Anthocyanin Absorption and Metabolism by Human Intestinal Caco-2 Cells—A Review
Abstract
:1. Introduction
2. Chemistry of Anthocyanins


3. Bioavailability of Anthocyanins: Absorption and Metabolism by Caco-2 Cells
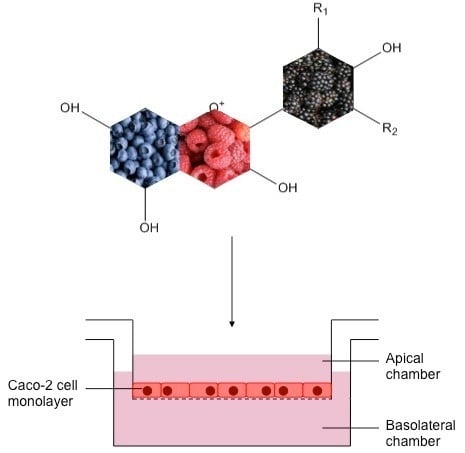
3.1. Caco-2 Cell Growth and Differentiation
3.2. Anthocyanin Transport through Caco-2 Cells
| Sample | Pre-Treatment | Anthocyanins | Anthocyanin Concentration | Cell Origin | Cell Differentiation | Incubation Time | Key Findings | Reference |
|---|---|---|---|---|---|---|---|---|
| Blueberry | Chemical extraction | Dp-3-Glu, Cy-3-Gal, Cy-3-Glu, Pt-3-Glu, Pn-3-Gal, Pn-3-Glu, Mv-3-Glu | 50 μg/mL | ATCC | 20–26 days | 0–120 min | Transport efficiency of ACNs averaged ca. 3%–4% (<1% in Dp-3-Glu); Glucose-based ACNs had higher bioavailability than galactose-based ACNs | [28] |
| Black currant extract | - | Dp-3-Glu, Dp-3-Rut, Cy-3-Glu, Cy-3-Rut | 180 μM | DSWZ | 19–21 days | 0–80 min | ACNs were not detected in any serosal solution | [41] |
| Red grape skin | Chemical extraction | Dp-3-Glu, Cy-3-Glu, Pt-3-Glu, Pn-3-Glu, Mv-3-Glu | 200 μg/mL | ATCC | 25 days | 4 days of pre-treatment + 6 min | Only ca. 1% of ACNs are transported; ACN transport significantly increased in the presence of ethanol; Cells pre-treated with ACNs showed ca. 50% increased transport; GLUT2 may be responsible for ACN transport | [42] |
| Açaí pulp | Chemical extraction | Cy-3-Rut, Cy-3-Glu | 50–500 μg/mL | ATCC | 21 days | 30–120 min | Transport efficiency of ACNs was 0.5%–4.9%; Presence of polymeric ACNs decreased transport of monomeric ACN glycosides (up to 40.3%) | [43] |
| Standard | - | Cat-Mv-3-Glu, Mv-3-Glu | 100 μM | n/a | 21 days | 30–120 min | Transport efficiency of Mv-3-Glu was 4%; Absorption efficiency of Cat-Mv-3-Glu was lower than Mv-3-Glu (ca. 3%) | [44] |
| Sour cherry fruit and nectar | Chemical extraction | Cy-3-Glu-Rut | 55 μM | ATCC | 23–24 days | 360 min | Cy-3-Glu-Rut recovery was ca. 0.5%–4%; Cy-3-Glu-Rut transported 3 times more efficiently from nectar than fruit; Sucrose and citric acid enhanced the transport of Cy-3-Glu-Rut (ca. 5-fold); SPE reduced the transport efficiency of Cy-3-Glu-Rut by 5–10-fold | [11] |
| Standard | Encapsulation | Cy-3-Glu | 37.5 μM | n/a | 20–26 days | 60 min | Nano-encapsulated Cy-3-Glu with apoferritin was more efficiently transported compared to free Cy-3-Glu | [29] |
| Standard | - | Cy-3-Glu | 10–40 μM | ATCC | 13 days | 30–120 min | Transport efficiency of Cy-3-Glu was 0.8%–2.4%; Phloridzin and phloretin inhibited the absorption of Cy-3-Glu; SGLT1 and GLUT2 are probably involved in the absorption of Cy-3-Glu | [45] |
| Açaí concentrate | Chemical extraction | Cy-3-Glu, Cy-3-Rut | 500 μg/mL | ATCC | 18–21 days | 0–120 min | Transport rate of ACNs was 1.22%; Phospholipids from soy lecithin and terpenes from cold pressed citrus oil increased the transport of ACNs | [46] |
| Strawberry | Chemical extraction + in vitro digestion | Pg-3-Glu, Pg-3-Mal-Glu, Cy-3-Glu | 16.3 mg/100 g | ATCC | 21 days | 120 min | Trace amount of Pg-3-Glu was transported | [47] |
| Grape | Chemical extraction | Mv-3-Glu, Pn-3-Glu, Pt-3-Glu, Cy-3-Glu, Dp-3-Glu | 1766.1 μg/mL | ATCC | 21 days | 30–240 min | Mv-3-Glu, Pn-3-Glu, Pt-3-Glu and Cy-3-Glu were transported, whereas Dp-3-Glu was not transported; Transport efficiency of major anthocyanin (Mv-3-Glu) was 0.35% | [48] |
| Grape/blueberry extract | - | Mv-3-Glu, Pn-3-Glu, Pt-3-Glu, Dp-3-Glu, Cy-3-Glu, Mv-3,5-DGlu, Pn-3,5-DGlu | 2613 μM | ATCC | 21 days | 0–90 min | Absorption rates of Mv-3-Glu, Pn-3-Glu, Pt-3-Glu, Dp-3-Glu and Cy-3-Glu were 0.005%–0.06%; Mv-3,5-DGlu and Pn-3,5-DGlu were not transported in quantifiable concentrations | [49] |
3.3. Anthocyanin Metabolism by Caco-2 Cells

3.4. Bioactive Properties of Anthocyanins on Caco-2 Cells
4. Conclusions and Future Perspectives
| Bioactivity | Sources | Assays/Markers | Anthocyanins | References |
|---|---|---|---|---|
| Antiproliferative | Arctic bramble, Black currant, Blueberry, Bilberry, Chokeberry juice, Cloudberry, Lingonberry, Peach, Plum, Potato, Purple rice, Red chicory, Standards, Strawberry, Strawberry guava | MTT, Trypan blue, Thymidine incorporation, CCK-8, LDH, SRB | Cy, Cy-3-Ara, Cy-3-Gal, Cy-3-Glu, Cy-3-Rut, Dp, Dp-3-Gal, Dp-3-Glu, Dp-3-Rut, Mv-3-Ara, Mv-3-Gal, Mv-3-Glu, Pg, Pn-3-Gal, Pn-3-Glu, Pt-3-Gal, Pt-3-Glu | [68,69,70,71,72,73,74,75,76,77,78,79] |
| Antioxidant | Bee pollen, Bilberry, Blackberry, Red chicory, Red orange, Wine | ROS, TBARS | Cy-3-Ara, Cy-3-Gal, Cy-3-Glu, Dp-3-Ara, Dp-3-Gal, Dp-3-Glu, Dp-3-Rut, Mv-3-Ace-Glu, Mv-3-Ara, Mv-3-Caf-Glu, Mv-3-Cou-Glu, Mv-3-Gal, Mv-3-Glu, Mv-3-Rut, Pn-3-Ara, Pn-3-Cou-Glu, Pn-3-Gal, Pn-3-Glu, Pt-3-Ara, Pt-3-Cou-Glu, Pt-3-Gal, Pt-3-Glu, Pt-3-Rut | [79,80,81,82,83,84,85] |
| Anti-inflammatory | Blackberry, Blueberry, Black raspberry, Grape, Raspberry | NF-κB, NO, IL-8, E-selectin, ICAM-1, VCAM-1 | Cy-3-Ara, Cy-3-Gal, Cy-3-Glu, Cy-3-Rut, Dp-3-Ara, Dp-3-Gal, Dp-3-Glu, Mv-3-Ara, Mv-3-Gal, Mv-3-Glu, Pn-3-Gal, Pn-3-Glu, Pt-3-Ara, Pt-3-Gal, Pt-3-Glu | [48,86,87] |
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Prior, R.L.; Wu, X. Anthocyanins: Structural characteristics that result in unique metabolic patterns and biological activities. Free Radic. Res. 2006, 40, 1014–1028. [Google Scholar] [CrossRef] [PubMed]
- He, J.; Giusti, M.M. Anthocyanins: Natural colorants with health-promoting properties. Annu. Rev. Food Sci. Technol. 2010, 1, 163–187. [Google Scholar] [CrossRef] [PubMed]
- Alvarez-Suarez, J.M.; Dekanski, D.; Ristic, S.; Radonjic, N.V.; Petronijevic, N.D.; Giampieri, F.; Astolfi, P.; González-Paramás, A.M.; Santos-Buelga, C.; Tulipani, S.; et al. Strawberry polyphenols attenuate ethanol-induced gastric lesions in rats by activation of antioxidant enzymes and attenuation of MDA increase. PLoS ONE 2011, 6, e25878. [Google Scholar] [CrossRef] [PubMed]
- Kamiloglu, S.; Serali, O.; Unal, N.; Capanoglu, E. Antioxidant activity and polyphenol composition of black mulberry (Morus nigra L.) products. J. Berry Res. 2013, 3, 41–51. [Google Scholar]
- Giampieri, F.; Alvarez-Suarez, J.M.; Battino, M. Strawberry and human health: Effects beyond antioxidant activity. J. Agric. Food Chem. 2014, 62, 3867–3876. [Google Scholar] [CrossRef] [PubMed]
- Howard, L.R.; Brownmiller, C.; Prior, R.L. Improved color and anthocyanin retention in strawberry puree by oxygen exclusion. J. Berry Res. 2014, 4, 107–116. [Google Scholar]
- McGhie, T.K.; Walton, M.C. The bioavailability and absorption of anthocyanins: Towards a better understanding. Mol. Nutr. Food Res. 2007, 51, 702–713. [Google Scholar] [CrossRef] [PubMed]
- Wallace, T.C. Anthocyanins in cardiovascular disease. Adv. Nutr. 2011, 2, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Mateus, N.; de Freitas, V. Anthocyanins as Food Colorants. In Anthocyanins; Winefield, C., Kevin Davies, K., et al., Eds.; Springer: New York, NY, USA, 2009; pp. 284–304. [Google Scholar]
- Pojer, E.; Mattivi, F.; Johnson, D.; Stockley, C.S. The case for anthocyanin consumption to promote human health: A review. Compr. Rev. Food Sci. Food Saf. 2013, 12, 483–508. [Google Scholar] [CrossRef]
- Toydemir, G.; Boyacioglu, D.; Capanoglu, E.; van der Meer, I.M.; Tomassen, M.M.; Hall, R.D.; Mes, J.J.; Beekwilder, J. Investigating the transport dynamics of anthocyanins from unprocessed fruit and processed fruit juice from sour cherry (Prunus cerasus L.) across intestinal epithelial cells. J. Agric. Food Chem. 2013, 61, 11434–11441. [Google Scholar] [CrossRef] [PubMed]
- Faria, A.; Fernandes, I.; Mateus, N.; Calhau, C. Bioavailability of anthocyanins. In Natural Products: Phytochemistry, Botany and Metabolism of Alkaloids, Phenolics and Terpenes; Ramawat, K.G., Merillon, J.M., Eds.; Springer: Berlin-Heidelberg, Germany, 2013; pp. 2465–2487. [Google Scholar]
- Forbes-Hernández, T.Y.; Giampieri, F.; Gasparrini, M.; Mazzoni, L.; Quiles, J.L.; Alvarez-Suarez, J.M.; Battino, M. The effects of bioactive compounds from plant foods on mitochondrial function: A focus on apoptotic mechanisms. Food Chem. Toxicol. 2014, 68, 154–182. [Google Scholar] [CrossRef] [PubMed]
- Ignat, I.; Volf, I.; Popa, V.I. A critical review of methods for characterisation of polyphenolic compounds in fruits and vegetables. Food Chem. 2011, 126, 1821–1835. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Beecher, G.R.; Holden, J.M.; Haytowitz, D.B.; Gebhardt, S.E.; Prior, R.L. Concentrations of anthocyanins in common foods in the United States and estimation of normal consumption. J. Agric. Food Chem. 2006, 54, 4069–4075. [Google Scholar] [CrossRef] [PubMed]
- Fang, J. Bioavailability of anthocyanins. Drug Metab. Rev. 2014, 46, 508–520. [Google Scholar] [CrossRef] [PubMed]
- Castaneda-Ovando, A.; de Lourdes Pacheco-Hernández, M.; Páez-Hernández, M.E.; Rodríguez, J.A.; Galán-Vidal, C.A. Chemical studies of anthocyanins: A review. Food Chem. 2009, 113, 859–871. [Google Scholar] [CrossRef]
- De Pascual-Teresa, S.; Sanchez-Ballesta, M.T.; García-Viguera, C. Anthocyanins. In Natural Products: Phytochemistry, Botany and Metabolism of Alkaloids, Phenolics and Terpenes; Ramawat, K.G., Merillon, J.M., Eds.; Springer: Berlin-Heidelberg, Germany, 2013; pp. 1803–1819. [Google Scholar]
- Fernandes, I.; Faria, A.; Calhau, C.; de Freitas, V.; Mateus, N. Bioavailability of anthocyanins and derivatives. J. Funct. Foods 2014, 7, 54–66. [Google Scholar] [CrossRef]
- Tsuda, T. Dietary anthocyanin-rich plants: Biochemical basis and recent progress in health benefits studies. Mol. Nutr. Food Res. 2012, 56, 159–170. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.L.; Shah, V.; Patnaik, R.; Adams, W.; Hussain, A.; Conner, D.; Mehta, M.; Malinowski, H.; Lazor, J.; Huang, S.H.; et al. Bioavailability and bioequivalence: An FDA regulatory overview. Pharm. Res. 2001, 18, 1645–1650. [Google Scholar] [CrossRef] [PubMed]
- Parada, J.; Aguilera, J.M. Food microstructure affects the bioavailability of several nutrients. J. Food Sci. 2007, 72, R21–R32. [Google Scholar] [CrossRef] [PubMed]
- Zucco, F.; Batto, A.; Bises, G.; Chambaz, J.; Chiusolo, A.; Consalvo, R.; Cross, H.; Dal Negro, G.D.; de Angelis, I.; Fabre, G.; et al. An inter-laboratory study to evaluate the effects of medium composition on the differentiation and barrier function of Caco-2 cell lines. Altern. Lab. Anim. 2005, 33, 603–618. [Google Scholar] [PubMed]
- Manna, C.; Galletti, P.; Maisto, G.; Cucciolla, V.; D’Angelo, S.; Zappia, V. Transport mechanism and metabolism of olive oil hydroxytyrosol in Caco-2 cells. FEBS Lett. 2000, 470, 341–344. [Google Scholar] [CrossRef]
- Deprez, S.; Mila, I.; Huneau, J.F.; Tome, D.; Scalbert, A. Transport of proanthocyanidin dimer, trimer, and polymer across monolayers of human intestinal epithelial Caco-2 cells. Antioxid. Redox Signal. 2001, 3, 957–967. [Google Scholar] [CrossRef] [PubMed]
- Konishi, Y.; Kobayashi, S. Transepithelial transport of chlorogenic acid, caffeic acid, and their colonic metabolites in intestinal Caco-2 cell monolayers. J. Agric. Food Chem. 2004, 52, 2518–2526. [Google Scholar] [CrossRef] [PubMed]
- Manzano, S.; Williamson, G. Polyphenols and phenolic acids from strawberry and apple decrease glucose uptake and transport by human intestinal Caco-2 cells. Mol. Nutr. Food Res. 2010, 54, 1773–1780. [Google Scholar] [CrossRef] [PubMed]
- Yi, W.; Akoh, C.C.; Fischer, J.; Krewer, G. Absorption of anthocyanins from blueberry extracts by Caco-2 human intestinal cell monolayers. J. Agric. Food Chem. 2006, 54, 5651–5658. [Google Scholar] [CrossRef] [PubMed]
- Zhang, T.; Lv, C.; Chen, L.; Bai, G.; Zhao, G.; Xu, C. Encapsulation of anthocyanin molecules within a ferritin nanocage increases their stability and cell uptake efficiency. Food Res. Int. 2014, 62, 183–192. [Google Scholar] [CrossRef]
- Sun, H.; Chow, E.C.; Liu, S.; Du, Y.; Pang, K.S. The Caco-2 cell monolayer: Usefulness and limitations. Expert Opin. Drug Metab. Toxicol. 2008, 4, 395–411. [Google Scholar] [CrossRef] [PubMed]
- Finotti, E.; Gezzi, R.; Nobili, F.; Garaguso, I.; Friedman, M. Effect of apple, baobab, red-chicory, and pear extracts on cellular energy expenditure and morphology of a Caco-2 cells using transepithelial electrical resistance (TEER) and scanning electron microscopy (SEM). RSC Adv. 2015, 5, 22490–22498. [Google Scholar] [CrossRef]
- Sabboh-Jourdan, H.; Valla, F.; Epriliati, I.; Gidley, M.J. Organic acid bioavailability from banana and sweet potato using an in vitro digestion and Caco-2 cell model. Eur. J. Nutr. 2011, 50, 31–40. [Google Scholar] [CrossRef] [PubMed]
- Sambuy, Y.; de Angelis, I.; Ranaldi, G.; Scarino, M.L.; Stammati, A.; Zucco, F. The Caco-2 cell line as a model of the intestinal barrier: Influence of cell and culture-related factors on Caco-2 cell functional characteristics. Cell Biol. Toxicol. 2005, 21, 1–26. [Google Scholar] [CrossRef] [PubMed]
- Barrington, R.; Williamson, G.; Bennett, R.N.; Davis, B.D.; Brodbelt, J.S.; Kroon, P.A. Absorption, conjugation and efflux of the flavonoids, kaempferol and galangin, using the intestinal CaCo-2/TC7 cell model. J. Funct. Foods 2009, 1, 74–87. [Google Scholar] [CrossRef] [PubMed]
- Pereira-Caro, G.; Mateos, R.; Saha, S.; Madrona, A.; Espartero, J.L.; Bravo, L.; Kroon, P.A. Transepithelial transport and metabolism of new lipophilic ether derivatives of hydroxytyrosol by enterocyte-like Caco-2/TC7 cells. J. Agric. Food Chem. 2010, 58, 11501–11509. [Google Scholar] [CrossRef] [PubMed]
- Soler, A.; Romero, M.P.; Macià, A.; Saha, S.; Furniss, C.S.; Kroon, P.A.; Motilva, M.J. Digestion stability and evaluation of the metabolism and transport of olive oil phenols in the human small-intestinal epithelial Caco-2/TC7 cell line. Food Chem. 2010, 119, 703–714. [Google Scholar] [CrossRef]
- Balimane, P.V.; Chong, S. Cell culture-based models for intestinal permeability: A critique. Drug Discov. Today 2005, 10, 335–343. [Google Scholar] [CrossRef]
- Zweibaum, A.; Pinto, M.; Chevalier, G.; Dussaulx, E.; Triadou, N.; Lacroix, B.; Haffen, K.; Brun, J.L.; Rousset, M. Enterocytic differentiation of a subpopulation of the human colon tumor cell line HT-29 selected for growth in sugar-free medium and its inhibition by glucose. J. Cell. Physiol. 1985, 122, 21–29. [Google Scholar] [CrossRef] [PubMed]
- Seeram, N.P.; Adams, L.S.; Hardy, M.L.; Heber, D. Total cranberry extract versus its phytochemical constituents: Antiproliferative and synergistic effects against human tumor cell lines. J. Agric. Food Chem. 2004, 52, 2512–2517. [Google Scholar] [CrossRef] [PubMed]
- Ackland, M.L.; van de Waarsenburg, S.; Jones, R. Synergistic antiproliferative action of the flavonols quercetin and kaempferol in cultured human cancer cell lines. In Vivo 2005, 19, 69–76. [Google Scholar] [PubMed]
- Steinert, R.E.; Ditscheid, B.; Netzel, M.; Jahreis, G. Absorption of black currant anthocyanins by monolayers of human intestinal epithelial Caco-2 cells mounted in ussing type chambers. J. Agric. Food Chem. 2008, 56, 4995–5001. [Google Scholar] [CrossRef] [PubMed]
- Faria, A.; Pestana, D.; Azevedo, J.; Martel, F.; de Freitas, V.; Azevedo, I.; Mateus, N.; Calhau, C. Absorption of anthocyanins through intestinal epithelial cells—Putative involvement of GLUT2. Mol. Nutr. Food Res. 2009, 53, 1430–1437. [Google Scholar] [CrossRef] [PubMed]
- Pacheco-Palencia, L.A.; Mertens-Talcott, S.U.; Talcott, S.T. In vitro absorption and antiproliferative activities of monomeric and polymeric anthocyanin fractions from açai fruit (Euterpe oleracea Mart.). Food Chem. 2010, 119, 1071–1078. [Google Scholar] [CrossRef]
- Fernandes, I.; Nave, F.; Gonçalves, R.; de Freitas, V.; Mateus, N. On the bioavailability of flavanols and anthocyanins: Flavanol—Anthocyanin dimers. Food Chem. 2012, 135, 812–818. [Google Scholar] [CrossRef] [PubMed]
- Zou, T.B.; Feng, D.; Song, G.; Li, H.W.; Tang, H.W.; Ling, W.H. The role of sodium-dependent glucose transporter 1 and glucose transporter 2 in the absorption of cyanidin-3-O-β-glucoside in Caco-2 cells. Nutrients 2014, 6, 4165–4177. [Google Scholar] [CrossRef] [PubMed]
- Cardona, J.A.; Mertens-Talcott, S.U.; Talcott, S.T. Phospholipids and terpenes modulate Caco-2 transport of açaí anthocyanins. Food Chem. 2015, 175, 267–272. [Google Scholar] [CrossRef] [PubMed]
- Kosińska-Cagnazzo, A.; Diering, S.; Prim, D.; Andlauer, W. Identification of bioaccessible and uptaken phenolic compounds from strawberry fruits in in vitro digestion/Caco-2 absorption model. Food Chem. 2015, 170, 288–294. [Google Scholar] [CrossRef] [PubMed]
- Kuntz, S.; Asseburg, H.; Dold, S.; Römpp, A.; Fröhling, B.; Kunz, C.; Rudloff, S. Inhibition of low-grade inflammation by anthocyanins from grape extract in an in vitro epithelial-endothelial co-culture model. Food Funct. 2015, 6, 1136–1149. [Google Scholar] [CrossRef] [PubMed]
- Kuntz, S.; Rudloff, S.; Asseburg, H.; Borsch, C.; Fröhling, B.; Unger, F.; Dold, S.; Spengler, B.; Römpp, A.; Kunz, C. Uptake and bioavailability of anthocyanins and phenolic acids from grape/blueberry juice and smoothie in vitro and in vivo. Br. J. Nutr. 2015, 113, 1044–1055. [Google Scholar] [CrossRef] [PubMed]
- Netzel, M.; Strass, G.; Janssen, M.; Bitsch, I.; Bitsch, R. Bioactive anthocyanins detected in human urine after ingestion of blackcurrant juice. J. Environ. Pathol. Toxicol. Oncol. 2001, 20, 89–95. [Google Scholar] [CrossRef] [PubMed]
- Frank, T.; Netzel, M.; Strass, G.; Bitsch, R.; Bitsch, I. Bioavailability of anthocyanidin-3-glucosides following consumption of red wine and red grape juice. Can. J. Physiol. Pharmacol. 2003, 81, 423–435. [Google Scholar] [CrossRef] [PubMed]
- Kurilich, A.C.; Clevidence, B.A.; Britz, S.J.; Simon, P.W.; Novotny, J.A. Plasma and urine responses are lower for acylated vs. nonacylated anthocyanins from raw and cooked purple carrots. J. Agric. Food Chem. 2005, 53, 6537–6542. [Google Scholar] [CrossRef] [PubMed]
- Charron, C.S.; Kurilich, A.C.; Clevidence, B.A.; Simon, P.W.; Harrison, D.J.; Britz, S.J.; Baer, D.; Novotny, J.A. Bioavailability of anthocyanins from purple carrot juice: Effects of acylation and plant matrix. J. Agric. Food Chem. 2009, 57, 1226–1230. [Google Scholar] [CrossRef] [PubMed]
- Milbury, P.E.; Vita, J.A.; Blumberg, J.B. Anthocyanins are bioavailable in humans following an acute dose of cranberry juice. J. Nutr. 2010, 140, 1099–1104. [Google Scholar] [CrossRef] [PubMed]
- Tian, X.J.; Yang, X.W.; Yang, X.; Wang, K. Studies of intestinal permeability of 36 flavonoids using Caco-2 cell monolayer model. Int. J. Pharm. 2009, 367, 58–64. [Google Scholar] [CrossRef] [PubMed]
- Bub, A.; Watzl, B.; Heeb, D.; Rechkemmer, G.; Briviba, K. Malvidin-3-glucoside bioavailability in humans after ingestion of red wine, dealcoholized red wine and red grape juice. Eur. J. Nutr. 2001, 40, 113–120. [Google Scholar] [CrossRef] [PubMed]
- Andlauer, W.; Stumpf, C.; Frank, K.; Fürst, P. Absorption and metabolism of anthocyanin cyanidin-3-glucoside in the isolated rat small intestine is not influenced by ethanol. Eur. J. Nutr. 2003, 42, 217–223. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, A.; Gomes, M.H.; Alexandre, E.M.; Poças, F.; Almeida, D.P.; Pintado, M. Phytochemicals preservation in strawberry as affected by pH modulation. Food Chem. 2015, 170, 74–83. [Google Scholar] [CrossRef] [PubMed]
- Kay, C.D.; Kroon, P.A.; Cassidy, A. The bioactivity of dietary anthocyanins is likely to be mediated by their degradation products. Mol. Nutr. Food Res. 2009, 53, S92–S101. [Google Scholar] [CrossRef] [PubMed]
- Alzaid, F.; Cheung, H.M.; Preedy, V.R.; Sharp, P.A. Regulation of glucose transporter expression in human intestinal Caco-2 cells following exposure to an anthocyanin-rich berry extract. PLoS ONE 2013, 8, e78932. [Google Scholar] [CrossRef] [PubMed]
- Betz, M.; Steiner, B.; Schantz, M.; Oidtmann, J.; Mäder, K.; Richling, E.; Kulozik, U. Antioxidant capacity of bilberry extract microencapsulated in whey protein hydrogels. Food Res. Int. 2012, 47, 51–57. [Google Scholar] [CrossRef]
- Oidtmann, J.; Schantz, M.; Mäder, K.; Baum, M.; Berg, S.; Betz, M.; Kulozik, U.; Leick, S.; Rehage, H.; Schwarz, K.; et al. Preparation and comparative release characteristics of three anthocyanin encapsulation systems. J. Agric. Food Chem. 2012, 60, 844–851. [Google Scholar] [CrossRef] [PubMed]
- Robert, P.; Fredes, C. The Encapsulation of anthocyanins from berry-type fruits. Trends in foods. Molecules 2015, 20, 5875–5888. [Google Scholar] [CrossRef] [PubMed]
- Cardona, F.; Andres-Lacueva, C.; Tulipani, S.; Tinahones, F.J.; Queipo-Ortuno, M.I. Benefits of polyphenols on gut microbiota and implications in human health. J. Nutr. Biochem. 2013, 24, 1415–1422. [Google Scholar] [CrossRef] [PubMed]
- Vitaglione, P.; Donnarumma, G.; Napolitano, A.; Galvano, F.; Gallo, A.; Scalfi, L.; Fogliano, V. Protocatechuic acid is the major human metabolite of cyanidin-glucosides. J. Nutr. 2007, 137, 2043–2048. [Google Scholar] [PubMed]
- Fernandes, I.; Marques, F.; de Freitas, V.; Mateus, N. Antioxidant and antiproliferative properties of methylated metabolites of anthocyanins. Food Chem. 2013, 141, 2923–2933. [Google Scholar] [CrossRef] [PubMed]
- Forester, S.C.; Waterhouse, A.L. Gut metabolites of anthocyanins, gallic acid, 3-O-methylgallic acid, and 2,4,6-trihydroxybenzaldehyde, inhibit cell proliferation of Caco-2 cells. J. Agric. Food Chem. 2010, 58, 5320–5327. [Google Scholar] [CrossRef] [PubMed]
- Lazzè, M.C.; Savio, M.; Pizzala, R.; Cazzalini, O.; Perucca, P.; Scovassi, A.I.; Stivala, L.A.; Bianchi, L. Anthocyanins induce cell cycle perturbations and apoptosis in different human cell lines. Carcinogenesis 2004, 25, 1427–1433. [Google Scholar] [CrossRef] [PubMed]
- Yi, W.; Fischer, J.; Krewer, G.; Akoh, C.C. Phenolic compounds from blueberries can inhibit colon cancer cell proliferation and induce apoptosis. J. Agric. Food Chem. 2005, 53, 7320–7329. [Google Scholar] [CrossRef] [PubMed]
- Bermúdez-Soto, M.J.; Larrosa, M.; Garcia-Cantalejo, J.M.; Espín, J.C.; Tomás-Barberan, F.A.; García-Conesa, M.T. Up-regulation of tumor suppressor carcinoembryonic antigen-related cell adhesion molecule 1 in human colon cancer Caco-2 cells following repetitive exposure to dietary levels of a polyphenol-rich chokeberry juice. J. Nutr. Biochem. 2007, 18, 259–271. [Google Scholar] [CrossRef] [PubMed]
- Lea, M.A.; Ibeh, C.; Vizzotto, M.; Cisneros-Zevallos, L.; Byrne, D.H.; Okie, W.R.; Moyer, M.P. Inhibition of growth and induction of differentiation of colon cancer cells by peach and plum phenolic compounds. Anticancer Res. 2008, 28, 2067–2076. [Google Scholar] [PubMed]
- McDougall, G.J.; Ross, H.A.; Ikeji, M.; Stewart, D. Berry extracts exert different antiproliferative effects against cervical and colon cancer cells grown in vitro. J. Agric. Food Chem. 2008, 56, 3016–3023. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Zhang, W.; Jing, H.; Popovich, D.G. Bog bilberry (Vaccinium uliginosum L.) extract reduces cultured Hep-G2, Caco-2, and 3T3-L1 cell viability, affects cell cycle progression, and has variable effects on membrane permeability. J. Food Sci. 2010, 75, H103–H107. [Google Scholar] [CrossRef] [PubMed]
- Holtung, L.; Grimmer, S.; Aaby, K. Effect of processing of black currant press-residue on polyphenol composition and cell proliferation. J. Agric. Food Chem. 2011, 59, 3632–3640. [Google Scholar] [CrossRef] [PubMed]
- Medina, A.L.; Haas, L.I.R.; Chaves, F.C.; Salvador, M.; Zambiazi, R.C.; Da Silva, W.P.; Nora, L.; Rombaldi, C.V. Araçá (Psidium cattleianum Sabine) fruit extracts with antioxidant and antimicrobial activities and antiproliferative effect on human cancer cells. Food Chem. 2011, 128, 916–922. [Google Scholar] [CrossRef]
- Wang, Q.; Chen, Q.; He, M.; Mir, P.; Su, J.; Yang, Q. Inhibitory effect of antioxidant extracts from various potatoes on the proliferation of human colon and liver cancer cells. Nutr. Cancer 2011, 63, 1044–1052. [Google Scholar] [CrossRef] [PubMed]
- Aaby, K.; Grimmer, S.; Holtung, L. Extraction of phenolic compounds from bilberry (Vaccinium myrtillus L.) press residue: Effects on phenolic composition and cell proliferation. LWT-Food Sci. Technol. 2013, 54, 257–264. [Google Scholar] [CrossRef]
- Chatthongpisut, R.; Schwartz, S.J.; Yongsawatdigul, J. Antioxidant activities and antiproliferative activity of Thai purple rice cooked by various methods on human colon cancer cells. Food Chem. 2015, 188, 99–105. [Google Scholar] [CrossRef] [PubMed]
- D’evoli, L.; Morroni, F.; Lombardi-Boccia, G.; Lucarini, M.; Hrelia, P.; Cantelli-Forti, G.; Tarozzi, A. Red chicory (Cichorium intybus L. cultivar) as a potential source of antioxidant anthocyanins for intestinal health. Oxid. Med. Cell. Longev. 2013, 2013. [Google Scholar] [CrossRef] [PubMed]
- Tarozzi, A.; Hrelia, S.; Angeloni, C.; Morroni, F.; Biagi, P.; Guardigli, M.; Cantelli-Forti, G.; Hrelia, P. Antioxidant effectiveness of organically and non-organically grown red oranges in cell culture systems. Eur. J. Nutr. 2006, 45, 152–158. [Google Scholar] [CrossRef] [PubMed]
- Elisia, I.; Kitts, D.D. Anthocyanins inhibit peroxyl radical-induced apoptosis in Caco-2 cells. Mol. Cell. Biochem. 2008, 312, 139–145. [Google Scholar] [CrossRef] [PubMed]
- Bornsek, S.M.; Ziberna, L.; Polak, T.; Vanzo, A.; Ulrih, N.P.; Abram, V.; Tramer, F.; Passamonti, S. Bilberry and blueberry anthocyanins act as powerful intracellular antioxidants in mammalian cells. Food Chem. 2012, 134, 1878–1884. [Google Scholar] [CrossRef] [PubMed]
- Deiana, M.; Loru, D.; Incani, A.; Rosa, A.; Atzeri, A.; Melis, M.P.; Cabboi, B.; Hollecker, L.; Pinna, M.B.; Argiolas, F.; et al. Wine extracts from Sardinian grape varieties attenuate membrane oxidative damage in Caco-2 cell monolayers. Food Chem. 2012, 134, 2105–2113. [Google Scholar] [CrossRef] [PubMed]
- Juadjur, A.; Mohn, C.; Schantz, M.; Baum, M.; Winterhalter, P.; Richling, E. Fractionation of an anthocyanin-rich bilberry extract and in vitro antioxidative activity testing. Food Chem. 2015, 167, 418–424. [Google Scholar] [CrossRef] [PubMed]
- Sousa, C.; Moita, E.; Valentão, P.; Fernandes, F.; Monteiro, P.; Andrade, P.B. Effects of colored and noncolored phenolics of Echium plantagineum L. bee pollen in Caco-2 cells under oxidative stress induced by tert-butyl hydroperoxide. J. Agric. Food Chem. 2015, 63, 2083–2091. [Google Scholar] [CrossRef] [PubMed]
- Taverniti, V.; Fracassetti, D.; del Bo, C.; Lanti, C.; Minuzzo, M.; Klimis-Zacas, D.; Riso, P.; Guglielmetti, S. Immunomodulatory effect of a wild blueberry anthocyanin-rich extract in human Caco-2 intestinal cells. J. Agric. Food Chem. 2014, 62, 8346–8351. [Google Scholar] [CrossRef] [PubMed]
- Jung, H.; Lee, H.J.; Cho, H.; Hwang, K.T. Anti-inflammatory activities of Rubus fruit anthocyanins in inflamed human intestinal epithelial cells. J. Food Biochem. 2015, 39, 300–309. [Google Scholar] [CrossRef]
- Kroon, P.A.; Clifford, M.N.; Crozier, A.; Day, A.J.; Donovan, J.L.; Manach, C.; Williamson, G. How should we assess the effects of exposure to dietary polyphenols in vitro? Am. J. Clin. Nutr. 2004, 80, 15–21. [Google Scholar] [PubMed]
- Fernandes, I.; de Freitas, V.; Reis, C.; Mateus, N. A new approach on the gastric absorption of anthocyanins. Food Funct. 2012, 3, 508–516. [Google Scholar] [CrossRef] [PubMed]
- Fang, J. Some anthocyanins could be efficiently absorbed across the gastrointestinal mucosa: Extensive presystemic metabolism reduces apparent bioavailability. J. Agric. Food Chem. 2014, 62, 3904–3911. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, H.; Fernandes, I.; Brás, N.F.; Faria, A.; de Freitas, V.; Calhau, C.; Mateus, N. Experimental and theoretical data on the mechanism by which red wine anthocyanins are transported through a human MKN-28 gastric cell model. J. Agric. Food Chem. 2015. [Google Scholar] [CrossRef] [PubMed]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kamiloglu, S.; Capanoglu, E.; Grootaert, C.; Van Camp, J. Anthocyanin Absorption and Metabolism by Human Intestinal Caco-2 Cells—A Review. Int. J. Mol. Sci. 2015, 16, 21555-21574. https://doi.org/10.3390/ijms160921555
Kamiloglu S, Capanoglu E, Grootaert C, Van Camp J. Anthocyanin Absorption and Metabolism by Human Intestinal Caco-2 Cells—A Review. International Journal of Molecular Sciences. 2015; 16(9):21555-21574. https://doi.org/10.3390/ijms160921555
Chicago/Turabian StyleKamiloglu, Senem, Esra Capanoglu, Charlotte Grootaert, and John Van Camp. 2015. "Anthocyanin Absorption and Metabolism by Human Intestinal Caco-2 Cells—A Review" International Journal of Molecular Sciences 16, no. 9: 21555-21574. https://doi.org/10.3390/ijms160921555
APA StyleKamiloglu, S., Capanoglu, E., Grootaert, C., & Van Camp, J. (2015). Anthocyanin Absorption and Metabolism by Human Intestinal Caco-2 Cells—A Review. International Journal of Molecular Sciences, 16(9), 21555-21574. https://doi.org/10.3390/ijms160921555