ookinetes. Gene 2014, 550, 245–252. [Google Scholar] [CrossRef] [PubMed]
- Omann, G.M.; Harter, J.M. Pertussis toxin effects on chemoattractant-induced response heterogeneity in human PMNs utilizing fluo-3 and flow cytometry. Cytometry 1991, 12, 252–259. [Google Scholar] [CrossRef] [PubMed]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tian, P.-J.; Li, B.-L.; Shan, Y.-J.; Zhang, J.-N.; Chen, J.-Y.; Yu, M.; Zhang, L.-W. Extraction of Peptidoglycan from L. paracasei subp. Paracasei X12 and Its Preliminary Mechanisms of Inducing Immunogenic Cell Death in HT-29 Cells. Int. J. Mol. Sci. 2015, 16, 20033-20049. https://doi.org/10.3390/ijms160820033
Tian P-J, Li B-L, Shan Y-J, Zhang J-N, Chen J-Y, Yu M, Zhang L-W. Extraction of Peptidoglycan from L. paracasei subp. Paracasei X12 and Its Preliminary Mechanisms of Inducing Immunogenic Cell Death in HT-29 Cells. International Journal of Molecular Sciences. 2015; 16(8):20033-20049. https://doi.org/10.3390/ijms160820033
Chicago/Turabian StyleTian, Pei-Jun, Bao-Long Li, Yu-Juan Shan, Jin-Na Zhang, Jing-Yu Chen, Min Yu, and Lan-Wei Zhang. 2015. "Extraction of Peptidoglycan from L. paracasei subp. Paracasei X12 and Its Preliminary Mechanisms of Inducing Immunogenic Cell Death in HT-29 Cells" International Journal of Molecular Sciences 16, no. 8: 20033-20049. https://doi.org/10.3390/ijms160820033
APA StyleTian, P.-J., Li, B.-L., Shan, Y.-J., Zhang, J.-N., Chen, J.-Y., Yu, M., & Zhang, L.-W. (2015). Extraction of Peptidoglycan from L. paracasei subp. Paracasei X12 and Its Preliminary Mechanisms of Inducing Immunogenic Cell Death in HT-29 Cells. International Journal of Molecular Sciences, 16(8), 20033-20049. https://doi.org/10.3390/ijms160820033