The Use of a Liposomal Formulation Incorporating an Antimicrobial Peptide from Tilapia as a New Adjuvant to Epirubicin in Human Squamous Cell Carcinoma and Pluripotent Testicular Embryonic Carcinoma Cells
Abstract
:1. Introduction

2. Results and Discussion
2.1. Results
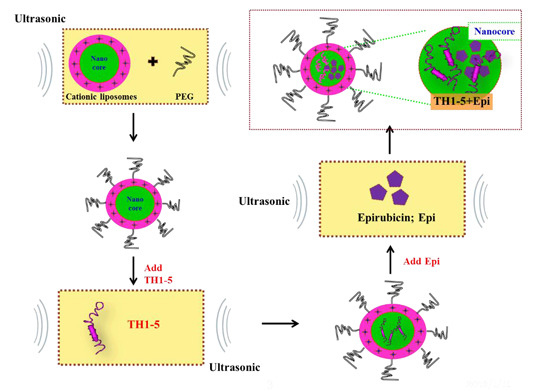
2.1.1. Determination of Encapsulation Efficiency, Particle Size, and Zeta Potential of PEGylated Liposomal TH1-5 or Epi
| Formulations | Particle Size (nm) | Zeta Potential (mV) | PDI a | EE% b |
|---|---|---|---|---|
| Lip | 93.12 ± 5.31 | 25.26 ± 2.88 | 0.101 ± 0.023 | - |
| Lip-Epi | 99.34 ± 2.26 | 26.32 ± 2.56 | 0.124 ± 0.027 | 91.26 ± 3.09 |
| Lip-TH1-5 | 100.89 ± 2.67 | 27.78 ± 2.35 | 0.152 ± 0.087 | 88.32 ± 2.15 |
| Lip-Epi+TH1-5 | 101.1 ± 4.67 | 30.66 ± 3.16 | 0.168 ± 0.089 | 87.28 ± 2.53 |
2.1.2. Epi and TH1-5 in PEGylated Liposomes Considerably Increased Epi Cytotoxicity



2.1.3. PEGylated Liposomal Epi and TH1-5 Enhanced ROS Generation in SCC15 and NT2D1 Cells

2.1.4. PEGylated Liposomal Epi and TH1-5 Diminished the mRNA Levels of ABC Transporters

2.1.5. PEGylated Liposomal TH1-5 Enhanced the Cellular Uptake of Epi into Cancer Cells

2.1.6. Epi and TH1-5 Encapsulated in PEGylated Liposomes Decreased the Mitochondrial Membrane Potential of SCC15 and NT2D1 Cells

2.1.7. Liposomal or Free TH1-5 and/or Epi Treatment Remarkably Enhanced Apoptosis of SCC15 and NT2D1 Cells


2.1.8. PEGylated Liposomal Epi and TH1-5 Modified mRNA Expressions of p53, Bax, and Bcl-2

2.1.9. PEGylated Liposomal Epi and TH1-5 Changed the mRNA Expressions and Activity Levels of Caspases

2.1.10. FREE or Liposomal TH1-5 and/or Epi Treatments Gave Rise to Morphological Changes Observed by Fluorescence Microscope


2.2. Discussion
3. Experimental Section
3.1. Materials
3.2. Cell Culture
3.3. Preparation of PEGylated Cationic Liposomal Formulations
3.4. Determination of Size Distribution, Zeta Potential, and Encapsulation Efficiency (EE%)
3.5. Cell Viability Assay
3.6. Measurement of Intracellular Hydrogen Peroxide and Superoxide Levels
3.7. Real-Time Quantitative PCR of MDR1, MRP1, MRP2, Bax, Bcl-2, and Caspases
3.8. Functional Assay of ABC Transporters
3.9. Determination of Mitochondrial Membrane Potential with 3,3′-Dihexyloxacarbocyanine Iodide (DiOC6)
3.10. Cell Cycle Analysis
3.11. Annexin V FITC Apoptosis Detection Assay
3.12. Caspases 3, 8, and 9 Activity Assay
3.13. Chromatin Condensation Imaged by Fluorescence Microscopy
3.14. Statistical Analyses
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Wang, Y.D.; Rajanbabu, V.; Chen, J.Y. Transcriptome analysis of medaka following epinecidin-1 and TH1–5 treatment of NNV infection. Fish Shellfish Immunol. 2015, 42, 121–131. [Google Scholar] [CrossRef] [PubMed]
- Ting, C.H.; Huang, H.N.; Huang, T.C.; Wu, C.J.; Chen, J.Y. The mechanisms by which pardaxin, a natural cationic antimicrobial peptide, targets the endoplasmic reticulum and induces c-FOS. Biomaterials 2014, 35, 3627–3640. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Hu, J.; Zeng, P.; Pan, F.; Yaseen, M.; Xu, H.; Lu, J.R. Molecular mechanisms of anticancer action and cell selectivity of short alpha-helical peptides. Biomaterials 2014, 35, 1552–1561. [Google Scholar] [CrossRef] [PubMed]
- Fadnes, B.; Uhlin-Hansen, L.; Lindin, I.; Rekdal, O. Small lytic peptides escape the inhibitory effect of heparan sulfate on the surface of cancer cells. BMC Cancer 2011, 11, 116. [Google Scholar] [CrossRef] [PubMed]
- Pan, C.Y.; Lin, C.N.; Chiou, M.T.; Yu, C.Y.; Chen, J.Y.; Chien, C.H. The antimicrobial peptide pardaxin exerts potent anti-tumor activity against canine perianal gland adenoma. Oncotarget 2015, 6, 2290–2301. [Google Scholar] [CrossRef] [PubMed]
- Oyinloye, B.E.; Adenowo, A.F.; Kappo, A.P. Reactive oxygen species, apoptosis, antimicrobial peptides and human inflammatory diseases. Pharmaceuticals 2015, 8, 151–175. [Google Scholar] [CrossRef] [PubMed]
- Suttmann, H.; Retz, M.; Paulsen, F.; Harder, J.; Zwergel, U.; Kamradt, J.; Wullich, B.; Unteregger, G.; Stockle, M.; Lehmann, J. Antimicrobial peptides of the Cecropin-family show potent antitumor activity against bladder cancer cells. BMC Urol. 2008, 8, 5. [Google Scholar] [CrossRef] [PubMed]
- Huang, P.H.; Chen, J.Y.; Kuo, C.M. Three different hepcidins from tilapia, Oreochromis mossambicus: Analysis of their expressions and biological functions. Mol. Immunol. 2007, 44, 1922–1934. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.H.; Yang, Y.L.; Tiao, M.M.; Kuo, H.C.; Huang, L.T.; Chuang, J.H. Hepcidin protects against lipopolysaccharide-induced liver injury in a mouse model of obstructive jaundice. Peptides 2012, 35, 212–217. [Google Scholar] [CrossRef] [PubMed]
- Lee, P.L.; Beutler, E. Regulation of hepcidin and iron-overload disease. Annu. Rev. Pathol. 2009, 4, 489–515. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.N.; Rajanbabu, V.; Pan, C.Y.; Chan, Y.L.; Hui, C.F.; Chen, J.Y.; Wu, C.J. Modulation of the immune-related gene responses to protect mice against Japanese encephalitis virus using the antimicrobial peptide, tilapia hepcidin 1–5. Biomaterials 2011, 32, 6804–6814. [Google Scholar] [CrossRef] [PubMed]
- Pan, C.Y.; Peng, K.C.; Lin, C.H.; Chen, J.Y. Transgenic expression of tilapia hepcidin 1–5 and shrimp chelonianin in zebrafish and their resistance to bacterial pathogens. Fish Shellfish Immunol. 2011, 31, 275–285. [Google Scholar] [CrossRef] [PubMed]
- Chang, T.W.; Lin, Y.M.; Wang, C.F.; Liao, Y.D. Outer membrane lipoprotein Lpp is Gram-negative bacterial cell surface receptor for cationic antimicrobial peptides. J. Biol. Chem. 2012, 287, 418–428. [Google Scholar] [CrossRef] [PubMed]
- Chang, W.T.; Pan, C.Y.; Rajanbabu, V.; Cheng, C.W.; Chen, J.Y. Tilapia (Oreochromis mossambicus) antimicrobial peptide, hepcidin 1–5, shows antitumor activity in cancer cells. Peptides 2011, 32, 342–352. [Google Scholar] [CrossRef] [PubMed]
- Baguley, B.C. Multiple drug resistance mechanisms in cancer. Mol. Biotechnol. 2010, 46, 308–316. [Google Scholar] [CrossRef] [PubMed]
- Lo, Y.L.; Wang, W.; Ho, C.T. 7,3ʹ,4ʹ-Trihydroxyisoflavone modulates multidrug resistance transporters and induces apoptosis via production of reactive oxygen species. Toxicology 2012, 302, 221–232. [Google Scholar] [CrossRef] [PubMed]
- Yamanaka, K.; Rocchi, P.; Miyake, H.; Fazli, L.; So, A.; Zangemeister-Wittke, U.; Gleave, M.E. Induction of apoptosis and enhancement of chemosensitivity in human prostate cancer LNCaP cells using bispecific antisense oligonucleotide targeting Bcl-2 and Bcl-xL genes. BJU Int. 2006, 97, 1300–1308. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Wang, C.; Qin, Y.W.; Yan, S.K.; Gao, Y.R. Simultaneous suppression of multidrug resistance and antiapoptotic cellular defense induces apoptosis in chemoresistant human acute myeloid leukemia cells. Leuk. Res. 2007, 31, 989–994. [Google Scholar] [CrossRef] [PubMed]
- Mangoni, M.L. Host-defense peptides: From biology to therapeutic strategies. Cell. Mol. Life Sci. 2011, 68, 2157–2159. [Google Scholar] [CrossRef] [PubMed]
- Lin, M.C.; Lin, S.B.; Chen, J.C.; Hui, C.F.; Chen, J.Y. Shrimp anti-lipopolysaccharide factor peptide enhances the antitumor activity of cisplatin in vitro and inhibits HeLa cells growth in nude mice. Peptides 2010, 31, 1019–1025. [Google Scholar] [CrossRef] [PubMed]
- Jin, S.; Zhang, Q.Y.; Kang, X.M.; Wang, J.X.; Zhao, W.H. Daidzein induces MCF-7 breast cancer cell apoptosis via the mitochondrial pathway. Ann. Oncol. 2010, 21, 263–268. [Google Scholar] [CrossRef] [PubMed]
- Kanamori, Y.; Murakami, M.; Matsui, T.; Funaba, M. The regulation of hepcidin expression by serum treatment: Requirements of the BMP response element and STAT- and AP-1-binding sites. Gene 2014, 551, 119–126. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hoskin, D.W.; Ramamoorthy, A. Studies on anticancer activities of antimicrobial peptides. Biochim. Biophys. Acta 2008, 1778, 357–375. [Google Scholar] [CrossRef] [PubMed]
- Simon, P.O., Jr.; McDunn, J.E.; Kashiwagi, H.; Chang, K.; Goedegebuure, P.S.; Hotchkiss, R.S.; Hawkins, W.G. Targeting AKT with the proapoptotic peptide, TAT-CTMP: A novel strategy for the treatment of human pancreatic adenocarcinoma. Int. J. Cancer 2009, 125, 942–951. [Google Scholar] [CrossRef] [PubMed]
- Mai, J.C.; Mi, Z.; Kim, S.H.; Ng, B.; Robbins, P.D. A proapoptotic peptide for the treatment of solid tumors. Cancer Res. 2001, 61, 7709–7712. [Google Scholar] [PubMed]
- Joshi, G.; Hardas, S.; Sultana, R.; St Clair, D.K.; Vore, M.; Butterfield, D.A. Glutathione elevation by gamma-glutamyl cysteine ethyl ester as a potential therapeutic strategy for preventing oxidative stress in brain mediated by in vivo administration of adriamycin: Implication for chemobrain. J. Neurosci. Res. 2007, 85, 497–503. [Google Scholar] [CrossRef] [PubMed]
- Wartenberg, M.; Gronczynska, S.; Bekhite, M.M.; Saric, T.; Niedermeier, W.; Hescheler, J.; Sauer, H. Regulation of the multidrug resistance transporter P-glycoprotein in multicellular prostate tumor spheroids by hyperthermia and reactive oxygen species. Int. J. Cancer 2005, 113, 229–240. [Google Scholar] [CrossRef] [PubMed]
- Pandey, V.; Chaube, B.; Bhat, M.K. Hyperglycemia regulates MDR-1, drug accumulation and ROS levels causing increased toxicity of carboplatin and 5-fluorouracil in MCF-7 cells. J. Cell. Biochem. 2011, 112, 2942–2952. [Google Scholar] [CrossRef] [PubMed]
- Lo, Y.L.; Wang, W. Formononetin potentiates epirubicin-induced apoptosis via ROS production in HeLa cells in vitro. Chem. Biol. Interact. 2013, 205, 188–197. [Google Scholar] [CrossRef] [PubMed]
- Hwang, B.; Hwang, J.S.; Lee, J.; Lee, D.G. The antimicrobial peptide, psacotheasin induces reactive oxygen species and triggers apoptosis in Candida albicans. Biochem. Biophys. Res. Commun. 2011, 405, 267–271. [Google Scholar] [CrossRef] [PubMed]
- Cho, J.; Lee, D.G. The antimicrobial peptide arenicin-1 promotes generation of reactive oxygen species and induction of apoptosis. Biochim. Biophys. Acta 2011, 1810, 1246–1251. [Google Scholar] [CrossRef] [PubMed]
- Cho, J.; Lee, D.G. Oxidative stress by antimicrobial peptide pleurocidin triggers apoptosis in Candida albicans. Biochimie 2011, 93, 1873–1879. [Google Scholar] [CrossRef] [PubMed]
- Changchien, J.J.; Chen, Y.J.; Huang, C.H.; Cheng, T.L.; Lin, S.R.; Chang, L.S. Quinacrine induces apoptosis in human leukemia K562 cells via p38 MAPK-elicited BCL2 down-regulation and suppression of ERK/c-Jun-mediated BCL2L1 expression. Toxicol. Appl. Pharmacol. 2015, 284, 33–41. [Google Scholar] [CrossRef] [PubMed]
- Checker, R.; Gambhir, L.; Sharma, D.; Kumar, M.; Sandur, S.K. Plumbagin induces apoptosis in lymphoma cells via oxidative stress mediated glutathionylation and inhibition of mitogen-activated protein kinase phosphatases (MKP1/2). Cancer Lett. 2015, 357, 265–278. [Google Scholar] [CrossRef] [PubMed]
- Li, S.D.; Huang, L. Targeted delivery of antisense oligodeoxynucleotide and small interference RNA into lung cancer cells. Mol. Pharm. 2006, 3, 579–588. [Google Scholar] [CrossRef] [PubMed]
- Lo, Y.L.; Liu, Y. Reversing multidrug resistance in Caco-2 by silencing MDR1, MRP1, MRP2, and BCL-2/BCL-xL using liposomal antisense oligonucleotides. PLoS ONE 2014, 9, 90180. [Google Scholar] [CrossRef] [PubMed]
- Lo, Y.L. Relationships between the hydrophilic-lipophilic balance values of pharmaceutical excipients and their multidrug resistance modulating effect in Caco-2 cells and rat intestines. J. Control. Release 2003, 90, 37–48. [Google Scholar] [CrossRef]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lo, Y.-L.; Lee, H.-P.; Tu, W.-C. The Use of a Liposomal Formulation Incorporating an Antimicrobial Peptide from Tilapia as a New Adjuvant to Epirubicin in Human Squamous Cell Carcinoma and Pluripotent Testicular Embryonic Carcinoma Cells. Int. J. Mol. Sci. 2015, 16, 22711-22734. https://doi.org/10.3390/ijms160922711
Lo Y-L, Lee H-P, Tu W-C. The Use of a Liposomal Formulation Incorporating an Antimicrobial Peptide from Tilapia as a New Adjuvant to Epirubicin in Human Squamous Cell Carcinoma and Pluripotent Testicular Embryonic Carcinoma Cells. International Journal of Molecular Sciences. 2015; 16(9):22711-22734. https://doi.org/10.3390/ijms160922711
Chicago/Turabian StyleLo, Yu-Li, Hsin-Pin Lee, and Wei-Chen Tu. 2015. "The Use of a Liposomal Formulation Incorporating an Antimicrobial Peptide from Tilapia as a New Adjuvant to Epirubicin in Human Squamous Cell Carcinoma and Pluripotent Testicular Embryonic Carcinoma Cells" International Journal of Molecular Sciences 16, no. 9: 22711-22734. https://doi.org/10.3390/ijms160922711
APA StyleLo, Y.-L., Lee, H.-P., & Tu, W.-C. (2015). The Use of a Liposomal Formulation Incorporating an Antimicrobial Peptide from Tilapia as a New Adjuvant to Epirubicin in Human Squamous Cell Carcinoma and Pluripotent Testicular Embryonic Carcinoma Cells. International Journal of Molecular Sciences, 16(9), 22711-22734. https://doi.org/10.3390/ijms160922711