Photodynamic Efficiency: From Molecular Photochemistry to Cell Death
Abstract
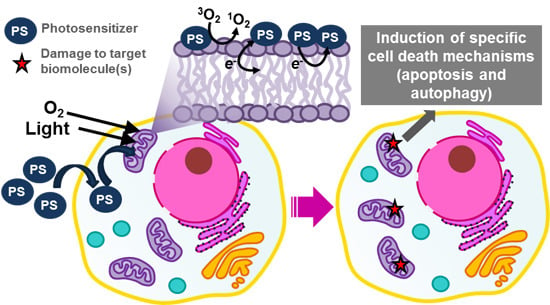
:1. Introduction
2. Biological Targets of Photooxidations
2.1. Photooxidation of Biomolecules

2.2. Consequences of Biomolecule Oxidation


| Photosensitizer (PS) | Subcellular Localization | Biological Consequences | Cell Death Mechanism | References |
|---|---|---|---|---|
| 9-Capronyloxytetrakis-(methoxyethyl)porphycene (CPO) | Endoplasmic reticulum (ER) | B-cell lymphoma 2 (Bcl-2) loss and release of Ca2+ | Apoptosis and autophagy | [73,98] |
| Sulfonated aluminum phthalocyanines (AlPcS2-4) | Lysosomes | Photodamage to mammalian target of rapamycin (mTOR) signaling network and release of lysosomal proteases, which activate caspase 3 | ND | [75,99] |
| Benzoporphyrin (BPD, Verteporfin) | Mitochondria | Decreases B-cell lymphoma-extra large (Bcl-xL) and increases the Bcl-2 associated X protein (Bax)/Bcl-xL ratio | Apoptosis | [100,101] |
| Cationic porphyrins | Plasma membrane and mitochondria | Plasma membrane disruption and mitochondrial inner membrane permeabilization, causing release of cytochrome c | Necrosis and apoptosis | [102,103,104] |
| Cationic zinc(II) phthalocyanines | Mitochondria | Destruction of the inner mitochondrial membrane | Apoptosis | [105] |
| Chlorophyllin e4 | Mitochondria and lysosomes | ND | Apoptosis and autophagy | [88,106] |
| Hypericin | ER | Loss of SERCA (sarco/endoplasmic reticulum Ca2+-ATPase) protein levels causing ER-Ca2+ depletion | Apoptosis and autophagy | [85,107,108] |
| Methylene blue (MB) | Mitochondria and lysosomes | Reduction of mitochondrial membrane potential and downregulation of the anti-apoptotic proteins Bcl-2 | Apoptosis | [31,42,109] |
| mTHPC, Foscan® | Mitochondria, golgi apparatus and ER | Photodamage to Bcl-2 protein and release of cytochrome c | Apoptosis | [110,111,112] |
| N-Aspartyl chlorin e6 (NPe6) | Lysosomes | Release of lysosomal proteases that cleave BH3-interacting domain death agonist (Bid) | Apoptosis | [113] |
| Photofrin® | Plasma membrane and mitochondria | Plasma membrane disruption and mitochondrial inner membrane permeabilization, causing release of cytochrome c | Necrosis and apoptosis | [114,115,116,117] |
| Rose bengal (RB) | Golgi apparatus | ND | Necrosis, apoptosis and autophagy | [118,119,120,121] |
| Silicon phthalocyanine (Pc4) | Mitochondria, ER and Golgi | Photodamage to Bcl-2 protein | Apoptosis | [122,123,124] |
| Tetrakis (p-sulfonatophenyl) porphyrin (TPPS4) | Lysosomes | Release of proteases causing cathepsin-mediated cleavage of Bid and inhibition of autolysosome formation | Apoptosis and autophagy | [72,125,126,127] |
3. Parameters Determining Photosensitizer (PS) Efficiency
3.1. Biological Environment Affects Triplet Reactivity

3.2. The Biological Outcome as a Function of PS Properties

4. Major Challenges
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Henderson, B.W.; Dougherty, T.J. How does photodynamic therapy work? Photochem. Photobiol. 1992, 55, 145–157. [Google Scholar] [CrossRef] [PubMed]
- Wainwright, M. Photodynamic antimicrobial chemotherapy (PACT). J. Antimicrob. Chemother. 1998, 42, 13–28. [Google Scholar] [CrossRef] [PubMed]
- Foote, C.S. Mechanisms of photosensitized oxidation. Science 1968, 162, 963–970. [Google Scholar] [CrossRef] [PubMed]
- Hamblin, M.R.; Hasan, T. Photodymamic therapy: A new antimicrobial approach to infectious disease? Photochem. Photobiol. Sci. 2014, 3, 436–450. [Google Scholar] [CrossRef] [PubMed]
- Dolmans, D.E.J.G.J.; Fukumurea, D.; Jain, R.K. Photodynamic therapy for cancer. Nat. Rev. Cancer 2003, 3, 380–387. [Google Scholar] [CrossRef] [PubMed]
- Baptista, M.S.; Wainwright, M. Photodynamic antimicrobial chemotherapy (PACT) for the treatment of malaria, leishmaniasis and trypanosomiasis. Braz. J. Med. Biol. Res. 2011, 44, 1–10. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tardivo, J.P.; del Giglio, A.; Paschoal, L.H.C.; Ito, A.S.; Baptista, M.S. Treatment of melanoma lesions using methylene blue and RL50 light source. Photodiagn. Photodyn. Ther. 2004, 1, 345–346. [Google Scholar] [CrossRef]
- Agostinis, P.; Berg, K.; Cengel, K.A.; Foster, T.H.; Girotti, A.W.; Gollnick, S.O.; Hahn, S.M.; Hamblin, M.R.; Juzeniene, A.; Kessel, D.; et al. Photodynamic therapy of cancer: An update. Am. Cancer Soc. 2011, 61, 250–281. [Google Scholar] [CrossRef] [PubMed]
- Marian, C.M. Spin-orbit coupling and intersystem crossing in molecules. Wiley Interdiscip. Rev. Comput. Mol. Sci. 2012, 2, 187–203. [Google Scholar] [CrossRef]
- Fang, J.; Chen, Y.-C. Nanomaterials for photohyperthermia: A review. Curr. Pharm. Des. 2013, 19, 6622–6634. [Google Scholar] [CrossRef] [PubMed]
- Hwang, S.; Nam, J.; Jung, S.; Song, J.; Doh, H.; Kim, S. Gold nanoparticle-mediated photothermal therapy: Current status and future perspective. Nanomedicine 2014, 9, 2003–2022. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Meng, D.; Hao, Y.; Zhao, Y.; Li, D.; Zhang, B.; Zhang, Y.; Zhang, Z. Gold nanostars mediated combined photothermal and photodynamic therapy and X-ray imaging for cancer theranostic applications. J. Biomater. Appl. 2015, 17, 928–934. [Google Scholar] [CrossRef] [PubMed]
- Braslavsky, S.E. Glossary of terms used in photochemistry. Pure Appl. Chem. 2007, 79, 293–465. [Google Scholar] [CrossRef]
- Frankel, E.N. Chemistry of free radical and singlet oxidation of lipids. Prog. Lipid Res. 1984, 23, 197–221. [Google Scholar] [CrossRef]
- Krinsky, N.I. Singlet oxygen in biological systems. Trends Biochem. Sci. 1977, 2, 35–38. [Google Scholar] [CrossRef]
- Girotti, A.W. Photosensitized oxidation of membrane lipids: Reaction pathways, cytotoxic effects, and cytoprotective mechanisms. J. Photochem. Photobiol. B Biol. 2001, 63, 103–113. [Google Scholar] [CrossRef]
- Davies, M.J. Singlet oxygen-mediated damage to proteins and its consequences. Biochem. Biophys. Res. Commun. 2003, 305, 761–770. [Google Scholar] [CrossRef]
- Michaeli, A.; Feitelson, J. Reactivity of singlet oxygen toward proteins: The effect of structure in basic pancreatic trypsin inhibitor and in ribonuclease A. Photochem. Photobiol. 1997, 65, 309–315. [Google Scholar] [CrossRef] [PubMed]
- Ravanat, J.L.; Cadet, J. Reaction of singlet oxygen with 2′-deoxyguanosine and DNA. Isolation and characterization of the main oxidation products. Chem. Res. Toxicol. 1995, 8, 379–388. [Google Scholar] [CrossRef] [PubMed]
- Sies, H.; Menck, C.F.M. Singlet oxyegn induced DNA damage. Mutat. Res. 1992, 275, 367–375. [Google Scholar] [CrossRef]
- Agnez-Lima, L.F.; Melo, J.T.A.; Silva, A.E.; Oliveira, A.H.S.; Timoteo, A.R.S.; Lima-Bessa, K.M.; Martinez, G.R.; Medeiros, M.H.G.; di Mascio, P.; Galhardo, R.S.; et al. DNA damage by singlet oxygen and cellular protective mechanisms. Mutat. Res. 2012, 751, 15–28. [Google Scholar] [CrossRef] [PubMed]
- Huang, L.; Xuan, Y.; Koide, Y.; Zhiyentayev, T.; Tanaka, M.; Hamblin, M.R. Type I and Type II mechanisms of antimicrobial photodynamic therapy: An in vitro study on gram-negative and gram-positive bacteria. Lasers Surg. Med. 2012, 44, 490–499. [Google Scholar] [CrossRef] [PubMed]
- Turro, N.J.; Ramamurthy, V.; Scaiano, J.C. Principles of Molecular Photochemistry: An Introduction, 1st ed.; University Science Books: Sausalito, CA, USA, 2009. [Google Scholar]
- Scurachio, R.S.; Skibsted, L.H.; Metzker, G.; Cardoso, D.R. Photodegradation of folate sensitized by riboflavin. Photochem. Photobiol. 2011, 87, 840–845. [Google Scholar] [CrossRef] [PubMed]
- De, O.R.; Arrivetti, L.; Scurachio, R.S.; Santos, W.G.; Papa, T.B.R.; Skibsted, L.H.; Cardoso, D.R. Photooxidation of other B-vitamins as sensitized by riboflavin. J. Agric. Food Chem. 2013, 61, 7615–7620. [Google Scholar]
- Huvaere, K.; Cardoso, D.R.; Homem-de-Mello, P.; Westermann, S.; Skibsted, L.H. Light-induced oxidation of unsaturated lipids as sensitized by flavins. J. Phys. Chem. B 2010, 114, 5583–5593. [Google Scholar] [CrossRef] [PubMed]
- Petroselli, G.; Dántola, M.L.; Cabrerizo, F.M.; Capparelli, A.L.; Lorente, C.; Oliveros, E.; Thomas, A.H. Oxidation of 2′-deoxyguanosine 5′-monophosphate photoinduced by pterin: Type I vs. type II mechanism. J. Am. Chem. Soc. 2008, 130, 3001–3011. [Google Scholar] [CrossRef] [PubMed]
- Cardoso, D.R.; Franco, D.W.; Olsen, K.; Andersen, M.L.; Skibsted, L.H. Reactivity of bovine whey proteins, peptides, and amino acids toward triplet riboflavin as studied by laser flash photolysis. J. Agric. Food Chem. 2004, 52, 6602–6606. [Google Scholar] [CrossRef] [PubMed]
- Tsaytler, P.A.; O’Flaherty, M.C.; Sakharov, D.V.; Krijgsveld, J.; Egmond, M.R. Immediate protein targets of photodynamic treatment in carcinoma cells. J. Proteome Res. 2008, 7, 3868–3878. [Google Scholar] [CrossRef] [PubMed]
- Magi, B.; Ettorre, A.; Liberatori, S.; Bini, L.; Andreassi, M.; Frosali, S.; Neri, P.; Pallini, V.; di Stefano, A. Selectivity of protein carbonylation in the apoptotic response to oxidative stress associated with photodynamic therapy: A cell biochemical and proteomic investigation. Cell Death Differ. 2004, 11, 842–852. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Zheng, W.; Li, Y.; Zhong, J.; Ji, J.; Shen, P. Apoptosis induced by methylene-blue-mediated photodynamic therapy in melanomas and the involvement of mitochondrial dysfunction revealed by proteomics. Cancer Sci. 2008, 99, 2019–2027. [Google Scholar] [CrossRef] [PubMed]
- Alves, E.; Santos, N.; Melo, T.; Maciel, E.; Dõria, M.L.; Faustino, M.A.F.; Tomé, J.P.C.; Neves, M.G.P.M.S.; Cavaleiro, J.A.S.; Cunha, Â.; et al. Photodynamic oxidation of Escherichia coli membrane phospholipids: New insights based on lipidomics. Rapid Commun. Mass Spectrom. 2013, 27, 2717–2728. [Google Scholar] [CrossRef] [PubMed]
- Halliwell, B.; Gutteridge, J.M.C. Free Radicals in Biology and Medicine, 3rd ed.; Oxford University Press: Oxford, UK, 2007. [Google Scholar]
- Buettner, G.R. The pecking order of free radicals and antioxidants: Lipid peroxidation, α-tocopherol, ascorbate. Arch. Biochem. Biophys. 1993, 300, 535–543. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, Y.J.; Forman, H.J.; Sevanian, A. Oxidants as stimulators of signal transduction. Free Radic. Biol. Med. 1996, 22, 269–285. [Google Scholar] [CrossRef]
- Silva, E.F.F.; Pedersen, B.W.; Breitenbach, T.; Toftegaard, R.; Kuimova, M.K.; Arnaut, L.G.; Ogilby, P.R. Irradiation- and sensitizer-dependent changes in the lifetime of intracellular singlet oxygen produced in a photosensitized process. J. Phys. Chem. B 2012, 116, 445–461. [Google Scholar] [CrossRef] [PubMed]
- Baier, J.; Maier, M.; Engl, R.; Landthaler, M.; Bäumler, W. Time-resolved investigations of singlet oxygen luminescence in water, in phosphatidylcholine, and in aqueous suspensions of phosphatidylcholine or HT29 cells. J. Phys. Chem. B 2005, 109, 3041–3046. [Google Scholar] [CrossRef] [PubMed]
- Wilkinson, F.; Helman, W.P.; Ross, A.B. Rate constants for the decay and reactions of the lowest electronically excited singlet state of molecular oxygen in solution. An expand and revised compilation. J. Phys. Chem. Ref. Data 1995, 24, 663–677. [Google Scholar] [CrossRef]
- Redmond, R.W.; Kochevar, I.E. Spatially resolved cellular responses to singlet oxygen. Photochem. Photobiol. 2006, 82, 1178–1186. [Google Scholar] [CrossRef] [PubMed]
- Schweitzer, C.; Schmidt, R. Physical mechanisms of generation and deactivation of singlet oxygen. Chem. Rev. 2003, 103, 1685–1757. [Google Scholar] [CrossRef] [PubMed]
- Chiarelli-Neto, O.; Pavani, C.; Ferreira, A.S.; Uchoa, A.F.; Severino, D.; Baptista, M.S. Generation and suppression of singlet oxygen in hair by photosensitization of melanin. Free Radic. Biol. Med. 2011, 51, 1195–1202. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, C.S.; Turchiello, R.; Kowaltowski, A.J.; Indig, G.L.; Baptista, M.S. Major determinants of photoinduced cell death: Subcellular localization versus photosensitization efficiency. Free Radic. Biol. Med. 2011, 51, 824–833. [Google Scholar] [CrossRef] [PubMed]
- Girotti, A.W. Lipid hydroperoxide generation, turnover, and effector action in biological systems. J. Lipid Res. 1998, 39, 1529–1542. [Google Scholar] [PubMed]
- Nagano, T. Bioimaging probes for reactive oxygen species and reactive nitrogen species. J. Clin. Biochem. Nutr. 2009, 45, 111–124. [Google Scholar] [CrossRef] [PubMed]
- Daghastanli, N.A.; Itri, R.; Baptista, M.S. Singlet oxygen reacts with 2′,7′-dichlorodihydrofluorescein and contributes to the formation of 2′,7′-dichlorofluorescein. Photochem. Photobiol. 2008, 84, 1238–1243. [Google Scholar] [CrossRef] [PubMed]
- Baker, A.; Kanofsky, J.R. Quenching of singlet oxygen by biomolecules from L1210 leukemia cells. Photochem. Photobiol. 1992, 55, 523–528. [Google Scholar] [CrossRef] [PubMed]
- Pavani, C.; Iamamoto, Y.; Baptista, M.S. Mechanism and efficiency of cell death of type II photosensitizers: Effect of zinc chelation. Photochem. Photobiol. 2012, 88, 774–781. [Google Scholar] [CrossRef] [PubMed]
- Ricchelli, F.; Franchi, L.; Miotto, G.; Borsetto, L.; Gobbo, S.; Nikolov, P.; Bommer, J.C.; Reddi, E. Meso-substituted tetra-cationic porphyrins photosensitize the death of human fibrosarcoma cells via lysosomal targeting. Int. J. Biochem. Cell Biol. 2005, 37, 306–319. [Google Scholar] [CrossRef] [PubMed]
- Ricchelli, F. Photophysical properties of porphyrins in biological membranes. J. Photochem. Photobiol. B Biol. 1995, 29, 109–118. [Google Scholar] [CrossRef]
- Cordeiro, R.M.; Miotto, R.; Baptista, M.S. Photodynamic efficiency of cationic meso-porphyrins at lipid bilayers: Insights from molecular dynamics simulations. J. Phys. Chem. B 2012, 116, 14618–14627. [Google Scholar] [CrossRef] [PubMed]
- Dzikovski, B.G.; Livshits, V.A.; Marsh, D. Oxygen permeation profile in lipid membranes: Comparison with transmembrane polarity profile. Biophys. J. 2003, 85, 1005–1012. [Google Scholar] [CrossRef]
- Subczynski, W.K.; Hyde, J.S. Concentration of oxygen in lipid bilayers using a spin-label method. Biophys. J. 1983, 41, 283–286. [Google Scholar] [CrossRef]
- Windrem, D.A.; Plachy, W.Z. The diffusion-solubility of oxygen in lipid bilayers. Biochim. Biophys. Acta 1980, 600, 655–665. [Google Scholar] [CrossRef]
- Wong-Ekkabut, J.; Xu, Z.; Triampo, W.; Tang, I.-M.; Tieleman, D.P.; Monticelli, L. Effect of lipid peroxidation on the properties of lipid bilayers: A molecular dynamics study. Biophys. J. 2007, 93, 4225–4236. [Google Scholar] [CrossRef] [PubMed]
- Weber, G.; Charitat, T.; Baptista, M.S.; Uchoa, A.F.; Pavani, C.; Junqueira, H.C.; Guo, Y.; Baulin, V.A.; Itri, R.; Marques, C.M.; et al. Lipid oxidation induces structural changes in biomimetic membranes. Soft Matter 2014, 10, 4241–4247. [Google Scholar] [CrossRef] [PubMed]
- Haluska, C.K.; Baptista, M.S.; Fernandes, A.U.; Schroder, A.P.; Marques, C.M.; Itri, R. Photo-activated phase separation in giant vesicles made from different lipid mixtures. Biochim. Biophys. Acta Biomembr. 2012, 1818, 666–672. [Google Scholar] [CrossRef] [PubMed]
- Gajate, C.; Gonzalez-Camacho, F.; Mollinedo, F. Lipid raft connection between extrinsic and intrinsic apoptotic pathways. Biochem. Biophys. Res. Commun. 2009, 380, 780–784. [Google Scholar] [CrossRef] [PubMed]
- Dykstra, M.; Cherukuri, A.; Sohn, H.W.; Tzeng, S.-J.; Pierce, S.K. Location is everything: Lipid rafts and immune cell signaling. Annu. Rev. Immunol. 2003, 21, 457–481. [Google Scholar] [CrossRef] [PubMed]
- Kawai, C.; Ferreira, J.C.; Baptista, M.S.; Nantes, I.L. Not only oxidation of cardiolipin affects the affinity of cytochrome c for lipid bilayers. J. Phys. Chem. B 2014, 118, 11863–11872. [Google Scholar] [CrossRef] [PubMed]
- Caetano, W.; Haddad, P.S.; Itri, R.; Severino, D.; Vieira, V.C.; Baptista, M.S.; Schröder, A.P.; Marques, C.M. Photo-induced destruction of giant vesicles in methylene blue solutions. Langmuir 2007, 23, 1307–1314. [Google Scholar] [CrossRef] [PubMed]
- Valenzeno, D.P. Photomodification of biological membranes with emphasis on singlet oxygen mechanisms. Photochem. Photobiol. 1987, 46, 147–160. [Google Scholar] [CrossRef] [PubMed]
- Anderson, S.M.; Krinsky, N.I.; Stone, M.J.; Clagett, D.C. Effect of singlet oxygen quenchers on oxidative damage to liposomes initiated by photosensitization or by radiofrequency discharge. Photochem. Photobiol. 1974, 20, 65–69. [Google Scholar] [CrossRef] [PubMed]
- Sankhagowit, S.; Wu, S.H.; Biswas, R.; Riche, C.T.; Povinelli, M.L.; Malmstadt, N. The dynamics of giant unilamellar vesicle oxidation probed by morphological transitions. Biochim. Biophys. Acta Biomembr. 2014, 1838, 2615–2624. [Google Scholar] [CrossRef] [PubMed]
- Riske, K.A.; Sudbrack, T.P.; Archilha, N.L.; Uchoa, A.F.; Schroder, A.P.; Marques, C.M.; Baptista, M.S.; Itri, R. Giant vesicles under oxidative stress induced by a membrane-anchored photosensitizer. Biophys. J. 2009, 97, 1362–1370. [Google Scholar] [CrossRef] [PubMed]
- Ytzhak, S.; Ehrenberg, B. The effect of photodynamic action on leakage of ions through liposomal membranes that contain oxidatively modified lipids. Photochem. Photobiol. 2014, 90, 796–800. [Google Scholar] [CrossRef] [PubMed]
- Runas, K.A.; Malmstadt, N. Low levels of lipid oxidation radically increase the passive permeability of lipid bilayers. Soft Matter 2015, 11, 499–505. [Google Scholar] [CrossRef] [PubMed]
- Hampton, M.B.; Morgan, P.E.; Davies, M.J. Inactivation of cellular caspases by peptide-derived tryptophan and tyrosine peroxides. FEBS Lett. 2002, 527, 289–292. [Google Scholar] [CrossRef]
- Escobar, J.A.; Rubio, M.A.; Lissi, E.A. SOD and catalase inactivation by singlet oxygen and peroxyl radicals. Free Radic. Biol. Med. 1996, 20, 285–290. [Google Scholar] [CrossRef]
- Silva, E.; de Landea, C.; Edwards, A.M.; Lissi, E. Lysozyme photo-oxidation by singlet oxygen: Properties of the partially inactivated enzyme. J. Photochem. Photobiol. B Biol. 2000, 55, 196–200. [Google Scholar] [CrossRef]
- Goosey, J.D.; Zigler, J.S.; Kinoshita, J.H. Cross-linking of lens crystallins in a photodynamic system: A process mediated by singlet oxygen. Science 1980, 208, 1278–1280. [Google Scholar] [CrossRef] [PubMed]
- Duprez, L.; Wirawan, E.; Berghe, T.V.; Vandenabeele, P. Major cell death pathways at a glance. Microbes Infect. 2009, 11, 1050–1062. [Google Scholar] [CrossRef] [PubMed]
- Mroz, P.; Yaroslavsky, A.; Kharkwal, G.B.; Hamblin, M.R. Cell death pathways in photodynamic therapy of cancer. Cancers (Basel) 2011, 3, 2516–2539. [Google Scholar] [CrossRef] [PubMed]
- Kessel, D.; Castelli, M.; Reiners, J.J. Ruthenium red-mediated suppression of Bcl-2 loss and Ca2+ release initiated by photodamage to the endoplasmic reticulum: Scavenging of reactive oxygen species. Cell Death Differ. 2005, 12, 502–511. [Google Scholar] [CrossRef] [PubMed]
- Inguscio, V.; Panzarini, E.; Dini, L. Autophagy contributes to the death/survival balance in cancer photodynamic therapy. Cells 2012, 1, 464–491. [Google Scholar] [CrossRef] [PubMed]
- Weyergang, A.; Berg, K.; Kaalhus, O.; Peng, Q.; Selbo, P.K. Photodynamic therapy targets the mTOR signaling network in vitro and in vivo. Mol. Pharm. 2008, 6, 255–264. [Google Scholar] [CrossRef] [PubMed]
- Reiners, J.J.; Agostinis, P.; Berg, K.; Oleinick, N.L.; Kessel, D. Assessing autophagy in the context of photodynamic therapy. Autophagy 2010, 6, 7–18. [Google Scholar] [CrossRef] [PubMed]
- Terasaki, M.; Song, J.; Wong, J.R.; Weiss, M.J.; Chen, L.B. Localization of endoplasmic reticulum in living and glutaraldehyde-fixed cells with fluorescent dyes. Cell 1984, 38, 101–108. [Google Scholar] [CrossRef]
- Barr, F.A.; Short, B. Golgins in the structure and dynamics of the Golgi apparatus. Curr. Opin. Cell Biol. 2003, 15, 405–413. [Google Scholar] [CrossRef]
- Rizzuto, R.; Pozzan, T. Microdomains of intracellular Ca2+: Molecular determinants and functional consequences. Physiol. Rev. 2006, 86, 369–408. [Google Scholar] [CrossRef] [PubMed]
- Trump, B.F.; Berezesky, I.K. The role of altered [Ca2+]i regulation in apoptosis, oncosis, and necrosis. Biochim. Biophys. Acta Mol. Cell Res. 1996, 1313, 173–178. [Google Scholar] [CrossRef]
- Zong, W.X.; Thompson, C.B. Necrotic death as a cell fate. Genes Dev. 2006, 20, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Kaufman, R.J.; Malhotra, J.D. Calcium trafficking integrates endoplasmic reticulum function with mitochondrial bioenergetics. Biochim. Biophys. Acta Mol. Cell Res. 2014, 1843, 2233–2239. [Google Scholar] [CrossRef] [PubMed]
- Agostinis, P.; Buytaert, E.; Breyssens, H.; Hendrickx, N. Regulatory pathways in photodynamic therapy induced apoptosis. Photochem. Photobiol. Sci. 2004, 3, 721–729. [Google Scholar] [CrossRef] [PubMed]
- Penning, L.C.; Rasch, M.H.; Ben-Hur, E.; Dubbelman, T.M.A.R.; Havelaar, A.C.; van der Zee, J.; van Steveninck, J. A role for the transient increase of cytoplasmic free calcium in cell rescue after photodynamic treatment. Biochim. Biophys. Acta Biomembr. 1992, 1107, 255–260. [Google Scholar] [CrossRef]
- Buytaert, E.; Callewaert, G.; Hendrickx, N.; Scorrano, L.; Hartmann, D.; Missiaen, L.; Vandenheede, J.R.; Heirman, I.; Grooten, J.; Agostinis, P. Role of endoplasmic reticulum depletion and multidomain proapoptotic BAX and BAK proteins in shaping cell death after hypericin-mediated photodynamic therapy. FASEB J. 2006, 20, 756–758. [Google Scholar] [CrossRef] [PubMed]
- Kessel, D.; Arroyo, A.S. Apoptotic and autophagic responses to Bcl-2 inhibition and photodamage. Photochem. Photobiol. Sci. 2007, 6, 1234–1245. [Google Scholar] [CrossRef] [PubMed]
- Andrzejak, M.; Price, M.; Kessel, D.H. Apoptotic and autophagic responses to photodynamic therapy in 1c1c7 murine hepatoma cells. Autophagy 2011, 7, 979–984. [Google Scholar] [CrossRef] [PubMed]
- Du, L.; Jiang, N.; Wang, G.; Chu, Y.; Lin, W.; Qian, J.; Zhang, Y.; Zheng, J.; Chen, G. Autophagy inhibition sensitizes bladder cancer cells to the photodynamic effects of the novel photosensitizer chlorophyllin e4. J. Photochem. Photobiol. B Biol. 2014, 133, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez, M.E.; Zhang, P.; Azizuddin, K.; Santos, G.B.D.; Chiu, S.; Xue, L.; Berlin, J.C.; Peng, X.; Wu, H.; Lam, M.; et al. Structural factors and mechanisms underlying the improved photodynamic cell killing with silicon phthalocyanine photosensitizers directed to lysosomes versus mitochondria. Photochem. Photobiol. 2009, 85, 1189–1200. [Google Scholar] [CrossRef] [PubMed]
- Saggu, S.; Hung, H.I.; Quiogue, G.; Lemasters, J.J.; Nieminen, A.L. Lysosomal signaling enhances mitochondria-mediated photodynamic therapy in a431 cancer cells: Role of iron. Photochem. Photobiol. 2012, 88, 461–468. [Google Scholar] [CrossRef] [PubMed]
- Kessel, D.; Reiners, J.J. Enhanced efficacy of photodynamic therapy via a sequential targeting protocol. Photochem. Photobiol. 2014, 90, 889–895. [Google Scholar] [CrossRef] [PubMed]
- Guicciardi, M.E.; Leist, M.; Gores, G.J. Lysosomes in cell death. Oncogene 2004, 23, 2881–2890. [Google Scholar] [CrossRef] [PubMed]
- Linder, S.; Shoshan, M.C. Lysosomes and endoplasmic reticulum: Targets for improved, selective anticancer therapy. Drug Resist. Updat. 2005, 8, 199–204. [Google Scholar] [CrossRef] [PubMed]
- Ravanat, J.L.; Mascio, P.D.; Martinez, G.R.; Medeiros, M.H.G.; Cadet, J. Singlet oxygen induces oxidation of cellular DNA. J. Biol. Chem. 2001, 276, 40601–40604. [Google Scholar] [CrossRef] [PubMed]
- Oleinick, N.L.; Evans, H.H. The photobiology of photodynamic therapy: Cellular targets and mechanisms. Radiat. Res. 1998, 150, S146–S156. [Google Scholar] [CrossRef] [PubMed]
- Castano, A.P.; Demidova, T.N.; Hamblin, M.R. Mechanisms in photodynamic therapy: Part two—Cellular signaling, cell metabolism and modes of cell death. Photodiagn. Photodyn. Ther. 2005, 2, 1–23. [Google Scholar] [CrossRef]
- Mohammad, R.M.; Muqbil, I.; Lowe, L.; Yedjou, C.; Hsu, H.-Y.; Lin, L.-T.; Siegelin, M.D.; Fimognari, C.; Kumar, N.B.; Dou, Q.P.; et al. Broad targeting of resistance to apoptosis in cancer. Semin. Cancer Biol. 2015, in press. [Google Scholar] [CrossRef] [PubMed]
- Kessel, D.; Vicente, M.G.H.; Reiners, J.J. Initiation of apoptosis and autophagy by photodynamic therapy. Autophagy 2006, 2, 289–290. [Google Scholar] [CrossRef] [PubMed]
- Moan, J.; Berg, K.; Bommer, J.C.; Western, A. Action spectra of phthalocyanines with respect to photosensitization of cells. Photochem. Photobiol. 1992, 56, 171–175. [Google Scholar] [CrossRef] [PubMed]
- Glidden, M.D.; Celli, J.P.; Massodi, I.; Rizvi, I.; Pogue, B.W.; Hasan, T. Image-based quantification of benzoporphyrin derivative uptake, localization, and photobleaching in 3D tumor models, for optimization of PDT parameters. Theranostics 2012, 2, 827–839. [Google Scholar] [CrossRef] [PubMed]
- Celli, J.P.; Solban, N.; Liang, A.; Pereira, S.P.; Hasan, T. Verteporfin-based photodynamic therapy overcomes gemcitabine insensitivity in a panel of pancreatic cancer cell lines. Lasers Surg. Med. 2011, 43, 565–574. [Google Scholar] [PubMed]
- Deda, D.K.; Pavani, C.; Caritá, E.; Baptista, M.S.; Toma, H.E.; Araki, K. Control of cytolocalization and mechanism of cell death by encapsulation of a photosensitizer. J. Biomed. Nanotechnol. 2013, 9, 1307–1317. [Google Scholar] [CrossRef] [PubMed]
- Pavani, C.; Uchoa, A.F.; Oliveira, C.S.; Iamamoto, Y.; Baptista, M.S. Effect of zinc insertion and hydrophobicity on the membrane interactions and PDT activity of porphyrin photosensitizers. Photochem. Photobiol. Sci. 2009, 8, 233–240. [Google Scholar] [CrossRef] [PubMed]
- Uchoa, A.F.; Oliveira, C.S.; Baptista, M.S. Relationship between structure and photoactivity of porphyrins derived from protoporphyrin IX. J. Porphyr. Phthalocyanines 2010, 14, 832–845. [Google Scholar] [CrossRef]
- Dummin, H.; Cernay, T.; Zimmermann, H.W. Selective photosensitization of mitochondria in HeLa cells by cationic Zn(II)phthalocyanines with lipophilic side-chains. J. Photochem. Photobiol. B Biol. 1997, 37, 219–229. [Google Scholar] [CrossRef]
- Lihuan, D.; Jingcun, Z.; Ning, J.; Guozeng, W.; Yiwei, C.; Wei, L.; Jing, Q.; Yuanfang, Z.; Gang, C. Photodynamic therapy with the novel photosensitizer chlorophyllin f induces apoptosis and autophagy in human bladder cancer cells. Lasers Surg. Med. 2014, 46, 319–324. [Google Scholar] [CrossRef] [PubMed]
- Buytaert, E.; Callewaert, G.; Vandenheede, J.R.; Agostinis, P. Deficiency in apoptotic effectors Bax and Bak reveals an autophagic cell death pathway initiated by photodamage to the endoplasmic reticulum. Autophagy 2006, 2, 238–240. [Google Scholar] [CrossRef] [PubMed]
- Buytaert, E.; Dewaele, M.; Agostinis, P. Molecular effectors of multiple cell death pathways initiated by photodynamic therapy. Biochim. Biophys. Acta Rev. Cancer 2007, 1776, 86–107. [Google Scholar] [CrossRef] [PubMed]
- Lim, E.J.; Oak, C.-H.; Heo, J.; Kim, Y.-H. Methylene blue-mediated photodynamic therapy enhances apoptosis in lung cancer cells. Oncol. Rep. 2013, 30, 856–862. [Google Scholar] [PubMed]
- Chen, J.Y.; Mak, N.K.; Yow, C.M.; Fung, M.C.; Chiu, L.C.; Leung, W.N.; Cheung, N.H. The binding characteristics and intracellular localization of temoporfin (mTHPC) in myeloid leukemia cells: Phototoxicity and mitochondrial damage. Photochem. Photobiol. 2000, 72, 541–547. [Google Scholar] [CrossRef]
- Teiten, M.H.; Bezdetnaya, L.; Morlière, P.; Santus, R.; Guillemin, F. Endoplasmic reticulum and Golgi apparatus are the preferential sites of Foscan localisation in cultured tumour cells. Br. J. Cancer 2003, 88, 146–152. [Google Scholar] [CrossRef] [PubMed]
- Marchal, S.; François, A.; Dumas, D.; Guillemin, F.; Bezdetnaya, L. Relationship between subcellular localization of Foscan and caspase activation in photosensitised MCF-7 cells. Br. J. Cancer 2007, 96, 944–951. [Google Scholar] [CrossRef] [PubMed]
- Reiners, J.J.; Caruso, J.A.; Mathieu, P.; Chelladurai, B.; Yin, X.-M.; Kessel, D. Release of cytochrome c and activation of pro-caspase-9 following lysosomal photodamage involves Bid cleavage. Cell Death Differ. 2002, 9, 934–944. [Google Scholar] [CrossRef] [PubMed]
- Yeh, S.-C.A.; Diamond, K.R.; Patterson, M.S.; Nie, Z.; Hayward, J.E.; Fang, Q. Monitoring photosensitizer uptake using two photon fluorescence life-time imaging microscopy. Theranostics 2012, 2, 817–826. [Google Scholar] [CrossRef] [PubMed]
- Wilson, B.C.; Olivo, M.; Singh, G. Subcellular localization of Photofrin and aminolevulinic acid and photodynamic cross-resistance in vitro in radiation-induced fibrosarcoma cells sensitive or resistant to photofrin-mediated photodynamic therapy. Photochem. Photobiol. 1997, 65, 166–176. [Google Scholar] [CrossRef] [PubMed]
- Hsieh, Y.J.; Wu, C.C.; Chang, C.J.; Yu, J.S. Subcellular localization of photofrin determines the death phenotype of human epidermoid carcinoma A431 cells triggered by photodynamic therapy: When plasma membranes are the main targets. J. Cell. Physiol. 2003, 194, 363–375. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.; Xing, D. Mechanism of mitochondrial membrane permeabilization during apoptosis under photofrin-mediated photodynamic therapy. J. X-ray Sci. Technol. 2012, 20, 363–372. [Google Scholar]
- Soldani, C.; Bottone, M.G.; Croce, A.C.; Fraschini, A.; Bottiroli, G.; Pellicciari, C. The Golgi apparatus is a primary site of intracellular damage after photosensitization with Rose Bengal acetate. Eur. J. Histochem. 2004, 48, 443–448. [Google Scholar] [CrossRef] [PubMed]
- Dini, L.; Inguscio, V.; Tenuzzo, B.; Panzarini, E. Rose bengal acetate photodynamic therapy-induced autophagy. Cancer Biol. Ther. 2010, 10, 1048–1056. [Google Scholar] [CrossRef] [PubMed]
- Panzarini, E.; Inguscio, V.; Dini, L. Overview of cell death mechanisms induced by rose bengal acetate-photodynamic therapy. Int. J. Photoenergy 2011, 2011. [Google Scholar] [CrossRef]
- Panzarini, E.; Inguscio, V.; Fimia, G.M.; Dini, L. Rose bengal acetate photodynamic therapy (RBAc-PDT) induces exposure and release of damage-associated molecular patterns (DAMPs) in human HeLa cells. PLoS ONE 2014, 9, e105778. [Google Scholar] [CrossRef] [PubMed]
- Singh, G.; Jeeves, W.P.; Wilson, B.C.; Jang, D. Mitochondrial photosensitization by Photofrin II. Photochem. Photobiol. 1987, 46, 645–649. [Google Scholar] [CrossRef] [PubMed]
- Xue, L.Y.; Chiu, S.M.; Oleinick, N.L. Photochemical destruction of the Bcl-2 oncoprotein during photodynamic therapy with the phthalocyanine photosensitizer Pc 4. Oncogene 2001, 20, 3420–3427. [Google Scholar] [CrossRef] [PubMed]
- Usuda, J.; Chiu, S.M.; Murphy, E.S.; Lam, M.; Nieminen, A.L.; Oleinick, N.L. Domain-dependent photodamage to Bcl-2: A membrane anchorage region is needed to form the target of phthalocyanine photosensitization. J. Biol. Chem. 2003, 278, 2021–2029. [Google Scholar] [CrossRef] [PubMed]
- Berg, K.; Western, A.; Bommer, J.C.; Moan, J. Intracellular localization of sulfonated meso-tetraphenylporphines in a human carcinoma cell line. Photochem. Photobiol. 1990, 52, 481–487. [Google Scholar] [CrossRef] [PubMed]
- Strømhaug, P.E.; Berg, T.O.; Berg, K.; Seglen, P.O. A novel method for the study of autophagy: Destruction of hepatocytic lysosomes, but not autophagosomes, by the photosensitizing porphyrin tetra(4-sulphonatophenyl)porphine. Biochem. J. 1997, 321 Pt 1, 217–225. [Google Scholar] [CrossRef] [PubMed]
- Berg, K.; Moan, J. Lysosomes as photochemical targets. Int. J. Cancer 1994, 59, 814–822. [Google Scholar] [CrossRef] [PubMed]
- Kochevar, I.E.; Lynch, M.C.; Zhuang, S.; Lambert, C.R. Singlet oxygen, but not oxidizing radicals, induces apoptosis in HL-60 cells. Photochem. Photobiol. 2000, 72, 548–553. [Google Scholar] [CrossRef]
- Ding, H.; Yu, H.; Dong, Y.; Tian, R.; Huang, G.; Boothman, D.A.; Sumer, B.D.; Gao, J. Photoactivation switch from Type II to Type I reactions by electron-rich micelles for improved photodynamic therapy of cancer cells under hypoxia. J. Control. Release 2011, 156, 276–280. [Google Scholar] [CrossRef] [PubMed]
- Bacellar, I.O.L.; Pavani, C.; Sales, E.M.; Itri, R.; Wainwright, M.; Baptista, M.S. Membrane damage efficiency of phenothiazinium photosensitizers. Photochem. Photobiol. 2014, 90, 801–813. [Google Scholar] [CrossRef] [PubMed]
- Vakrat-Haglili, Y.; Weiner, L.; Brumfeld, V.; Brandis, A.; Salomon, Y.; McIlroy, B.; Wilson, B.C.; Pawlak, A.; Rozanowska, M.; Sarna, T.; et al. The microenvironment effect on the generation of reactive oxygen species by Pd-bacteriopheophorbide. J. Am. Chem. Soc. 2005, 127, 6487–6497. [Google Scholar] [CrossRef] [PubMed]
- Silva, E.F.F.; Serpa, C.; Da̧browski, J.M.; Monteiro, C.J.P.; Formosinho, S.J.; Stochel, G.; Urbanska, K.; Simões, S.; Pereira, M.M.; Arnaut, L.G. Mechanisms of singlet-oxygen and superoxide-ion generation by porphyrins and bacteriochlorins and their implications in photodynamic therapy. Chemistry 2010, 16, 9273–9286. [Google Scholar] [CrossRef] [PubMed]
- Engelmann, F.M.; Mayer, I.; Gabrielli, D.S.; Toma, H.E.; Kowaltowski, A.J.; Araki, K.; Baptista, M.S. Interaction of cationic meso-porphyrins with liposomes, mitochondria and erythrocytes. J. Bioenerg. Biomembr. 2007, 39, 175–185. [Google Scholar] [CrossRef] [PubMed]
- Rokitskaya, T.I.; Block, M.; Antonenko, Y.N.; Kotova, E.A.; Pohl, P. Photosensitizer binding to lipid bilayers as a precondition for the photoinactivation of membrane channels. Biophys. J. 2000, 78, 2572–2580. [Google Scholar] [CrossRef] [PubMed]
- Ali-Seyed, M.; Bhuvaneswari, R.; Soo, K.C.; Olivo, M. Photolon™-photosensitization induces apoptosis via ROS-mediated cross-talk between mitochondria and lysosomes. Int. J. Oncol. 2011, 39, 821–831. [Google Scholar] [PubMed]
- Sun, X.; Leung, W.N. Photodynamic therapy with pyropheophorbide-a methyl ester in human lung carcinoma cancer cell: Efficacy, localization and apoptosis. Photochem. Photobiol. 2007, 75, 644–651. [Google Scholar] [CrossRef]
- MacDonald, I.J.; Morgan, J.; Bellnier, D.A.; Paszkiewicz, G.M.; Whitaker, J.E.; Litchfield, D.J.; Dougherty, T.J. Subcellular localization patterns and their relationship to photodynamic activity of pyropheophorbide-a derivatives. Photochem. Photobiol. 1999, 70, 789–797. [Google Scholar] [CrossRef] [PubMed]
- Kessel, D.; Luo, Y.; Deng, Y.; Chang, C.K. The role of subcellular localization in initiation of apoptosis by photodynamic therapy. Photochem. Photobiol. 1997, 65, 422–426. [Google Scholar] [CrossRef] [PubMed]
- Kuimova, M.K.; Yahioglu, G.; Ogilby, P.R. Singlet oxygen in a cell: Spatially dependent lifetimes and quenching rate constants. J. Am. Chem. Soc. 2009, 131, 332–340. [Google Scholar] [CrossRef] [PubMed]
- DeRosa, M.C.; Crutchley, R.J. Photosensitized singlet oxygen and its applications. Coord. Chem. Rev. 2002, 233–234, 351–371. [Google Scholar] [CrossRef]
- Junqueira, H.C.; Severino, D.; Dias, L.G.; Gugliotti, M.S.; Baptista, M.S. Modulation of methylene blue photochemical properties based on adsorption at aqueous micelle interfaces. Phys. Chem. Chem. Phys. 2002, 4, 2320–2328. [Google Scholar] [CrossRef]
- Severino, D.; Junqueira, H.C.; Gugliotti, M.; Gabrielli, D.S.; Baptista, M.S. Influence of negatively charged interfaces on the ground and excited state properties of methylene blue. Photochem. Photobiol. 2003, 77, 459–468. [Google Scholar] [CrossRef]
- Maisch, T.; Baier, J.; Franz, B.; Maier, M.; Landthaler, M.; Szeimies, R.-M.; Bäumler, W. The role of singlet oxygen and oxygen concentration in photodynamic inactivation of bacteria. Proc. Natl. Acad. Sci. USA. 2007, 104, 7223–7228. [Google Scholar] [CrossRef] [PubMed]
- Foster, T.H.; Murant, R.S.; Bryant, R.G. Oxygen consumption and diffusion effects in photodynamic therapy. Radiat. Res. 1991, 126, 296–303. [Google Scholar] [CrossRef] [PubMed]
- Fernandez, D.A.; Awruch, J.; Dicelio, L.E. Photophysical and aggregation studies of t-butyl-substituted Zn phthalocyanines. Photochem. Photobiol. 1996, 63, 784–792. [Google Scholar] [CrossRef]
- Montes de Oca, M.N.; Vara, J.; Milla, L.; Rivarola, V.; Ortiz, C.S. Physicochemical properties and photodynamic activity of novel derivatives of triarylmethane and thiazine. Arch. Pharm. 2013, 346, 255–265. [Google Scholar] [CrossRef] [PubMed]
- Nuñez, S.C.; Yoshimura, T.M.; Ribeiro, M.S.; Junqueira, H.C.; Maciel, C.; Coutinho-Neto, M.D.; Baptista, M.S. Urea enhances the photodynamic efficiency of methylene blue. J. Photochem. Photobiol. B Biol. 2015, in press. [Google Scholar] [CrossRef] [PubMed]
- Tsubone, T.M.; Braga, G.; Vilsinski, B.H.; Gerola, A.P.; Hioka, N.; Caetano, W. Aggregation of aluminum phthalocyanine hydroxide in water/ethanol mixtures. J. Braz. Chem. Soc. 2014, 25, 890–897. [Google Scholar] [CrossRef]
- Aveline, B.M.; Hasan, T.; Redmond, R.W. The effects of aggregation, protein binding and cellular incorporation on the photophysical properties of benzoporphyrin derivative monoacid ring A (BPDMA). J. Photochem. Photobiol. B Biol. 1995, 30, 161–169. [Google Scholar] [CrossRef]
- Choi, M.T.M.; Li, P.P.S.; Ng, D.K.P. A direct comparison of the aggregation behavior of phthalocyanines and 2,3-naphthalocyanines. Tetrahedron 2000, 56, 3881–3887. [Google Scholar] [CrossRef]
- Gabrielli, D.; Belisle, E.; Severino, D.; Kowaltowski, A.J.; Baptista, M.S. Binding, aggregation and photochemical properties of methylene blue in mitochondrial suspensions. Photochem. Photobiol. 2004, 79, 227–232. [Google Scholar] [CrossRef] [PubMed]
- Turbay, B.M.E.; Rey, V.; Argañaraz, N.M.; Morán, F.E.; Aspée, A.; Lissi, E.A.; Borsarelli, C.D. Biology Effect of dye localization and self-interactions on the photosensitized generation of singlet oxygen by rose bengal bound to bovine serum albumin. J. Photochem. Photobiol. B Biol. 2014, 141, 275–282. [Google Scholar] [CrossRef] [PubMed]
- Baptista, M.S.; Indig, G.L. Effect of BSA binding on photophysical and photochemical properties of triarylmethane dyes. J. Phys. Chem. B 1998, 102, 4678–4688. [Google Scholar] [CrossRef]
- Núñez, S.C.; Garcez, A.S.; Kato, I.T.; Yoshimura, T.M.; Gomes, L.; Baptista, M.S.; Ribeiro, M.S. Effects of ionic strength on the antimicrobial photodynamic efficiency of methylene blue. Photochem. Photobiol. Sci. 2014, 13, 595–602. [Google Scholar] [CrossRef] [PubMed]
- Vilsinski, B.H.; Gerola, A.P.; Enumo, J.A.; Campanholi, K.D.S.S.; Pereira, P.C.D.S.; Braga, G.; Hioka, N.; Kimura, E.; Tessaro, A.L.; Caetano, W. Formulation of aluminum chloride phthalocyanine in pluronic™ P-123 and F-127 block copolymer micelles: Photophysical properties and photodynamic inactivation of microorganisms. Photochem. Photobiol. 2015, 91, 518–525. [Google Scholar] [CrossRef] [PubMed]
- Gerola, A.P.; Tsubone, T.M.; Santana, A.; de Oliveira, H.P.M.; Hioka, N.; Caetano, W. Properties of chlorophyll and derivatives in homogeneous and microheterogeneous systems. J. Phys. Chem. B 2011, 115, 7364–7373. [Google Scholar] [CrossRef] [PubMed]
- Tada, D.B.; Baptista, M.S. Photosensitizing nanoparticles and the modulation of ROS generation. Front. Chem. 2015, 3, 33. [Google Scholar] [CrossRef] [PubMed]
- Rossi, L.M.; Silva, P.R.; Vono, L.L. R.; Fernandes, A.U.; Tada, D.B.; Baptista, M.S. Protoporphyrin IX nanoparticle carrier: Preparation, optical properties and singlet oxygen generation. Langmuir 2008, 24, 12534–12538. [Google Scholar] [CrossRef] [PubMed]
- Tada, D.B.; Vono, L.L.R.; Duarte, E.L.; Itri, R.; Kiyohara, P.K.; Baptista, M.S.; Rossi, L.M. Methylene blue-containing silica-coated magnetic particles: A potential magnetic carrier for pHotodynamic therapy. Langmuir 2007, 23, 8194–8199. [Google Scholar] [CrossRef] [PubMed]
- Tada, D.B.; Rossi, L.M.; Leite, C.A.P.; Itri, R.; Baptista, M.S. Nanoparticle platform to modulate reaction mechanism of phenothiazine photosensitizers. J. Nanosci. Nanotechnol. 2010, 10, 3100–3108. [Google Scholar] [CrossRef] [PubMed]
- Yoon, H.K.; Lou, X.; Chen, Y.C.; Koo Lee, Y.E.; Yoon, E.; Kopelman, R. Nanophotosensitizers engineered to generate a tunable mix of reactive oxygen species, for optimizing photodynamic therapy, using a microfluidic device. Chem. Mater. 2014, 26, 1592–1600. [Google Scholar] [CrossRef] [PubMed]
- Taquet, J.-P.; Frochot, C.; Manneville, V.; Barberi-Heyob, M. Phthalocyanines covalently bound to biomolecules for a targeted photodynamic therapy. Curr. Med. Chem. 2007, 14, 1673–1687. [Google Scholar] [CrossRef] [PubMed]
- Uchoa, A.F.; de Oliveira, K.T.; Baptista, M.S.; Bortoluzzi, A.J.; Iamamoto, Y.; Serra, O.A. Chlorin photosensitizers sterically designed to prevent self-aggregation. J. Org. Chem. 2011, 76, 8824–8832. [Google Scholar] [CrossRef] [PubMed]
- Mikata, Y.; Sawaguchi, T.; Kakuchi, T.; Gottschaldt, M.; Schubert, U.S.; Ohi, H.; Yano, S. Control of the aggregation properties of tris(maltohexaose)-linked porphyrins with an alkyl chain. Eur. J. Org. Chem. 2010, 1, 663–671. [Google Scholar] [CrossRef]
- Dos Santos, F.A.B.; Uchoa, A.F.; Baptista, M.S.; Iamamoto, Y.; Serra, O.A.; Brocksom, T.J.; de Oliveira, K.T. Synthesis of functionalized chlorins sterically-prevented from self-aggregation. Dyes Pigment. 2013, 99, 402–411. [Google Scholar] [CrossRef]
- Russell, J.; Diamond, K. Characterization of fluorescence lifetime of Photofrin and delta-aminolevulinic acid induced protoporphyrin IX in living cells using single-and two-photon excitation. IEEE J. Sel. Top. Quantum Electron. 2008, 14, 158–166. [Google Scholar] [CrossRef]
- Connelly, J.P.; Botchway, S.W.; Kunz, L.; Pattison, D.; Parker, A.W.; MacRobert, A.J. Time-resolved fluorescence imaging of photosensitiser distributions in mammalian cells using a picosecond laser line-scanning microscope. J. Photochem. Photobiol. A Chem. 2001, 142, 169–175. [Google Scholar] [CrossRef]
- Kress, M.; Meier, T.; Steiner, R.; Dolp, F.; Erdmann, R.; Ortmann, U.; Rück, A. Time-resolved microspectrofluorometry and fluorescence lifetime imaging of photosensitizers using picosecond pulsed diode lasers in laser scanning microscopes. J. Biomed. Opt. 2003, 8, 26–32. [Google Scholar] [CrossRef] [PubMed]
- Lassalle, H.P.; Wagner, M.; Bezdetnaya, L.; Guillemin, F.; Schneckenburger, H. Fluorescence imaging of Foscan® and Foslip in the plasma membrane and in whole cells. J. Photochem. Photobiol. B Biol. 2008, 92, 47–53. [Google Scholar] [CrossRef] [PubMed]
- Maas, A.L.; Carter, S.L.; Wiley, E.P.; Miller, J.; Yuan, M.; Yu, G.; Durham, A.C.; Busch, T.M. Tumor vascular microenvironment determines responsiveness to photodynamic therapy. Cancer Res. 2012, 72, 2079–2088. [Google Scholar] [CrossRef] [PubMed]
- Hovhannisyan, V.; Guo, H.W.; Hovhannisyan, A.; Ghukasyan, V.; Buryakina, T.; Chen, Y.F.; Dong, C.Y. Photo-induced processes in collagen-hypericin system revealed by fluorescence spectroscopy and multiphoton microscopy. Biomed. Opt. Express 2014, 5, 1355–1362. [Google Scholar] [CrossRef] [PubMed]
- Sharman, W.M.; van Lier, J.E.; Allen, C.M. Targeted photodynamic therapy via receptor mediated delivery systems. Adv. Drug Deliv. Rev. 2004, 56, 53–76. [Google Scholar] [CrossRef] [PubMed]
- Jori, G.; Reddi, E. The role of lipoproteins in the delivery of tumour-targeting photosensitizers. Int. J. Biochem. 1993, 25, 1369–1375. [Google Scholar] [CrossRef]
- Zhou, C.; Milanesi, C.; Jori, G. An ultrastructural comparative evaluation of tumors photosensitized by porphyrins administered in aqueous solution, bound to liposomes or to lipoproteins. Photochem. Photobiol. 1988, 48, 487–492. [Google Scholar] [CrossRef] [PubMed]
- Josefsen, L.B.; Boyle, R.W. Unique diagnostic and therapeutic roles of porphyrins and phthalocyanines in photodynamic therapy, imaging and theranostics. Theranostics 2012, 2, 916–966. [Google Scholar] [CrossRef] [PubMed]
- Ochsner, M. Photophysical and photobiological processes in the photodynamic therapy of tumours. J. Photochem. Photobiol. B Biol. 1997, 39, 1–18. [Google Scholar] [CrossRef]
- Chen, X.; Hui, L.; Foster, D.A.; Drain, C.M. Efficient synthesis and photodynamic activity of porphyrin-saccharide conjugates: Targeting and incapacitating cancer cells. Biochemistry 2004, 43, 10918–10929. [Google Scholar] [CrossRef] [PubMed]
- Hirohara, S.; Nishida, M.; Sharyo, K.; Obata, M.; Ando, T.; Tanihara, M. Synthesis, photophysical properties and photocytotoxicity of mono-, di-, tri- and tetra-glucosylated fluorophenylporphyrins. Bioorg. Med. Chem. 2010, 18, 1526–1535. [Google Scholar] [CrossRef] [PubMed]
- Moylan, C.; Scanlan, E.M.; Senge, M.O. Chemical synthesis and medicinal applications of glycoporphyrins. Curr. Med. Chem. 2015, 22, 2238–2348. [Google Scholar] [CrossRef] [PubMed]
- Pereira, P.M.R.; Carvalho, J.J.; Silva, S.; Cavaleiro, J.A.S.; Schneider, R.J.; Fernandes, R.; Tomé, J.P.C. Porphyrin conjugated with serum albumins and monoclonal antibodies boosts efficiency in targeted destruction of human bladder cancer cells. Org. Biomol. Chem. 2014, 12, 1804–1811. [Google Scholar] [CrossRef] [PubMed]
- Xu, D.; Chen, X.; Chen, K.; Peng, Y.; Li, Y.; Ke, Y.; Gan, D. Tetra-sulfonate phthalocyanine zinc-bovine serum albumin conjugate-mediated photodynamic therapy of human glioma. J. Biomater. Appl. 2014, 29, 378–385. [Google Scholar] [CrossRef] [PubMed]
- Dozzo, P.; Koo, M.S.; Berger, S.; Forte, T.M.; Kahl, S.B. Synthesis, characterization, and plasma lipoprotein association of a nucleus-targeted boronated porphyrin. J. Med. Chem. 2005, 48, 357–359. [Google Scholar] [CrossRef] [PubMed]
- Marotta, D.E.; Cao, W.; Wileyto, E.P.; Li, H.; Corbin, I.; Rickter, E.; Glickson, J.D.; Chance, B.; Zheng, G.; Busch, T.M. Evaluation of bacteriochlorophyll-reconstituted low-density lipoprotein nanoparticles for photodynamic therapy efficacy in vivo. Nanomedicine 2011, 6, 475–487. [Google Scholar] [CrossRef] [PubMed]
- Gijsens, A.; Missiaen, L.; Merlevede, W.; de Witte, P. Epidermal growth factor-mediated targeting of chlorin e6 selectively potentiates its photodynamic activity. Cancer Res. 2000, 60, 2197–2202. [Google Scholar] [PubMed]
- Marchal, S.; Dolivet, G.; Lassalle, H.-P.; Guillemin, F.; Bezdetnaya, L. Targeted photodynamic therapy in head and neck squamous cell carcinoma: Heading into the future. Lasers Med. Sci. 2015. [Google Scholar] [CrossRef] [PubMed]
- Serebrovskaya, E.O.; Ryumina, A.P.; Boulina, M.E.; Shirmanova, M.V.; Zagaynova, E.V.; Bogdanova, E.A.; Lukyanov, S.A.; Lukyanov, K.A. Phototoxic effects of lysosome-associated genetically encoded photosensitizer KillerRed. J. Biomed. Opt. 2014, 19, 071403. [Google Scholar] [CrossRef] [PubMed]
- Obaid, G.; Chambrier, I.; Cook, M.J.; Russell, D.A. Cancer targeting with biomolecules: A comparative study of photodynamic therapy. Photochem. Photobiol. Sci. 2015, 14, 737–747. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gravier, J.; Schneider, R.; Frochot, C.; Bastogne, T.; Schmitt, F.; Didelon, J.; Guillemin, F.; Barberi-Heyob, M. Improvement of meta-tetra(hydroxyphenyl)chlorin-like photosensitizer selectivity with folate-based targeted delivery. Synthesis and in vivo delivery studies. J. Med. Chem. 2008, 51, 3867–3877. [Google Scholar] [CrossRef] [PubMed]
- Li, P.-X.; Mu, J.-H.; Xiao, H.-L.; Li, D.-H. Antitumor effect of photodynamic therapy with a novel targeted photosensitizer on cervical carcinoma. Oncol. Rep. 2015, 33, 125–132. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Zeng, F.; Wu, H.; Hu, C.; Wu, S. Enhanced photodynamic efficiency achieved via a dual-targeted strategy based on photosensitizer/micelle structure. Biomacromolecules 2014, 15, 4249–4259. [Google Scholar] [CrossRef] [PubMed]
- Li, X.-S.; Ke, M.-R.; Huang, W.; Ye, C.-H.; Huang, J.-D. A pH-responsive layered double hydroxide (LDH)-phthalocyanine nanohybrid for efficient photodynamic therapy. Chemistry 2015, 21, 3310–3317. [Google Scholar] [CrossRef] [PubMed]
- Shi, J.; Liu, Y.; Wang, L.; Gao, J.; Zhang, J.; Yu, X.; Ma, R.; Liu, R.; Zhang, Z. A tumoral acidic pH-responsive drug delivery system based on a novel photosensitizer (fullerene) for in vitro and in vivo chemo-photodynamic therapy. Acta Biomater. 2014, 10, 1280–1291. [Google Scholar] [CrossRef] [PubMed]
- Jeong, S.; Park, W.; Lee, C.-S.; Na, K. A cancer-recognizing polymeric photosensitizer based on the tumor extracellular pH response of conjugated polymers for targeted cancer photodynamic therapy. Macromol. Biosci. 2014, 14, 1688–1695. [Google Scholar] [CrossRef] [PubMed]
- Kolemen, S.; Işık, M.; Kim, G.M.; Kim, D.; Geng, H.; Buyuktemiz, M.; Karatas, T.; Zhang, X.-F.; Dede, Y.; Yoon, J.; et al. Intracellular modulation of excited-state dynamics in a chromophore dyad: Differential enhancement of photocytotoxicity targeting cancer cells. Angew. Chem. 2015, 127, 5430–5434. [Google Scholar] [CrossRef]
- Xu, J.; Zeng, F.; Wu, H.; Yu, C.; Wu, S. Dual-targeting nanosystem for enhancing photodynamic therapy efficiency. ACS Appl. Mater. Interfaces 2015, 7, 9287–9296. [Google Scholar] [CrossRef] [PubMed]
- Albani, B.A.; Pena, B.; Leed, N.A.; de Paula, N.A.B.G.; Pavani, C.; Baptista, M.S.; Dunbar, K.R.; Turro, C. Marked improvement in photoinduced cell death by a new tris-heteroleptic complex with dual action: Singlet oxygen sensitization and ligand dissociation. J. Am. Chem. Soc. 2014, 136, 17095–17101. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Chen, Z.; Zheng, W.; Zhu, H.; Lu, S.; Ma, E.; Tu, D.; Zhou, S.; Huang, M.; Chen, X. Lanthanide-doped upconversion nanoparticles electrostatically coupled with photosensitizers for near-infrared-triggered photodynamic therapy. Nanoscale 2014, 6, 8274–8282. [Google Scholar] [CrossRef] [PubMed]
- Dou, Q.; Teng, C.; Ye, E.; Loh, X. Effective near-infrared photodynamic therapy assisted by upconversion nanoparticles conjugated with photosensitizers. Int. J. Nanomed. 2015, 10, 419–432. [Google Scholar]
- Wang, X.; Liu, K.; Yang, G.; Cheng, L.; He, L.; Liu, Y.; Li, Y.; Guo, L.; Liu, Z. Near-infrared light triggered photodynamic therapy in combination with gene therapy using upconversion nanoparticles for effective cancer cell killing. Nanoscale 2014, 6, 9198–9205. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Tian, J.; He, W.; Guo, Z. H2O2-activatable and O2-evolving nanoparticles for highly efficient and selective photodynamic therapy against hypoxic tumor cells. J. Am. Chem. Soc. 2015, 137, 1539–1547. [Google Scholar] [CrossRef] [PubMed]
- Huang, L.; St. Denis, T.G.; Xuan, Y.; Huang, Y.Y.; Tanaka, M.; Zadlo, A.; Sarna, T.; Hamblin, M.R. Paradoxical potentiation of methylene blue-mediated antimicrobial photodynamic inactivation by sodium azide: Role of ambient oxygen and azide radicals. Free Radic. Biol. Med. 2012, 53, 2062–2071. [Google Scholar] [CrossRef] [PubMed]
- Yin, R.; Wang, M.; Huang, Y.; Landi, G.; Vecchio, D.; Chiang, L.Y.; Hamblin, M.R. Antimicrobial photodynamic inactivation with decacationic functionalized fullerenes: Oxygen-independent photokilling in presence of azide and new mechanistic insights. Free Radic. Biol. Med. 2015, 79, 14–27. [Google Scholar] [CrossRef] [PubMed]
- Kasimova, K.R.; Sadasivam, M.; Landi, G.; Sarna, T.; Hamblin, M.R. Potentiation of photoinactivation of Gram-positive and Gram-negative bacteria mediated by six phenothiazinium dyes by addition of azide ion. Photochem. Photobiol. Sci. 2014, 13, 1541–1548. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Dai, T.; Wang, M.; Vecchio, D.; Chiang, L.Y.; Hamblin, M.R. Potentiation of antimicrobial photodynamic inactivation mediated by a cationic fullerene by added iodide: In vitro and in vivo studies. Nanomedicine 2015, 10, 603–614. [Google Scholar] [CrossRef] [PubMed]
- Vecchio, D.; Gupta, A.; Huang, L.; Landi, G.; Avci, P.; Rodas, A.; Hamblin, M.R. Bacterial photodynamic inactivation mediated by methylene blue and red light is enhanced by synergistic effect of potassium iodide. Antimicrob. Agents Chemother. 2015, 59, 5203–5212. [Google Scholar] [CrossRef] [PubMed]
- Ezzeddine, R.; Al-Banaw, A.; Tovmasyan, A.; Craik, J.D.; Batinic-Haberle, I.; Benov, L.T. Effect of molecular characteristics on cellular uptake, subcellular localization, and phototoxicity of Zn(II) N-alkylpyridylporphyrins. J. Biol. Chem. 2013, 288, 36579–36788. [Google Scholar] [CrossRef] [PubMed]
- Bonneau, S.; Vever-Bizet, C. Tetrapyrrole photosensitisers, determinants of subcellular localisation and mechanisms of photodynamic processes in therapeutic approaches. Expert Opin. Ther. Pat. 2008, 18, 1011–1025. [Google Scholar] [CrossRef]
- Boyle, R.W.; Dolphin, D. Structure and biodistribution relationships of photodynamic sensitizers. Photochem. Photobiol. 1996, 64, 469–485. [Google Scholar] [CrossRef] [PubMed]
- Castano, A.P.; Demidova, T.N.; Hamblin, M.R. Mechanisms in photodynamic therapy: Part One—Photosensitizers, photochemistry and cellular localization. Photodiagn. Photodyn. Ther. 2004, 1, 279–293. [Google Scholar] [CrossRef]
- Doherty, G.J.; McMahon, H.T. Mechanisms of endocytosis. Annu. Rev. Biochem. 2009, 78, 857–902. [Google Scholar] [CrossRef] [PubMed]
- Soriano, J.; Villanueva, A.; Stockert, J.C.; Cañete, M. Vehiculization determines the endocytic internalization mechanism of Zn(II)-phthalocyanine. Histochem. Cell Biol. 2013, 139, 149–160. [Google Scholar] [CrossRef] [PubMed]
- Feofanov, A.; Sharonov, G.; I, A.G.; Karmakova, T.; Pljutinskaya, A.; Lebedeva, V.; Ruziyev, R.; Yakub, R.; Mironov, A.; Refregier, M.; Maurizot, J.; Vigny, P. Comparative study of photodynamic properties of 13,15-N-cycloimide derivatives of chlorin p6. Photochem. Photobiol. 2004, 79, 172–188. [Google Scholar] [CrossRef]
- Jensen, T.J.; Vicente, M.G.H.; Luguya, R.; Norton, J.; Fronczek, F.R.; Smith, K.M. Effect of overall charge and charge distribution on cellular uptake, distribution and phototoxicity of cationic porphyrins in HEp2 cells. J. Photochem. Photobiol. B Biol. 2010, 100, 100–111. [Google Scholar] [CrossRef] [PubMed]
- Oseroff, A.R.; Ohuoha, D.; Ara, G.; McAuliffe, D.; Foley, J.; Cincotta, L. Intramitochondrial dyes allow selective in vitro photolysis of carcinoma cells. Proc. Natl. Acad. Sci. USA 1986, 83, 9729–9733. [Google Scholar] [CrossRef] [PubMed]
- Beckman, W.C.; Powers, S.K.; Brown, J.T.; Gillespie, G.Y.; Bigner, D.D.; Camps, J.L. Differential retention of rhodamine 123 by avian sarcoma virus-induced glioma and normal brain tissue of the rat in vivo. Cancer 1987, 59, 266–270. [Google Scholar] [CrossRef]
- Kandela, R.K.; Bartlett, J.A.; Indig, G.L. Effect of molecular structure on the selective phototoxicity of triarylmethane dyes towards tumor cells. Photochem. Photobiol. Sci. 2002, 1, 309–314. [Google Scholar] [CrossRef] [PubMed]
- Andrzejak, M.; Santiago, M.; Kessel, D. Effects of endosomal photodamage on membrane recycling and endocytosis. Photochem. Photobiol. 2011, 87, 699–706. [Google Scholar] [CrossRef] [PubMed]
- Roberts, W.G.; Berns, M.W. In vitro photosensitization I. Cellular uptake and subcellular localization of mono-l-aspartyl chlorin e6, chloro-aluminum sulfonated phthalocyanine, and photofrin II. Lasers Surg. Med. 1989, 9, 90–101. [Google Scholar] [CrossRef] [PubMed]
- Woodburn, K.W.; Vardaxis, N.J.; Hill, J.S.; Kaye, A.H.; Phillips, D.R. Subcellular localization of porphyrins using confocal laser scanning microscopy. Photochem. Photobiol. 1991, 54, 725–732. [Google Scholar] [CrossRef] [PubMed]
- Xue, L.; Chiu, S.; Fiebig, A.; Andrews, D.W.; Oleinick, N.L. Photodamage to multiple Bcl-xL isoforms by photodynamic therapy with the phthalocyanine photosensitizer Pc 4. Oncogene 2003, 22, 9197–9204. [Google Scholar] [CrossRef] [PubMed]
- Boya, P.; Kroemer, G. Lysosomal membrane permeabilization in cell death. Oncogene 2008, 27, 6434–6451. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zong, D.; Zielinska-Chomej, K.; Juntti, T.; Mörk, B.; Lewensohn, R.; Hååg, P.; Viktorsson, K. Harnessing the lysosome-dependent antitumor activity of phenothiazines in human small cell lung cancer. Cell Death Dis. 2014, 5, e1111. [Google Scholar] [CrossRef] [PubMed]
- Raben, N.; Shea, L.; Hill, V.; Plotz, P. Monitoring autophagy in lysosomal storage disorders. Methods Enzymol. 2009, 453, 417–449. [Google Scholar] [PubMed]
- Kessel, D.; Luguya, R.; Vicente, M.G.H. Localization and photodynamic efficacy of two cationic porphyrins varying in charge distributions. Photochem. Photobiol. 2003, 78, 431–435. [Google Scholar] [CrossRef]
- Kessel, D. Relocalization of cationic porphyrins during photodynamic therapy. Photochem. Photobiol. Sci. 2002, 1, 837–840. [Google Scholar] [CrossRef] [PubMed]
- Berg, K.; Selbo, P.K.; Weyergang, A.; Dietze, A.; Prasmickaite, L.; Bonsted, A.; Engesaeter, B.; Angell-Petersen, E.; Warloe, T.; Frandsen, N.; et al. Porphyrin-related photosensitizers for cancer imaging and therapeutic applications. J. Microsc. 2005, 218, 133–147. [Google Scholar] [CrossRef] [PubMed]
- Berg, K.; Selbo, P.K.; Prasmickaite, L.; Tjelle, T.E.; Sandvig, K.; Moan, J.; Gaudernack, G.; Fodstad, Ø.; Kjølsrud, S.; Anholt, H.; et al. Photochemical internalization: A novel technology for delivery of macromolecules into cytosol. Cancer Res. 1999, 59, 1180–1183. [Google Scholar] [PubMed]
- Selbo, P.K.; Sandvig, K.; Kirveliene, V.; Berg, K. Release of gelonin from endosomes and lysosomes to cytosol by photochemical internalization. Biochim. Biophys. Acta 2000, 1475, 307–313. [Google Scholar] [CrossRef]
- Berstad, M.B.; Weyergang, A.; Berg, K. Photochemical internalization (PCI) of HER2-targeted toxins: Synergy is dependent on the treatment sequence. Biochim. Biophys. Acta 2012, 1820, 1849–1858. [Google Scholar] [CrossRef] [PubMed]
- Høgset, A.; Prasmickaite, L.; Selbo, P.K.; Hellum, M.; Engesæter, B.; Bonsted, A.; Berg, K. Photochemical internalisation in drug and gene delivery. Adv. Drug Deliv. Rev. 2004, 56, 95–115. [Google Scholar] [CrossRef] [PubMed]
- Selbo, P.K.; Weyergang, A.; Høgset, A.; Norum, O.J.; Berstad, M.B.; Vikdal, M.; Berg, K. Photochemical internalization provides time- and space-controlled endolysosomal escape of therapeutic molecules. J. Control. Release 2010, 148, 2–12. [Google Scholar] [CrossRef] [PubMed]
- Hadjur, C.; Lange, N.; Rebstein, J.; Monnier, P.; van den Bergh, H.; Wagnières, G. Spectroscopic studies of photobleaching and photoproduct formation of meta(tetrahydroxyphenyl)chlorin (m-THPC) used in photodynamic therapy. The production of singlet oxygen by m-THPC. J. Photochem. Photobiol. B Biol. 1998, 45, 170–178. [Google Scholar] [CrossRef]
- Moan, J. Effect of bleaching of porphyrin sensitizers during photodynamic therapy. Cancer Lett. 1986, 33, 45–53. [Google Scholar] [CrossRef]
- Wainwright, M.; Giddens, R.M. Phenothiazinium photosensitisers: Choices in synthesis and application. Dyes Pigment. 2003, 57, 245–257. [Google Scholar] [CrossRef]
- Wainwright, M.; Phoenix, D.A.; Rice, L.; Burrow, S.M.; Waring, J. Increased cytotoxicity and phototoxicity in the methylene blue series via chromophore methylation. J. Photochem. Photobiol. B Biol. 1997, 40, 233–239. [Google Scholar] [CrossRef]
- Acedo, P.; Stockert, J.C.; Cañete, M.; Villanueva, A. Two combined photosensitizers: A goal for more effective photodynamic therapy of cancer. Cell Death Dis. 2014, 5, e1122. [Google Scholar] [CrossRef] [PubMed]
- Lau, J.T.F.; Lo, P.C.; Fong, W.P.; Ng, D.K.P. A zinc(II) phthalocyanine conjugated with an oxaliplatin derivative for dual chemo- and photodynamic therapy. J. Med. Chem. 2012, 55, 5446–5454. [Google Scholar] [CrossRef] [PubMed]
- Lottner, C.; Knuechel, R.; Bernhardt, G.; Brunner, H. Combined chemotherapeutic and photodynamic treatment on human bladder cells by hematoporphyrin-platinum(II) conjugates. Cancer Lett. 2004, 203, 171–180. [Google Scholar] [CrossRef] [PubMed]
- Dietze, A.; Peng, Q.; Selbo, P.K.; Kaalhus, O.; Müller, C.; Bown, S.; Berg, K. Enhanced photodynamic destruction of a transplantable fibrosarcoma using photochemical internalisation of gelonin. Br. J. Cancer 2005, 92, 2004–2009. [Google Scholar] [CrossRef] [PubMed]
- Adigbli, D.K.; Wilson, D.G.G.; Farooqui, N.; Sousi, E.; Risley, P.; Taylor, I.; Macrobert, A.J.; Loizidou, M. Photochemical internalisation of chemotherapy potentiates killing of multidrug-resistant breast and bladder cancer cells. Br. J. Cancer 2007, 97, 502–512. [Google Scholar] [CrossRef] [PubMed]
- Savellano, M.D.; Hasan, T. Targeting cells that overexpress the epidermal growth factor receptor with polyethylene glycolated BPD verteporfin photosensitizer immunoconjugates. Photochem. Photobiol. 2003, 77, 431–439. [Google Scholar] [CrossRef]
- Krumova, K.; Greene, L.E.; Cosa, G. Fluorogenic α-tocopherol analogue for monitoring the antioxidant status within the inner mitochondrial membrane of live cells. J. Am. Chem. Soc. 2013, 135, 17135–17143. [Google Scholar] [CrossRef] [PubMed]
- Khatchadourian, A.; Krumova, K.; Boridy, S.; An, T.N.; Maysinger, D.; Cosa, G. Molecular imaging of lipid peroxyl radicals in living cells with a BODIPY–α-Tocopherol adduct. Biochemistry 2009, 48, 5658–5668. [Google Scholar] [CrossRef] [PubMed]
- Liu, K.-H.; Wang, C.-P.; Chang, M.-F.; Chung, Y.-W.; Lou, P.-J.; Lin, J.-H. Molecular characterization of photosensitizer-mediated photodynamic therapy by gene expression profiling. Hum. Exp. Toxicol. 2013, 33, 629–637. [Google Scholar] [CrossRef] [PubMed]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bacellar, I.O.L.; Tsubone, T.M.; Pavani, C.; Baptista, M.S. Photodynamic Efficiency: From Molecular Photochemistry to Cell Death. Int. J. Mol. Sci. 2015, 16, 20523-20559. https://doi.org/10.3390/ijms160920523
Bacellar IOL, Tsubone TM, Pavani C, Baptista MS. Photodynamic Efficiency: From Molecular Photochemistry to Cell Death. International Journal of Molecular Sciences. 2015; 16(9):20523-20559. https://doi.org/10.3390/ijms160920523
Chicago/Turabian StyleBacellar, Isabel O. L., Tayana M. Tsubone, Christiane Pavani, and Mauricio S. Baptista. 2015. "Photodynamic Efficiency: From Molecular Photochemistry to Cell Death" International Journal of Molecular Sciences 16, no. 9: 20523-20559. https://doi.org/10.3390/ijms160920523
APA StyleBacellar, I. O. L., Tsubone, T. M., Pavani, C., & Baptista, M. S. (2015). Photodynamic Efficiency: From Molecular Photochemistry to Cell Death. International Journal of Molecular Sciences, 16(9), 20523-20559. https://doi.org/10.3390/ijms160920523