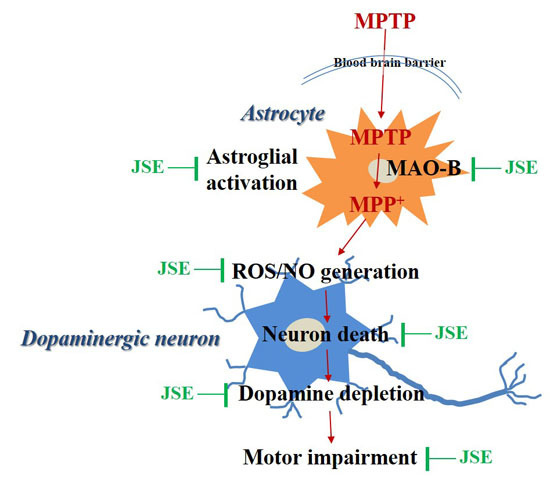
In Vitro and in Vivo Neuroprotective Effects of Walnut (Juglandis Semen) in Models of Parkinson’s Disease
Abstract
:1. Introduction
2. Results
2.1. Determination of Caffeic Acid in Extract of Juglandis Semen (JSE) Using High Performance Liquid Chromatography (HPLC)

2.2. Effects of JSE on Monoamine Oxidase B (MAO-B) Activity in Vitro
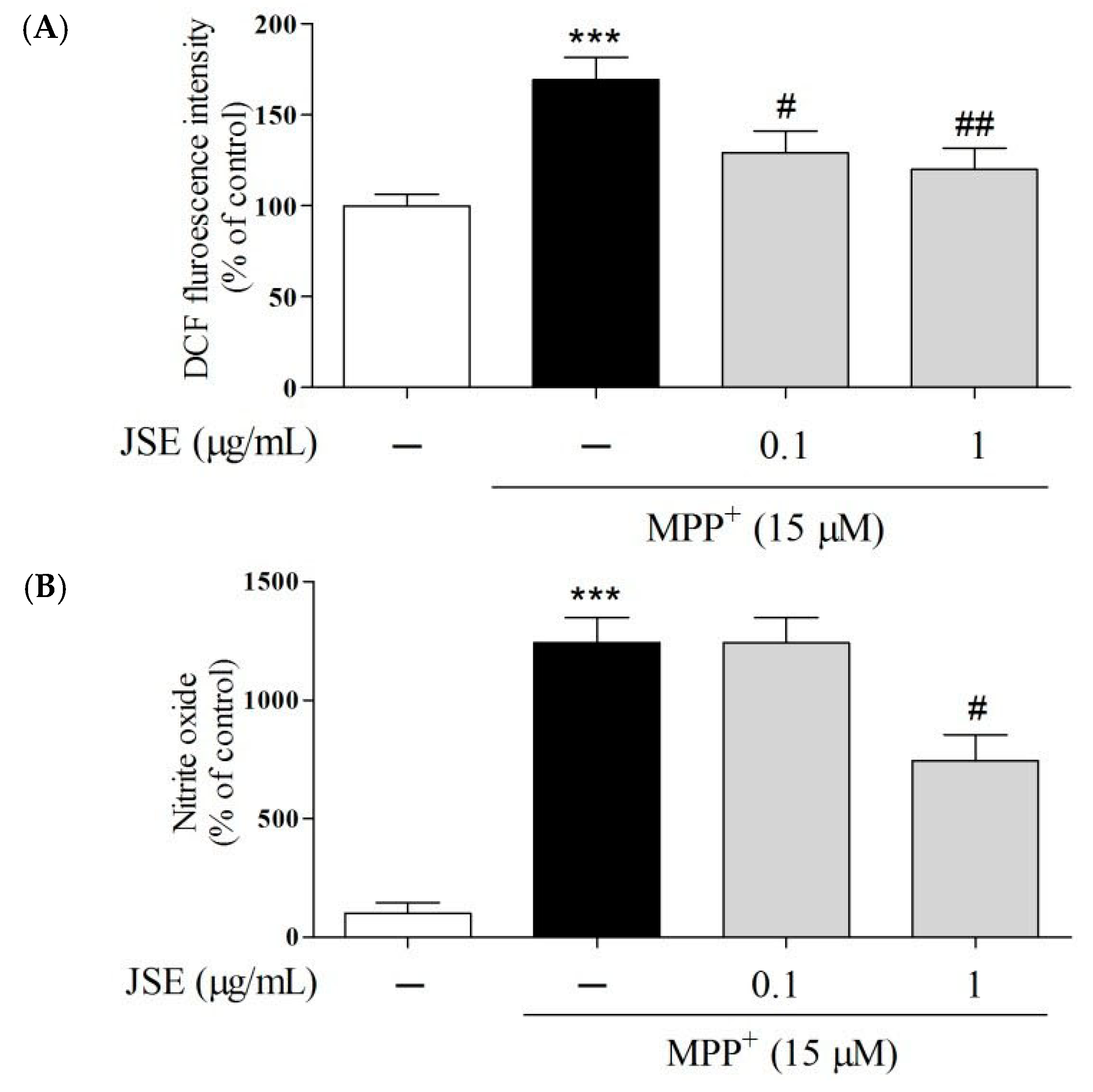
2.3. Effects of JSE on 1-Methyl-4-phenylpyridinium (MPP+)-Induced Reactive Oxygen Species (ROS) and NO Generation in Vitro

2.4. Effects of JSE against MPP+ or 1-Methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP)-Induced Dopaminergic Cell Death in Vitro and in Vivo

2.5. Effects of JSE on MAO Activity in Vivo


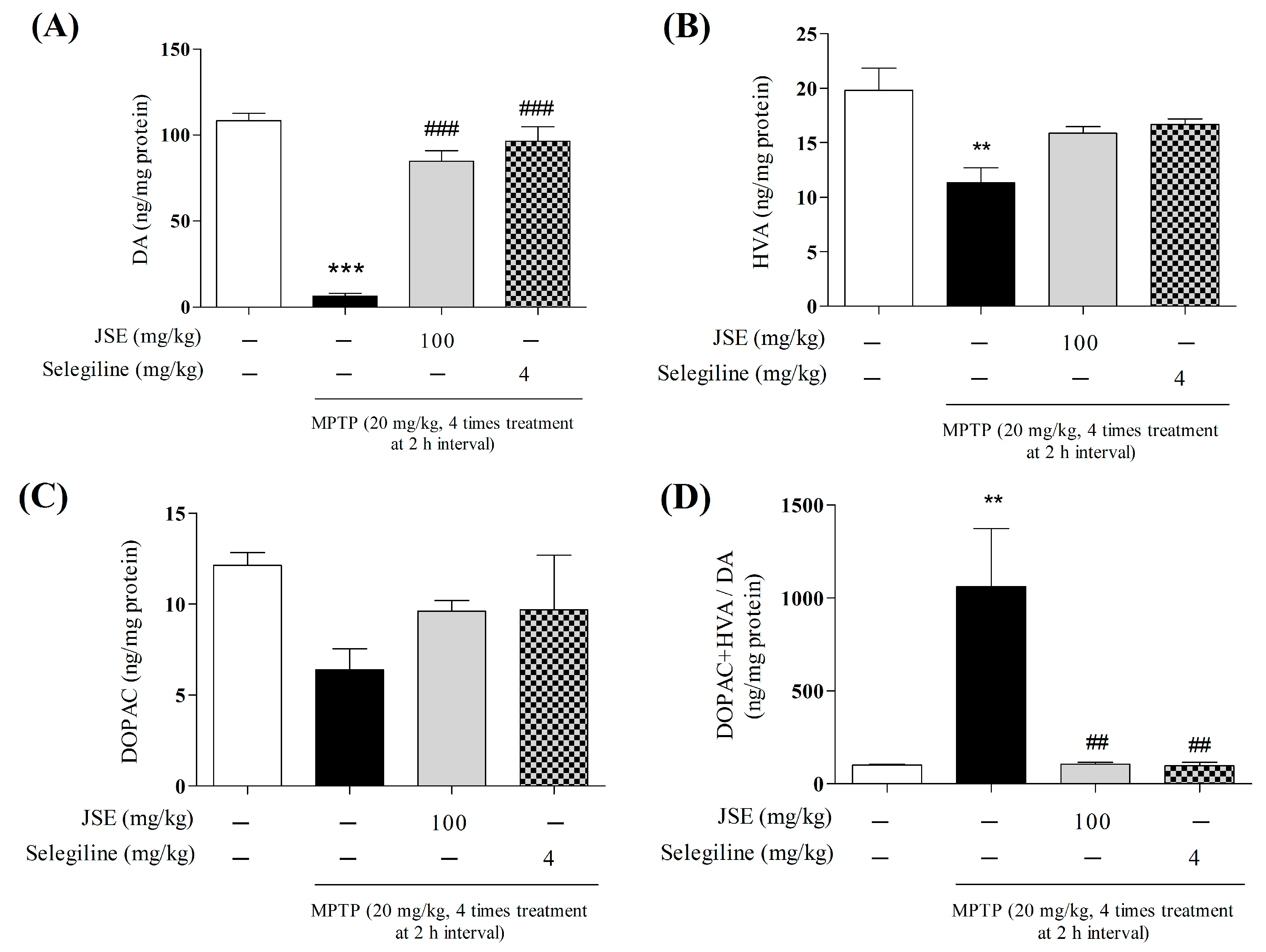
2.6. Effects of JSE against 1-Methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP)-Induced DA Availability in the ST of Mouse Brain
2.7. Effects of JSE against MPTP-Induced Locomotor Ataxia
2.8. Effects of JSE against MPTP-Induced Movement Impairment in the Pole Test
2.9. Effects of JSE against MPTP-Induced Movement Impairment in the Rotarod Test

3. Discussion
4. Experimental Section
4.1. Chemicals
4.2. Preparation of the JSE and Standardization
4.3. Primary Rat Mesencephalic Neuron/Glia Mixed Cultures and Treatment
4.4. Assessment of MAO-B Activity and Expressions Levels in in Vitro System
4.5. Assessment of Intracellular ROS and NO Generation
4.6. Animals
4.7. Drug Administration
4.8. Behavioral Evaluation
4.8.1. Locomotor Activity
4.8.2. Pole Test and Rotarod Test
4.8.3. Brain Tissue Preparation
4.9. Measurement of Anti-Tyrosine Hydroxylase (TH)-Positive Dopaminergic Neurons and GFAP/MAO-B Co-Localizaiton
4.10. Assessment of Activity of MAO Isoforms in the in Vivo System
4.11. Measurement of Neurotransmitter Levels by HPLC
4.12. Statistical Analysis
5. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Dawson, T.M.; Dawson, V.L. Molecular pathways of neurodegeneration in Parkinson’s disease. Science 2003, 302, 819–822. [Google Scholar] [CrossRef] [PubMed]
- Moore, D.J.; Dawson, T.M. Value of genetic models in understanding the cause and mechanisms of Parkinson’s disease. Curr. Neurol. Neurosci. Rep. 2008, 8, 288–296. [Google Scholar] [CrossRef] [PubMed]
- Leegwater-Kim, J.; Bortan, E. The role of rasagiline in the treatment of Parkinson’s disease. Clin. Interv. Aging 2010, 5, 149–156. [Google Scholar] [CrossRef] [PubMed]
- Chen, K.; Wu, H.F.; Shih, J.C. The deduced amino acid sequences of human platelet and frontal cortex monoamine oxidase b are identical. J. Neurochem. 1993, 61, 187–190. [Google Scholar] [CrossRef] [PubMed]
- Siddiqui, A.; Mallajosyula, J.K.; Rane, A.; Andersen, J.K. Ability to delay neuropathological events associated with astrocytic mao-b increase in a parkinsonian mouse model: Implications for early intervention on disease progression. Neurobiol. Dis. 2010, 40, 444–448. [Google Scholar] [CrossRef] [PubMed]
- Tatton, W.; Chalmers-Redman, R.; Tatton, N. Neuroprotection by deprenyl and other propargylamines: Glyceraldehyde-3-phosphate dehydrogenase rather than monoamine oxidase b. J. Neural Transm. 2003, 110, 509–515. [Google Scholar] [CrossRef] [PubMed]
- Mallajosyula, J.K.; Kaur, D.; Chinta, S.J.; Rajagopalan, S.; Rane, A.; Nicholls, D.G.; di Monte, D.A.; Macarthur, H.; Andersen, J.K. MAO-B elevation in mouse brain astrocytes results in Parkinson’s pathology. PLoS ONE 2008, 3, e1616. [Google Scholar] [CrossRef] [PubMed]
- Damier, P.; Kastner, A.; Agid, Y.; Hirsch, E.C. Does monoamine oxidase type b play a role in dopaminergic nerve cell death in Parkinson’s disease? Neurology 1996, 46, 1262–1269. [Google Scholar] [CrossRef] [PubMed]
- Nagatsu, T.; Sawada, M. Molecular mechanism of the relation of monoamine oxidase b and its inhibitors to Parkinson’s disease: Possible implications of glial cells. J. Neural Transm. Suppl. 2006, 71, 53–65. [Google Scholar] [PubMed]
- Schapira, A.H. Monoamine oxidase b inhibitors for the treatment of Parkinson’s disease: A review of symptomatic and potential disease-modifying effects. CNS Drugs 2011, 25, 1061–1071. [Google Scholar] [CrossRef] [PubMed]
- Weinreb, O.; Amit, T.; Bar-Am, O.; Youdim, M.B. Rasagiline: A novel anti-parkinsonian monoamine oxidase-b inhibitor with neuroprotective activity. Prog. Neurobiol. 2010, 92, 330–344. [Google Scholar] [CrossRef] [PubMed]
- Naoi, M.; Maruyama, W.; Inaba-Hasegawa, K. Revelation in the neuroprotective functions of rasagiline and selegiline: The induction of distinct genes by different mechanisms. Expert Rev. Neurother. 2013, 13, 671–684. [Google Scholar] [CrossRef] [PubMed]
- Tieu, K. A guide to neurotoxic animal models of Parkinson’s disease. Cold Spring Harb. Perspect. Med. 2011, 1. [Google Scholar] [CrossRef] [PubMed]
- Fritz, R.R.; Abell, C.W.; Patel, N.T.; Gessner, W.; Brossi, A. Metabolism of the neurotoxin in MPTP by human liver monoamine oxidase b. FEBS Lett. 1985, 186, 224–228. [Google Scholar] [CrossRef]
- Kitayama, S.; Mitsuhata, C.; Davis, S.; Wang, J.B.; Sato, T.; Morita, K.; Uhl, G.R.; Dohi, T. MPP+ toxicity and plasma membrane dopamine transporter: Study using cell lines expressing the wild-type and mutant rat dopamine transporters. Biochim. Biophys. Acta 1998, 1404, 305–313. [Google Scholar] [CrossRef]
- Brooks, W.J.; Jarvis, M.F.; Wagner, G.C. Astrocytes as a primary locus for the conversion MPTP into MPP+. J. Neural Transm. 1989, 76, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Nicklas, W.J.; Vyas, I.; Heikkila, R.E. Inhibition of nadh-linked oxidation in brain mitochondria by 1-methyl-4-phenyl-pyridine, a metabolite of the neurotoxin, 1-methyl-4-phenyl-1,2,5,6-tetrahydropyridine. Life Sci. 1985, 36, 2503–2508. [Google Scholar] [CrossRef]
- Mizuno, Y.; Sone, N.; Saitoh, T. Effects of 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine and 1-methyl-4-phenylpyridinium ion on activities of the enzymes in the electron transport system in mouse brain. J. Neurochem. 1987, 48, 1787–1793. [Google Scholar] [CrossRef] [PubMed]
- Keane, P.C.; Kurzawa, M.; Blain, P.G.; Morris, C.M. Mitochondrial dysfunction in Parkinson’s disease. Parkinson's Dis. 2011, 2011. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Ni, Y.Y.; Liu, J.; Lu, J.W.; Wang, F.; Wu, X.L.; Gu, M.M.; Lu, Z.Y.; Wang, Z.G.; Ren, Z.H. Dopamine receptor 3 might be an essential molecule in 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine-induced neurotoxicity. BMC Neurosci. 2013, 14. [Google Scholar] [CrossRef] [PubMed]
- Singer, T.P.; Ramsay, R.R.; McKeown, K.; Trevor, A.; Castagnoli, N.E., Jr. Mechanism of the neurotoxicity of 1-methyl-4-phenylpyridinium (MPP+), the toxic bioactivation product of 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP). Toxicology 1988, 49, 17–23. [Google Scholar] [CrossRef]
- Hirsch, E.C.; Faucheux, B.A. Iron metabolism and Parkinson’s disease. Mov. Disord. 1998, 13 (Suppl. 1), 39–45. [Google Scholar] [PubMed]
- Gutteridge, J.M. Iron and oxygen radicals in brain. Ann. Neurol. 1992, 32 (Suppl. 1), S16–S21. [Google Scholar] [CrossRef] [PubMed]
- Papoutsi, Z.; Kassi, E.; Chinou, I.; Halabalaki, M.; Skaltsounis, L.A.; Moutsatsou, P. Walnut extract (Juglans regia L.) and its component ellagic acid exhibit anti-inflammatory activity in human aorta endothelial cells and osteoblastic activity in the cell line ks483. Br. J. Nutr. 2008, 99, 715–722. [Google Scholar] [CrossRef] [PubMed]
- Park, G.; Kim, H.G.; Hong, S.P.; Kim, S.Y.; Oh, M.S. Walnuts (seeds of Juglandis sinensis L.) protect human epidermal keratinocytes against UVB-induced mitochondria-mediated apoptosis through upregulation of ros elimination pathways. Skin Pharmacol. Physiol. 2014, 27, 132–140. [Google Scholar] [CrossRef] [PubMed]
- Anderson, K.J.; Teuber, S.S.; Gobeille, A.; Cremin, P.; Waterhouse, A.L.; Steinberg, F.M. Walnut polyphenolics inhibit in vitro human plasma and ldl oxidation. J. Nutr. 2001, 131, 2837–2842. [Google Scholar] [PubMed]
- Haque, R.; Bin-Hafeez, B.; Parvez, S.; Pandey, S.; Sayeed, I.; Ali, M.; Raisuddin, S. Aqueous extract of Walnut (Juglans regia L.) protects mice against cyclophosphamide-induced biochemical toxicity. Hum. Exp. Toxicol. 2003, 22, 473–480. [Google Scholar] [CrossRef] [PubMed]
- Mazzio, E.A.; Harris, N.; Soliman, K.F. Food constituents attenuate monoamine oxidase activity and peroxide levels in C6 astrocyte cells. Planta Medica 1998, 64, 603–606. [Google Scholar] [CrossRef] [PubMed]
- Ma, Z.; Wei, X.; Fontanilla, C.; Noelker, C.; Dodel, R.; Hampel, H.; Du, Y. Caffeic acid phenethyl ester blocks free radical generation and 6-hydroxydopamine-induced neurotoxicity. Life Sci. 2006, 79, 1307–1311. [Google Scholar] [CrossRef] [PubMed]
- Willis, L.M.; Bielinski, D.F.; Fisher, D.R.; Matthan, N.R.; Joseph, J.A. Walnut extract inhibits LPS-induced activation of BV-2 microglia via internalization of TLR4: Possible involvement of phospholipase D2. Inflammation 2010, 33, 325–333. [Google Scholar] [CrossRef] [PubMed]
- Asadi-Shekaari, M.; Kalantaripour, T.P.; Nejad, F.A.; Namazian, E.; Eslami, A. The anticonvulsant and neuroprotective effects of Walnuts on the neurons of rat brain cortex. Avicenna J. Med. Biotechnol. 2012, 4, 155–158. [Google Scholar] [PubMed]
- Carey, A.N.; Fisher, D.R.; Joseph, J.A.; Shukitt-Hale, B. The ability of Walnut extract and fatty acids to protect against the deleterious effects of oxidative stress and inflammation in hippocampal cells. Nutr. Neurosci. 2013, 16, 13–20. [Google Scholar] [CrossRef] [PubMed]
- Chauhan, N.; Wang, K.C.; Wegiel, J.; Malik, M.N. Walnut extract inhibits the fibrillization of amyloid β-protein, and also defibrillizes its preformed fibrils. Curr. Alzheimer Res. 2004, 1, 183–188. [Google Scholar] [CrossRef] [PubMed]
- Muthaiyah, B.; Essa, M.M.; Lee, M.; Chauhan, V.; Kaur, K.; Chauhan, A. Dietary supplementation of Walnuts improves memory deficits and learning skills in transgenic mouse model of Alzheimer’s disease. J. Alzheimer's Dis. 2014, 42, 1397–1405. [Google Scholar]
- Harfenist, M.; Joyner, C.T.; Mize, P.D.; White, H.L. Selective inhibitors of monoamine oxidase. 2. Arylamide sar. J. Med. Chem. 1994, 37, 2085–2089. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.G.; Park, G.; Piao, Y.; Kang, M.S.; Pak, Y.K.; Hong, S.P.; Oh, M.S. Effects of the root bark of paeonia suffruticosa on mitochondria-mediated neuroprotection in an MPTP-induced model of Parkinson’s disease. Food Chem. Toxicol. 2014, 65, 293–300. [Google Scholar] [CrossRef] [PubMed]
- Ju, M.S.; Kim, H.G.; Choi, J.G.; Ryu, J.H.; Hur, J.; Kim, Y.J.; Oh, M.S. Cassiae semen, a seed of cassia obtusifolia, has neuroprotective effects in Parkinson’s disease models. Food Chem. Toxicol. 2010, 48, 2037–2044. [Google Scholar] [CrossRef] [PubMed]
- Park, G.; Park, Y.J.; Yang, H.O.; Oh, M.S. Ropinirole protects against 1-methyl-4-phenyl-1, 2, 3, 6-tetrahydropyridine (MPTP)-induced neurotoxicity in mice via anti-apoptotic mechanism. Pharmacol. Biochem. Behav. 2013, 104, 163–168. [Google Scholar] [CrossRef] [PubMed]
- Stocchi, F.; Vacca, L.; Radicati, F.G. How to optimize the treatment of early stage Parkinson’s disease. Transl. Neurodegener. 2015, 4. [Google Scholar] [CrossRef] [PubMed]
- Ossig, C.; Reichmann, H. Treatment strategies in early and advanced parkinson disease. Neurol. Clin. 2015, 33, 19–37. [Google Scholar] [CrossRef] [PubMed]
- Mizuno, Y.; Kondo, T.; Kuno, S.; Nomoto, M.; Yanagisawa, N. Early addition of selegiline to L-Dopa treatment is beneficial for patients with parkinson disease. Clin. Neuropharmacol. 2010, 33, 1–4. [Google Scholar] [CrossRef] [PubMed]
- Marconi, S.; Zwingers, T. Comparative efficacy of selegiline vs. rasagiline in the treatment of early Parkinson’s disease. Eur. Rev. Med. Pharmacol. Sci. 2014, 18, 1879–1882. [Google Scholar] [PubMed]
- Caslake, R.; Macleod, A.; Ives, N.; Stowe, R.; Counsell, C. Monoamine oxidase B inhibitors vs. other dopaminergic agents in early Parkinson’s disease. Cochrane Database Syst. Rev. 2009, 4. [Google Scholar] [CrossRef]
- Zhao, Q.; Cai, D.; Bai, Y. Selegiline rescues gait deficits and the loss of dopaminergic neurons in a subacute MPTP mouse model of Parkinson’s disease. Int. J. Mol. Med. 2013, 32, 883–891. [Google Scholar] [CrossRef] [PubMed]
- Fox, S.H. Non-dopaminergic treatments for motor control in Parkinson’s disease. Drugs 2013, 73, 1405–1415. [Google Scholar] [CrossRef] [PubMed]
- Takahata, K.; Shimazu, S.; Katsuki, H.; Yoneda, F.; Akaike, A. Effects of selegiline on antioxidant systems in the nigrostriatum in rat. J. Neural Transm. 2006, 113, 151–158. [Google Scholar] [CrossRef] [PubMed]
- Kumar, M.J.; Andersen, J.K. Perspectives on MAO-B in aging and neurological disease: Where do we go from here? Mol. Neurobiol. 2004, 30, 77–89. [Google Scholar] [CrossRef]
- Chen, J.J.; Wilkinson, J.R. The monoamine oxidase type b inhibitor rasagiline in the treatment of parkinson disease: Is tyramine a challenge? J. Clin. Pharmacol. 2012, 52, 620–628. [Google Scholar] [CrossRef] [PubMed]
- Eruslanov, E.; Kusmartsev, S. Identification of ros using oxidized dcfda and flow-cytometry. Methods Mol. Biol. 2010, 594, 57–72. [Google Scholar] [PubMed]
- Meiser, J.; Weindl, D.; Hiller, K. Complexity of dopamine metabolism. Cell Commun. Signal. 2013, 11, 34. [Google Scholar] [CrossRef] [PubMed]
- Siddiqui, A.; Hanson, I.; Andersen, J.K. MAO-B elevation decreases Parkin’s ability to efficiently clear damaged mitochondria: Protective effects of rapamycin. Free Radic. Res. 2012, 46, 1011–1018. [Google Scholar] [CrossRef] [PubMed]
- Weidinger, A.; Kozlov, A.V. Biological activities of reactive oxygen and nitrogen species: Oxidative stress versus signal transduction. Biomolecules 2015, 5, 472–484. [Google Scholar] [CrossRef] [PubMed]
- Fujita, K.; Nakabeppu, Y.; Noda, M. Therapeutic effects of hydrogen in animal models of Parkinson’s disease. Parkinson's Dis. 2011, 2011. [Google Scholar] [CrossRef] [PubMed]
- White, H.L.; Scates, P.W.; Cooper, B.R. Extracts of ginkgo biloba leaves inhibit monoamine oxidase. Life Sci. 1996, 58, 1315–1321. [Google Scholar] [CrossRef]
- Ou, X.M.; Chen, K.; Shih, J.C. Monoamine oxidase A and repressor R1 are involved in apoptotic signaling pathway. Proc. Natl. Acad. Sci. USA 2006, 103, 10923–10928. [Google Scholar] [CrossRef] [PubMed]
- Yi, H.; Akao, Y.; Maruyama, W.; Chen, K.; Shih, J.; Naoi, M. Type A monoamine oxidase is the target of an endogenous dopaminergic neurotoxin, N-methyl(R)salsolinol, leading to apoptosis in SH-SY5Y cells. J. Neurochem. 2006, 96, 541–549. [Google Scholar] [CrossRef] [PubMed]
- Fitzgerald, J.C.; Ufer, C.; Billett, E.E. A link between monoamine oxidase-A and apoptosis in serum deprived human SH-SY5Y neuroblastoma cells. J. Neural Transm. 2007, 114, 807–810. [Google Scholar] [CrossRef] [PubMed]
- Michael, J.K.; Pastor, R.C.; Philip, D.L. Biosynthesis of Catecholamines, 6th ed.; Lippincott-Raven Publisher: Philadelphia, PA, USA, 1999. [Google Scholar]
- Kopin, I.J.; White, J.H.; Bankiewicz, K. A new approach to biochemical evaluation of brain dopamine metabolism. Cell. Mol. Neurobiol. 1988, 8, 171–179. [Google Scholar] [CrossRef] [PubMed]
- Sedelis, M.; Hofele, K.; Auburger, G.W.; Morgan, S.; Huston, J.P.; Schwarting, R.K. MPTP susceptibility in the mouse: Behavioral, neurochemical, and histological analysis of gender and strain differences. Behav. Genet. 2000, 30, 171–182. [Google Scholar] [CrossRef] [PubMed]
- Piggott, M.A.; Marshall, E.F.; Thomas, N.; Lloyd, S.; Court, J.A.; Jaros, E.; Costa, D.; Perry, R.H.; Perry, E.K. Dopaminergic activities in the human striatum: Rostrocaudal gradients of uptake sites and of D1 and D2 but not of D3 receptor binding or dopamine. Neuroscience 1999, 90, 433–445. [Google Scholar] [CrossRef]
- Matsuura, K.; Kabuto, H.; Makino, H.; Ogawa, N. Pole test is a useful method for evaluating the mouse movement disorder caused by striatal dopamine depletion. J. Neurosci. Methods 1997, 73, 45–48. [Google Scholar] [CrossRef]
- McIlwain, K.L.; Merriweather, M.Y.; Yuva-Paylor, L.A.; Paylor, R. The use of behavioral test batteries: Effects of training history. Physiol. Behav. 2001, 73, 705–717. [Google Scholar] [CrossRef]
- Shiotsuki, H.; Yoshimi, K.; Shimo, Y.; Funayama, M.; Takamatsu, Y.; Ikeda, K.; Takahashi, R.; Kitazawa, S.; Hattori, N. A rotarod test for evaluation of motor skill learning. J. Neurosci. Methods 2010, 189, 180–185. [Google Scholar] [CrossRef] [PubMed]
- Lakso, M.; Vartiainen, S.; Moilanen, A.M.; Sirvio, J.; Thomas, J.H.; Nass, R.; Blakely, R.D.; Wong, G. Dopaminergic neuronal loss and motor deficits in caenorhabditis elegans overexpressing human α-synuclein. J. Neurochem. 2003, 86, 165–172. [Google Scholar] [CrossRef] [PubMed]
- Essa, M.M.; Subash, S.; Dhanalakshmi, C.; Manivasagam, T.; Al-Adawi, S.; Guillemin, G.J.; Justin Thenmozhi, A. Dietary supplementation of Walnut partially reverses 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine induced neurodegeneration in a mouse model of Parkinson’s disease. Neurochem. Res. 2015, 40, 1283–1293. [Google Scholar] [CrossRef] [PubMed]
- Maraldi, T.; Vauzour, D.; Angeloni, C. Dietary polyphenols and their effects on cell biochemistry and pathophysiology 2013. Oxid. Med. Cell. Longev. 2014, 2014, 576363. [Google Scholar] [CrossRef] [PubMed]
- Muthaiyah, B.; Essa, M.M.; Chauhan, V.; Chauhan, A. Protective effects of Walnut extract against amyloid β peptide-induced cell death and oxidative stress in pc12 cells. Neurochem. Res. 2011, 36, 2096–2103. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.G.; Ju, M.S.; Shim, J.S.; Kim, M.C.; Lee, S.H.; Huh, Y.; Kim, S.Y.; Oh, M.S. Mulberry fruit protects dopaminergic neurons in toxin-induced Parkinson’s disease models. Br. J. Nutr. 2010, 104, 8–16. [Google Scholar] [CrossRef] [PubMed]
- Holt, A.; Sharman, D.F.; Baker, G.B.; Palcic, M.M. A continuous spectrophotometric assay for monoamine oxidase and related enzymes in tissue homogenates. Anal. Biochem. 1997, 244, 384–392. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.G.; Kim, T.M.; Park, G.; Lee, T.H.; Oh, M.S. Repeated heat exposure impairs nigrostriatal dopaminergic neurons in mice. Biol. Pharm. Bull. 2013, 36, 1556–1561. [Google Scholar] [CrossRef] [PubMed]
- Silver, H.; Youdim, M.B. MAO-A and MAO-B activities in rat striatum, frontal cortex and liver are unaltered after long-term treatment with fluvoxamine and desipramine. Eur. Neuropsychopharmacol. 2000, 10, 125–128. [Google Scholar] [CrossRef]
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Choi, J.G.; Park, G.; Kim, H.G.; Oh, D.-S.; Kim, H.; Oh, M.S. In Vitro and in Vivo Neuroprotective Effects of Walnut (Juglandis Semen) in Models of Parkinson’s Disease. Int. J. Mol. Sci. 2016, 17, 108. https://doi.org/10.3390/ijms17010108
Choi JG, Park G, Kim HG, Oh D-S, Kim H, Oh MS. In Vitro and in Vivo Neuroprotective Effects of Walnut (Juglandis Semen) in Models of Parkinson’s Disease. International Journal of Molecular Sciences. 2016; 17(1):108. https://doi.org/10.3390/ijms17010108
Chicago/Turabian StyleChoi, Jin Gyu, Gunhyuk Park, Hyo Geun Kim, Dal-Seok Oh, Hocheol Kim, and Myung Sook Oh. 2016. "In Vitro and in Vivo Neuroprotective Effects of Walnut (Juglandis Semen) in Models of Parkinson’s Disease" International Journal of Molecular Sciences 17, no. 1: 108. https://doi.org/10.3390/ijms17010108
APA StyleChoi, J. G., Park, G., Kim, H. G., Oh, D.-S., Kim, H., & Oh, M. S. (2016). In Vitro and in Vivo Neuroprotective Effects of Walnut (Juglandis Semen) in Models of Parkinson’s Disease. International Journal of Molecular Sciences, 17(1), 108. https://doi.org/10.3390/ijms17010108