Lung Regeneration: Endogenous and Exogenous Stem Cell Mediated Therapeutic Approaches
Abstract
:1. Introduction
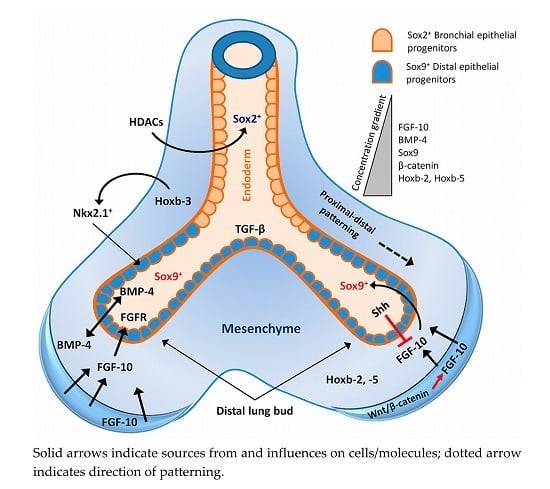
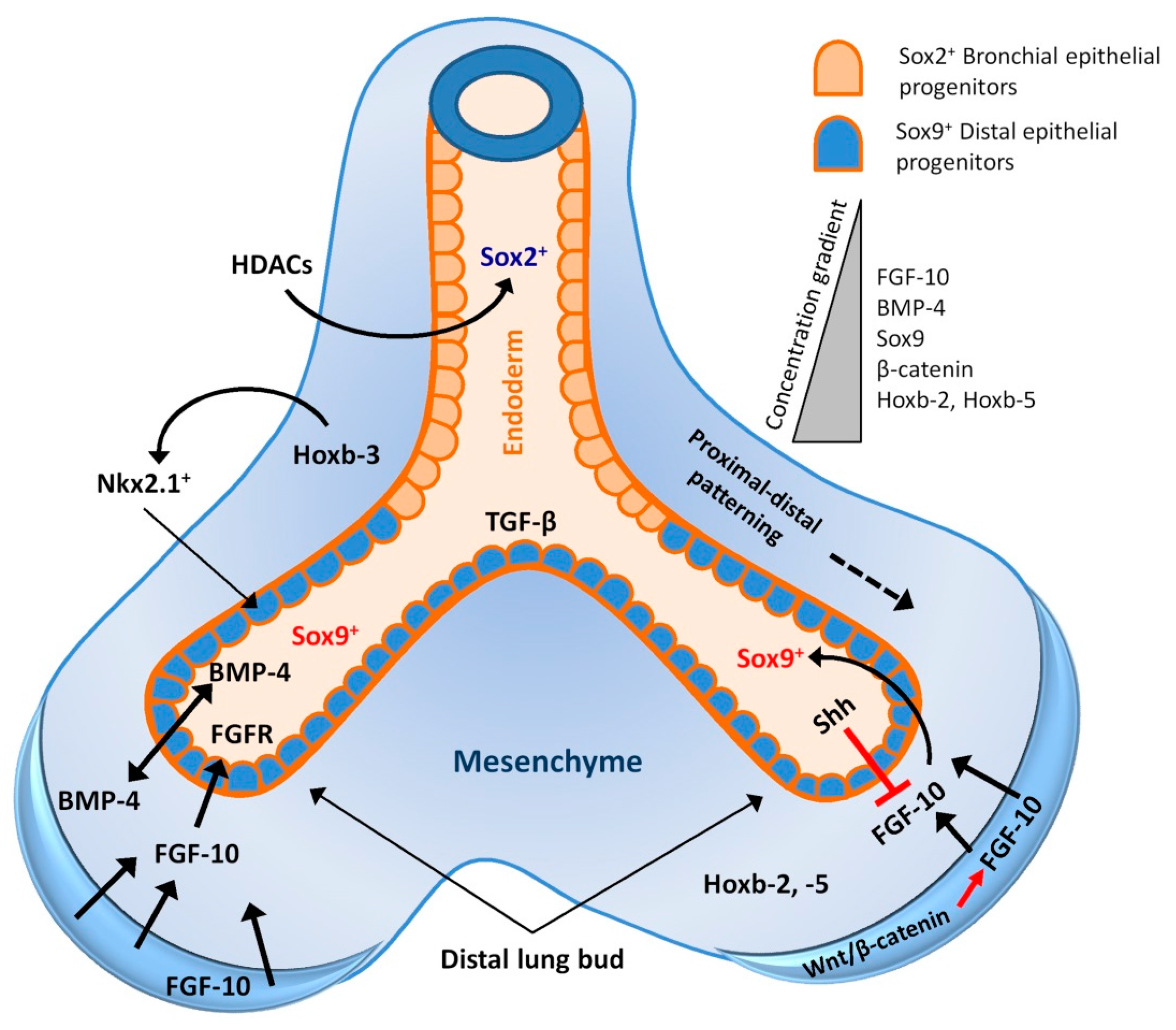
2. Lung Development and Associated Signalling Pathways
3. Endogenous Stem Cell Population of the Respiratory System
3.1. Trachea and Proximal Airways Stem Cells
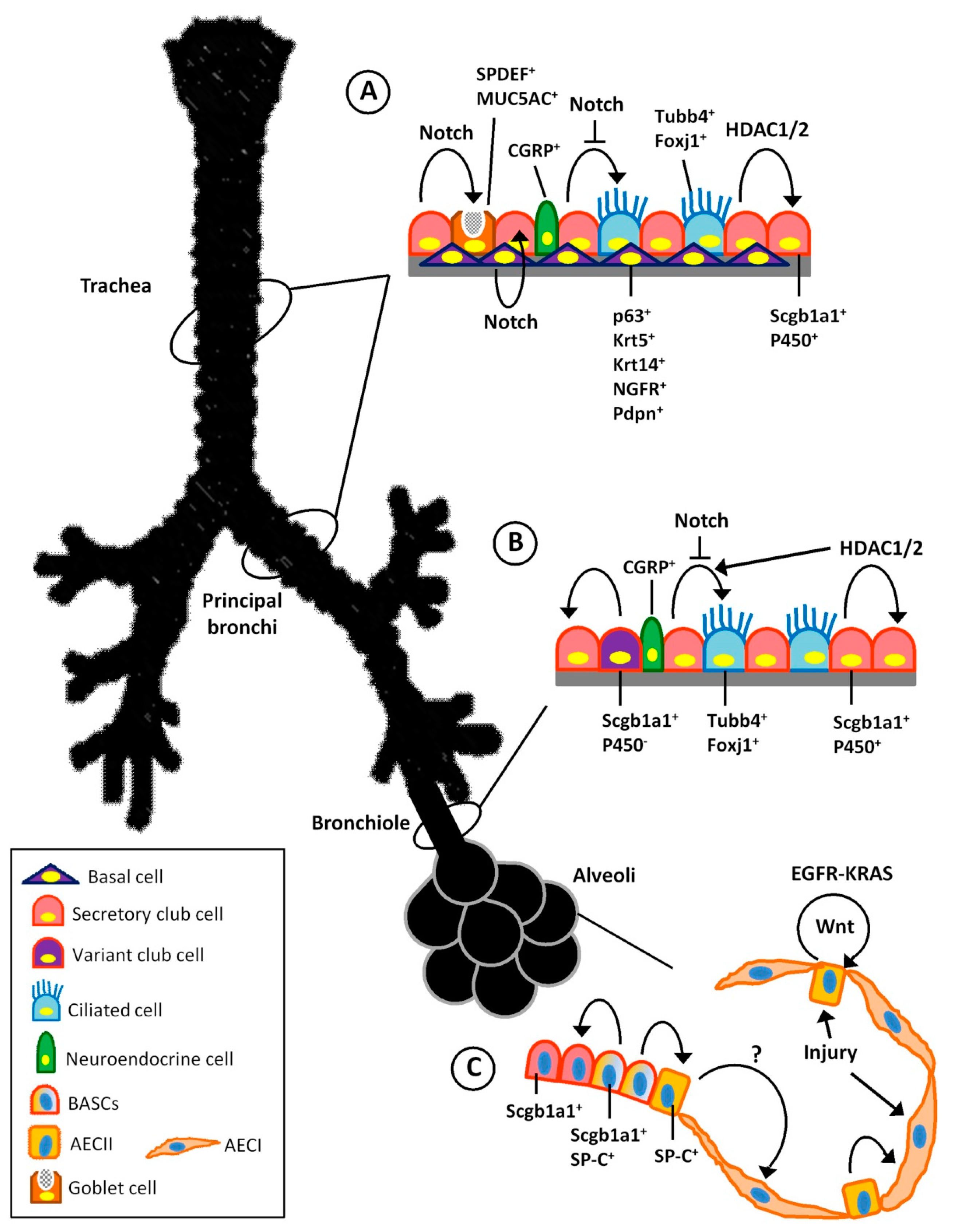
3.2. Distal Airway Stem Cells
3.3. Stem Cells of the Alveolar Region
3.4. Lung Mesenchymal Stem Cells
4. Signalling Pathways Involved in Postnatal Lung Injury Repair and Regeneration
4.1. Wnt Signalling
4.2. Notch Signalling
4.3. Histone Deacetylases (HDACs) Signalling
4.4. miRNAs and lncRNAs Regulation
5. Effects of Matrix Components in Lung Regeneration
6. Exogenous Stem Cell Interventions in Regenerative Therapies
6.1. Differentiation of ESCs and iPSCs into Pulmonary Epithelium
6.2. MSC-Mediated Regenerative Therapies
7. Stem Cells in Lung Tissue Engineering
8. Conclusions
Acknowledgments
Conflicts of Interest
References
- Beers, M.F.; Morrisey, E.E. The three R’s of lung health and disease: Repair, remodeling, and regeneration. J. Clin. Investig. 2011, 121, 2065–2073. [Google Scholar] [CrossRef] [PubMed]
- Wansleeben, C.; Barkauskas, C.E.; Rock, J.R.; Hogan, B.L. Stem cells of the adult lung: Their development and role in homeostasis, regeneration, and disease. Wiley Interdiscip. Rev. Dev. Biol. 2013, 2, 131–148. [Google Scholar] [CrossRef] [PubMed]
- Pillai, S.G.; Ge, D.; Zhu, G.; Kong, X.; Shianna, K.V.; Need, A.C.; Feng, S.; Hersh, C.P.; Bakke, P.; Gulsvik, A.; et al. ICGN Investigators. A genome-wide association study in chronic obstructive pulmonary disease (COPD): Identification of two major susceptibility loci. PLoS Genet. 2009, 5, e1000421. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Zhou, H.; Yang, J.; Xiao, J.; Liang, B.; Li, D.; Zhou, H.; Zeng, Q.; Fang, C.; Rao, Z.; et al. Association of HHIP polymorphisms with COPD and COPD-related phenotypes in a Chinese Han population. Gene 2013, 531, 101–105. [Google Scholar] [CrossRef] [PubMed]
- Wilk, J.B.; Chen, T.H.; Gottlieb, D.J.; Walter, R.E.; Nagle, M.W.; Brandler, B.J.; Myers, R.H.; Borecki, I.B.; Silverman, E.K.; Weiss, S.T.; et al. A genome-wide association study of pulmonary function measures in the Framingham heart study. PLoS Genet. 2009, 5, e1000429. [Google Scholar] [CrossRef] [PubMed]
- Kotton, D.N.; Morrisey, E.E. Lung regeneration: Mechanisms, applications and emerging stem cell populations. Nat. Med. 2014, 20, 822–832. [Google Scholar] [CrossRef] [PubMed]
- Boyden, E.A. Development and growth of the airways. In Development of the Lung; Hodson, W.A., Ed.; Marcel Dekker: New York, NY, USA, 1977; pp. 3–35. [Google Scholar]
- Ten Have-Opbroek, A.A. Lung development in the mouse embryo. Exp. Lung Res. 1991, 17, 111–130. [Google Scholar] [CrossRef] [PubMed]
- Loosli, C.G.; Potter, E.L. Pre- and postnatal development of the respiratory portion of the human lung. Am. Rev. Respir. Dis. 1959, 80, 5–23. [Google Scholar] [PubMed]
- Bucher, U.; Reid, L.M. Development of intrasegmental bronchial tree: The pattern of branching and development of cartilage at various stages of intra-uterine life. Thorax 1961, 16, 207–218. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Boyden, E.A. Development of human lung. In Brenneman’s Practice of Paediatrics; Kelley, V., Ed.; Harper & Row: Hagerstown, MD, USA, 1980; pp. 1–17. [Google Scholar]
- Langston, C.; Kida, K.; Reed, M.; Thurlbeck, W.M. Human lung growth in late gestation and in the neonate. Am. Rev. Respir. Dis. 1984, 129, 607–613. [Google Scholar] [PubMed]
- Warburton, D.; El-Hashash, A.; Carraro, G.; Tiozzo, C.; Sala, F.; Rogers, O.; de Langhe, S.; Kemp, P.J.; Riccardi, D.; Torday, J.; et al. Lung organogenesis. Curr. Top. Dev. Biol. 2010, 90, 73–158. [Google Scholar] [PubMed]
- Alescio, T.; Cassini, A. Induction in vitro of tracheal buds by pulmonary mesenchyme grafted on tracheal epithelium. J. Exp. Zool. 1962, 150, 83–94. [Google Scholar] [CrossRef] [PubMed]
- Spooner, B.S.; Wessells, N.K. Mammalian lung development: Interactions in primordium formation and bronchial morphogenesis. J. Exp. Zool. 1970, 175, 445–454. [Google Scholar] [CrossRef] [PubMed]
- Wessells, N.K. Mammalian lung development: Interactions in formation and morphogenesis of tracheal buds. J. Exp. Zool. 1970, 175, 455–466. [Google Scholar] [CrossRef] [PubMed]
- Hilfer, S.R.; Rayner, R.M.; Brown, J.W. Mesenchymal control of branching pattern in the fetal mouse lung. Tissue Cell 1985, 17, 523–538. [Google Scholar] [CrossRef]
- Nogawa, H.; Ito, T. Branching morphogenesis of embryonic mouse lung epithelium in mesenchyme-free culture. Development 1995, 121, 1015–1022. [Google Scholar] [PubMed]
- Deterding, R.R.; Shannon, J.M. Proliferation and differentiation of fetal rat pulmonary epithelium in the absence of mesenchyme. J. Clin. Investig. 1995, 95, 2963–2972. [Google Scholar] [CrossRef] [PubMed]
- Bellusci, S.; Grindley, J.; Emoto, H.; Itoh, N.; Hogan, B.L. Fibroblast growth factor 10 (FGF10) and branching morphogenesis in the embryonic mouse lung. Development 1997, 124, 4867–4878. [Google Scholar] [PubMed]
- Masters, J.R.W. Epithelial-mesenchymal interaction during lung development: The effect of mesenchymal mass. Dev. Biol. 1976, 51, 98–108. [Google Scholar] [CrossRef]
- Shannon, J.M. Induction of alveolar type II cell differentiation in fetal tracheal epithelium by grafted distal lung mesenchyme. Dev. Biol. 1994, 166, 600–614. [Google Scholar] [CrossRef] [PubMed]
- Shannon, J.M.; Nielson, L.D.; Gebb, S.A.; Randell, S.H. Mesenchyme specifies epithelial differentiation in reciprocal recombinants of embryonic lung and trachea. Dev. Dyn. 1998, 212, 482–494. [Google Scholar] [CrossRef]
- Deutsch, G.H.; Pinar, H. Prenatal lung development. In Chronic Obstructive Lung Diseases; Voelkel, N.F., MacNee, W., Eds.; BC Decker Inc.: London, UK, 2002; Chapter 2; pp. 1–14. [Google Scholar]
- Ayers, M.M.; Jeffery, P.K. Proliferation and differentiation in mammalian airway epithelium. Eur. Respir. J. 1988, 1, 58–80. [Google Scholar] [PubMed]
- Weaver, T.E.; Whitsett, J.A. Function and regulation of expression of pulmonary surfactant-associated proteins. Biochem. J. 1991, 273, 249–264. [Google Scholar] [CrossRef] [PubMed]
- Wert, S.; Glasser, S.; Korfhagen, T.; Whitsett, J.A. Transcriptional elements from the human SP-C gene direct expression in the primordial respiratory epithelium of transgenic mice. Dev. Biol. 1993, 156, 426–443. [Google Scholar] [CrossRef] [PubMed]
- Singh, G.; Singh, J.; Katyal, S.L.; Brown, W.E.; Kramps, J.A.; Paradis, I.L.; Dauber, J.H.; Macpherson, T.A.; Squeglia, N. Identification, cellular localization, isolation, and characterization of human Clara cell-specific 10 KD protein. J. Histochem. Cytochem. 1988, 36, 73–80. [Google Scholar] [CrossRef] [PubMed]
- Hackett, B.P.; Shimizu, N.; Gitlin, J.D. Clara cell secretory protein gene expression in bronchiolar epithelium. Am. J. Physiol. 1992, 262, L399–L404. [Google Scholar] [PubMed]
- Wuenschell, C.W.; Sunday, M.E.; Singh, G.; Minoo, P.; Slavkin, H.C.; Warburton, D. Embryonic mouse lung progenitor cells co-express immunohistochemical markers of diverse mature cell lineages. J. Histochem. Cytochem. 1996, 44, 113–123. [Google Scholar] [CrossRef] [PubMed]
- Guazzi, S.; Lonigro, R.; Pintonello, L.; Boncinelli, E.; di Lauro, R.; Mavilio, F. The thyroid transcription factor-1 gene is a candidate target for regulation by Hox proteins. EMBO J. 1994, 13, 3339–3347. [Google Scholar] [PubMed]
- Minoo, P.; Hamdan, H.; Bu, D.; Warburton, D.; Stepanik, P.; deLemos, R. TTF-1 regulates lung epithelial morphogenesis. Dev. Biol. 1995, 172, 694–698. [Google Scholar] [CrossRef] [PubMed]
- Kimura, S.; Yoshinobu, H.; Pineau, T.; Fernandez-Salguero, P.; Fox, C.H.; Ward, J.M.; Gonzalez, F.J. The T/EBP null mouse: Thyroid-specific enhancer-binding protein is essential for the organogenesis of the thyroid, lung, ventral forebrain, and pituitary. Genes Dev. 1996, 10, 60–69. [Google Scholar] [CrossRef] [PubMed]
- Minoo, P.; Su, G.; Drum, H.; Bringas, P.; Kimura, S. Defects in tracheoesophageal and lung morphogenesis in Nkx2.1(-/-) mouse embryos. Dev. Biol. 1999, 209, 60–71. [Google Scholar] [CrossRef] [PubMed]
- Bohinski, R.J.; DiLauro, R.; Whitsett, J.A. The lung-specific surfactant protein B gene promoter is a target for thyroid transcription factor 1 and hepatocyte nuclear factor 3, indicating common factors for organ-specific gene expression along the foregut axis. Mol. Cell. Biol. 1994, 14, 5671–5681. [Google Scholar] [CrossRef] [PubMed]
- Bruno, M.D.; Bohinski, R.J.; Huelsman, K.M.; Whitsett, J.A.; Korfhagen, T.R. Lung cell specific expression of the murine surfactant protein A (SP-A) gene is mediated by interactions between the SPA promoter and thyroid transcription factor-1. J. Biol. Chem. 1995, 270, 6531–6536. [Google Scholar] [PubMed]
- Yan, C.; Sever, Z.; Whitsett, J.A. Upstream enhancer activity in the surfactant protein B gene is mediated by thyroid transcription factor 1. J. Biol. Chem. 1995, 270, 24852–24857. [Google Scholar] [CrossRef] [PubMed]
- Kelly, S.E.; Bachurski, C.J.; Burhans, M.S.; Glasser, S.W. Transcription of the lung-specific surfactant protein C gene is mediated by thyroid transcription factor 1. J. Biol. Chem. 1996, 271, 6881–6888. [Google Scholar] [PubMed]
- Zhang, L.; Whitsett, J.A.; Stripp, B.R. Regulation of Clara cell secretory protein gene transcription by thyroid transcription factor-1. Biochim. Biophys. Acta 1997, 1350, 359–367. [Google Scholar] [CrossRef]
- Lazzaro, D.; Price, M.; DeFelice, M.; DiLauro, R. The transcription factor TTF-1 is expressed at the onset of thyroid and lung morphogenesis and in restricted regions of the foetal brain. Development 1991, 113, 1093–1104. [Google Scholar] [PubMed]
- Ikeda, K.; Clark, J.C.; Shaw-White, J.R.; Stahlman, M.T.; Boutell, C.J.; Whitsett, J.A. Gene structure and expression of human thyroid transcription factor-1 in respiratory epithelial cells. J. Biol. Chem. 1995, 270, 8108–8114. [Google Scholar] [CrossRef] [PubMed]
- Kimura, S.; Ward, J.M.; Minoo, P. Thyroid-specific enhancerbinding protein/thyroid transcription factor 1 is not required for the initial specification of the thyroid and lung primordia. Biochimie 1999, 81, 321–327. [Google Scholar] [CrossRef]
- Lai, E.; Clark, K.L.; Burley, S.K.; Darnell, J.E., Jr. Hepatocyte nuclear factor 3/forkhead or “winged helix” proteins: A family of transcription factors of diverse biologic function. Proc. Natl. Acad. Sci. USA 1993, 90, 10421–10423. [Google Scholar] [CrossRef] [PubMed]
- Ikeda, K.; Shaw-White, J.R.; Wert, S.E.; Whitsett, J.A. Hepatocyte nuclear factor 3 activates transcription of thyroid transcription factor 1 in respiratory epithelial cells. Mol. Cell. Biol. 1996, 16, 3626–3636. [Google Scholar] [CrossRef] [PubMed]
- Shaw-White, J.R.; Bruno, M.D.; Whitsett, J.A. GATA-6 activates transcription of thyroid transcription factor-1. J. Biol. Chem. 1999, 274, 2658–2664. [Google Scholar] [CrossRef] [PubMed]
- Monaghan, A.P.; Kaestner, K.H.; Grau, E.; Schutz, G. Postimplantation expression patterns indicate a role for the mouse forkhead/HNF-3 α, β and γ genes in determination of the definitive endoderm, chordamesoderm and neuroectoderm. Development 1993, 119, 563–578. [Google Scholar]
- Ang, S.-L.; Wierda, A.; Wong, D.; Stevens, K.A.; Cascio, S.; Rossant, J.; Zaret, K.S. The formation and maintenance of the definitive endoderm lineage in the mouse: Involvement of HNF-3/forkhead proteins. Development 1993, 119, 1301–1315. [Google Scholar] [PubMed]
- Kuo, C.T.; Morrisey, E.E.; Anandappa, R.; Sigrist, K.; Lu, M.M.; Parmacek, M.S.; Soudais, C.; Leiden, J.M. GATA4 transcription factor is required for ventral morphogenesis and heart tube formation. Genes Dev. 1997, 11, 1048–1060. [Google Scholar] [CrossRef] [PubMed]
- Alcorn, J.L.; Gao, E.; Chen, Q.; Smith, M.E.; Gerard, R.D.; Mendelson, C.R. Genomic elements involved in transcriptional regulation of the surfactant protein-A gene. Mol. Endocrinol. 1993, 7, 1072–1085. [Google Scholar] [PubMed]
- Bingle, C.D.; Hackett, B.P.; Moxley, M.; Longmore, W.; Gitlin, J.D. Role of HNF-3 α and HNF-3 β in Clara cell secretory protein gene expression in bronchiolar epithelium. Biochem. J. 1995, 308, 197–202. [Google Scholar] [CrossRef] [PubMed]
- Ang, S.-L.; Rossant, J. HNF-3B is essential for node and notochord formation in mouse development. Cell 1994, 78, 561–74. [Google Scholar] [CrossRef]
- Bogue, C.W.; Lou, L.J.; Vasavada, H.; Wilson, C.M.; Jacobs, H.C. Expression of Hoxb genes in the developing mouse foregut and lung. Am. J. Respir. Cell Mol. Biol. 1996, 15, 163–171. [Google Scholar] [CrossRef] [PubMed]
- Kappen, C. Hox genes in the lung. Am. J. Respir. Cell Mol. Biol. 1996, 15, 156–162. [Google Scholar] [CrossRef] [PubMed]
- Volpe, M.V.; Martin, A.; Vosatka, R.J.; Mazzoni, C.L.; Nielsen, H.C. Hoxb-5 expression in the developing mouse lung suggests a role in branching morphogenesis and epithelial cell fate. Histochem. Cell Biol. 1997, 108, 495–504. [Google Scholar] [CrossRef] [PubMed]
- Bogue, C.W.; Gross, I.; Vasavada, H.; Dynia, D.W.; Wilson, C.M.; Jacobs, H.C. Identification of Hox genes in newborn lung and effects of gestational age and retinoic acid on their expression. Am. J. Physiol. 1994, 266, L448–L454. [Google Scholar] [PubMed]
- Chinoy, M.R.; Volpe, M.V.; Cilley, R.E.; Zgleszewski, S.E.; Vosatka, R.J.; Martin, A.; Nielsen, H.C.; Krummel, T.M. Growth factors and dexamethasone regulate Hoxb5 protein in cultured murine fetal lungs. Am. J. Physiol. 1998, 274, L610–L620. [Google Scholar] [PubMed]
- Cardoso, W.V.; Williams, M.C.; Mitsialis, S.A.; Joyce-Brady, M.; Rishi, A.K.; Brody, J.S. Retinoic acid induces changes in the pattern of airway branching and alters epithelial cell differentiation in the developing lung in vitro. Am. J. Respir. Cell Mol. Biol. 1995, 12, 464–476. [Google Scholar] [CrossRef] [PubMed]
- Bellusci, S.; Henderson, R.; Winnier, G.; Oikawa, T.; Hogan, B.L. Evidence from normal expression and targeted misexpression that bone morphogenetic protein-4 (BMP-4) plays a role in mouse embryonic lung morphogenesis. Development 1996, 122, 1693–1702. [Google Scholar] [PubMed]
- Weaver, M.; Yingling, Y.M.; Dunn, N.R.; Bellusci, S.; Hogan, B.L. BMP signaling regulates proximal-distal differentiation of endoderm in mouse lung development. Development 1999, 126, 4005–4015. [Google Scholar] [PubMed]
- Metzger, R.J.; Klein, O.D.; Martin, G.R.; Krasnow, M.A. The branching programme of mouse lung development. Nature 2008, 453, 745–750. [Google Scholar] [CrossRef] [PubMed]
- Tompkins, D.H.; Besnard, V.; Lange, A.W.; Keiser, A.R.; Wert, S.E.; Bruno, M.D.; Whitsett, J.A. Sox2 activates cell proliferation and differentiation in the respiratory epithelium. Am. J. Respir. Cell Mol. Biol. 2011, 45, 101–110. [Google Scholar] [CrossRef] [PubMed]
- Tompkins, D.H.; Besnard, V.; Lange, A.W.; Wert, S.E.; Keiser, A.R.; Smith, A.N.; Lang, R.; Whitsett, J.A. Sox2 is required for maintenance and differentiation of bronchiolar Clara, ciliated, and goblet cells. PLoS ONE 2009, 4, e8248. [Google Scholar] [CrossRef] [PubMed]
- Que, J.; Luo, X.; Schwartz, R.J.; Hogan, B.L. Multiple roles for Sox2 in the developing and adult mouse trachea. Development 2009, 136, 1899–1907. [Google Scholar] [CrossRef] [PubMed]
- Que, J.; Okubo, T.; Goldenring, J.R.; Nam, K.T.; Kurotan, I.R.; Morrisey, E.E.; Taranova, O.; Pevny, L.H.; Hogan, B.L. Multiple dose-dependent roles for Sox2 in the patterning and differentiation of anterior foregut endoderm. Development 2007, 134, 2521–2531. [Google Scholar] [CrossRef] [PubMed]
- Rockich, B.E.; Hrycaj, S.M.; Shih, H.P.; Nagy, M.S.; Ferguson, M.A.; Kopp, J.L.; Sander, M.; Wellik, D.M.; Spence, J.R. Sox9 plays multiple roles in the lung epithelium during branching morphogenesis. Proc. Natl. Acad. Sci. USA 2013, 110, E4456–E4464. [Google Scholar] [CrossRef] [PubMed]
- Chen, F.; Cao, Y.; Qian, J.; Shao, F.; Niederreither, K.; Cardoso, W.V. A retinoic acid-dependent network in the foregut controls formation of the mouse lung primordium. J. Clin. Investig. 2010, 120, 2040–2048. [Google Scholar] [CrossRef] [PubMed]
- De Langhe, S.P.; Carraro, G.; Tefft, D.; Li, C.; Xu, X.; Chai, Y.; Minoo, P.; Hajihosseini, M.K.; Drouin, J.; Kaartinen, V.; et al. Formation and differentiation of multiple mesenchymal lineages during lung development is regulated by β-catenin signaling. PLoS ONE 2008, 3, e1516. [Google Scholar] [CrossRef] [PubMed]
- Goss, A.M.; Tian, Y.; Cheng, L.; Yang, J.; Zhou, D.; Cohen, E.D.; Morrisey, E.E. Wnt2 signaling is necessary and sufficient to activate the airway smooth muscle program in the lung by regulating myocardin/Mrtf-B and Fgf10 expression. Dev. Biol. 2011, 356, 541–552. [Google Scholar] [CrossRef] [PubMed]
- Nyeng, P.; Norgaard, G.A.; Kobberup, S.; Jensen, J. FGF10 maintains distal lung bud epithelium and excessive signaling leads to progenitor state arrest, distalization, and goblet cell metaplasia. BMC Dev. Biol. 2008, 8. [Google Scholar] [CrossRef] [PubMed]
- Ramasamy, S.K.; Mailleux, A.A.; Gupte, V.V.; Mata, F.; Sala, F.G.; Veltmaat, J.M.; del Moral, P.M.; de Langhe, S.; Parsa, S.; Kelly, L.K.; et al. Fgf10 dosage is critical for the amplification of epithelial cell progenitors and for the formation of multiple mesenchymal lineages during lung development. Dev. Biol. 2007, 307, 237–247. [Google Scholar] [CrossRef] [PubMed]
- Rawlins, E.L.; Clark, C.P.; Xue, Y.; Hogan, B.L. The Id2+ distal tip lung epithelium contains individual multipotent embryonic progenitor cells. Development 2009, 136, 3741–3745. [Google Scholar] [CrossRef] [PubMed]
- Volckaert, T.; Campbell, A.; Dill, E.; Li, C.; Minoo, P.; de Langhe, S. Localized Fgf10 expression is not required for lung branching morphogenesis but prevents differentiation of epithelial progenitors. Development 2013, 140, 3731–3742. [Google Scholar] [CrossRef] [PubMed]
- Izvolsky, K.I.; Shoykhet, D.; Yang, Y.; Yu, Q.; Nugent, M.A.; Cardoso, W.V. Heparan sulfate-FGF10 interactions during lung morphogenesis. Dev. Biol. 2003, 258, 185–200. [Google Scholar] [CrossRef]
- Izvolsky, K.I.; Zhong, L.; Wei, L.; Yu, Q.; Nugent, M.A.; Cardoso, W.V. Heparan sulfates expressed in the distal lung are required for Fgf10 binding to the epithelium and for airway branching. Am. J. Physiol. Lung Cell. Mol. Physiol. 2003, 285, L838–L846. [Google Scholar] [CrossRef] [PubMed]
- Sedita, J.; Izvolsky, K.; Cardoso, W.V. Differential expression of heparan sulfate 6-O-sulfotransferase isoforms in the mouse embryo suggests distinctive roles during organogenesis. Dev. Dyn. 2004, 231, 782–794. [Google Scholar] [CrossRef] [PubMed]
- McKeehan, W.L.; Wang, F.; Kan, M. The heparan sulfate-fibroblast growth factor family: Diversity of structure and function. Prog. Nucleic Acid Res. Mol. Biol. 1998, 59, 135–176. [Google Scholar] [PubMed]
- Chang, D.R.; Alanis, D.M.; Miller, R.K.; Ji, H.; Akiyama, H.; McCrea, P.D.; Chen, J. Lung epithelial branching program antagonizes alveolar differentiation. Proc. Natl. Acad. Sci. USA 2013, 110, 18042–18051. [Google Scholar] [CrossRef] [PubMed]
- Volckaert, T.; de Langhe, S. Lung epithelial stem cells and their niches: Fgf10 takes center stage. Fibrogenesis Tissue Repair. 2014, 8. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Tian, Y.; Morley, M.P.; Lu, M.M.; Demayo, F.J.; Olson, E.N.; Morrisey, E.E. Development and regeneration of Sox2+ endoderm progenitors are regulated by a Hdac1/2-Bmp4/Rb1 regulatory pathway. Dev. Cell 2013, 24, 345–358. [Google Scholar] [CrossRef] [PubMed]
- Shiratori, M.; Oshika, E.; Ung, L.P.; Singh, G.; Shinozuka, H.; Warburton, D.; Michalopoulos, G.; Katyal, S.L. Keratinocyte growth factor and lung morphogenesis. Am. J. Respir. Cell Mol. Biol. 1996, 15, 328–338. [Google Scholar] [CrossRef] [PubMed]
- Cardoso, W.V.; Itoh, A.; Nogawa, H.; Mason, I.; Brody, J.S. FGF-1 and FGF-7 induce distinct patterns of growth and differentiation in embryonic lung epithelium. Dev. Dyn. 1997, 8, 398–405. [Google Scholar] [CrossRef]
- Shannon, J.M.; Gebb, S.A.; Nielson, L.D. Induction of alveolar type II cell differentiation in embryonic tracheal epithelium in mesenchyme-free culture. Development 1999, 126, 1675–1688. [Google Scholar] [PubMed]
- Weinstein, M.; Xu, X.; Ohyama, K.; Deng, C.-X. FGFR-3 and FGFR-4 function cooperatively to direct alveogenesis in the murine lung. Development 1998, 125, 3615–3623. [Google Scholar] [PubMed]
- Plopper, C.G.; st George, J.A.; Read, L.C.; Nishio, S.J.; Weir, A.J.; Edwards, L.; Tarantal, A.F.; Pinkerton, K.E.; Merritt, T.A.; Whitsett, J.A.; et al. Acceleration of type II cell differentiation in fetal rhesus monkey lung by administration of EGF. Am. J. Physiol. 1992, 262, L313–L321. [Google Scholar] [PubMed]
- Kolios, G.; Moodley, Y. Introduction to stem cells and regenerative medicine. Respiration 2013, 85, 3–10. [Google Scholar] [CrossRef] [PubMed]
- Potten, C.; Loeffler, M. Stem cells: Attributes, cycles, spirals, pitfalls and uncertainties. Lessons for and from the crypt. Development 1990, 110, 1001–1020. [Google Scholar] [PubMed]
- Leeman, K.T.; Fillmore, C.M.; Kim, C.F. Lung stem and progenitor cells in tissue homeostasis and disease. Curr. Top. Dev. Biol. 2014, 107, 207–233. [Google Scholar] [PubMed]
- Adamson, I.Y.; Bowden, D.H. The type 2 cell as progenitor of alveolar epithelial regeneration. A cytodynamic study in mice after exposure to oxygen. Lab. Investig. 1974, 30, 35–42. [Google Scholar] [PubMed]
- Adamson, I.Y.; Bowden, D.H. Origin of ciliated alveolar epithelial cells in bleomycin-induced lung injury. Am. J. Pathol. 1977, 87, 569–580. [Google Scholar] [PubMed]
- Adamson, I.Y.; Bowden, D.H. Bleomycin-induced injury and metaplasia of alveolar type 2 cells. Relationship of cellular responses to drug presence in the lung. Am. J. Pathol. 1979, 96, 531–544. [Google Scholar] [PubMed]
- Bowden, D.H.; Adamson, I.Y.; Wyatt, J.P. Reaction of the lung cells to a high concentration of oxygen. Arch. Pathol. 1968, 86, 671–675. [Google Scholar] [PubMed]
- Evans, M.J.; Cabral, L.C.; Stephens, R.J.; Freeman, G. Acute kinetic response and renewal of the alveolar epithelium following injury by nitrogen dioxide. Chest 1974, 65, 62S–65S. [Google Scholar] [CrossRef] [PubMed]
- Evans, M.J.; Cabral, L.J.; Stephens, R.J.; Freeman, G. Renewal of alveolar epithelium in the rat following exposure to NO2. Am. J. Pathol. 1973, 70, 175–198. [Google Scholar] [PubMed]
- Evans, M.J.; Cabral, L.J.; Stephens, R.J.; Freeman, G. Transformation of alveolar type 2 cells to type 1 cells following exposure to NO2. Exp. Mol. Pathol. 1975, 22, 142–150. [Google Scholar] [CrossRef]
- Evans, M.J.; Dekker, N.P.; Cabral-Anderson, L.J.; Freeman, G. Quantitation of damage to the alveolar epithelium by means of type 2 cell proliferation. Am. Rev. Respir. Dis. 1978, 118, 787–790. [Google Scholar] [CrossRef] [PubMed]
- Rawlins, E.L.; Hogan, B.L. Epithelial stem cells of the lung: Privileged few or opportunities for many? Development 2006, 133, 2455–2465. [Google Scholar] [CrossRef] [PubMed]
- Rawlins, E.L.; Ostrowski, L.E.; Randell, S.H.; Hogan, B.L. Lung development and repair: Contribution of the ciliated lineage. Proc. Natl. Acad. Sci. USA 2007, 104, 410–417. [Google Scholar] [CrossRef] [PubMed]
- Rawlins, E.L.; Hogan, B.L. Ciliated epithelial cell lifespan in the mouse trachea and lung. Am. J. Physiol. Lung Cell. Mol. Physiol. 2008, 295, L231–L234. [Google Scholar] [CrossRef] [PubMed]
- Rawlins, E.L.; Okubo, T.; Que, J.; Xue, Y.; Clark, C.; Luo, X.; Hogan, B.L. Epithelial stem/progenitor cells in lung postnatal growth, maintenance, and repair. Cold Spring Harb. Symp. Quant. Biol. 2008, 73, 291–295. [Google Scholar] [CrossRef] [PubMed]
- Borthwick, D.W.; Shahbazian, M.; Krantz, Q.T.; Dorin, J.R.; Randell, S.H. Evidence for stem-cell niches in the tracheal epithelium. Am. J. Respir. Cell Mol. Biol. 2001, 24, 662–670. [Google Scholar] [CrossRef] [PubMed]
- Rock, J.; Randell, S.; Hogan, B. Airway basal stem cells: A perspective on their roles in epithelial homeostasis and remodeling. Dis. Models Mech. 2010, 3, 545–556. [Google Scholar] [CrossRef] [PubMed]
- Rock, J.R.; Onaitis, M.W.; Rawlins, E.L.; Lu, Y.; Clark, C.P.; Xue, Y.; Randell, S.H.; Hogan, B.L. Basal cells as stem cells of the mouse trachea and human airway epithelium. Proc. Natl. Acad. Sci. USA 2009, 106, 12771–12775. [Google Scholar] [CrossRef] [PubMed]
- Cole, B.B.; Smith, R.W.; Jenkins, K.M.; Graham, B.B.; Reynolds, P.R.; Reynolds, S.D. Tracheal basal cells: A facultative progenitor cell pool. Am. J. Pathol. 2010, 177, 362–376. [Google Scholar] [CrossRef] [PubMed]
- Hong, K.U.; Reynolds, S.D.; Watkins, S.; Fuchs, E.; Stripp, B.R. In vivo differentiation potential of tracheal basal cells: Evidence for multipotent and unipotent subpopulations. Am. J. Physiol. Lung Cell. Mol. Physiol. 2004, 286, L643–L649. [Google Scholar] [CrossRef] [PubMed]
- Kumar, P.A.; Hu, Y.; Yamamoto, Y.; Hoe, N.B.; Wei, T.S.; Mu, D.; Hoe, N.B.; Wei, T.S.; Mu, D.; Sun, Y.; et al. Distal airway stem cells yield alveoli in vitro and during lung regeneration following H1N1 influenza infection. Cell 2011, 147, 525–538. [Google Scholar] [CrossRef] [PubMed]
- Pardo-Saganta, A.; Law, B.M.; Gonzalez-Celeiro, M.; Vinarsky, V.; Rajagopal, J. Ciliated cells of pseudostratified airway epithelium do not become mucous cells after ovalbumin challenge. Am. J. Respir. Cell Mol. Biol. 2013, 48, 364–373. [Google Scholar] [CrossRef] [PubMed]
- Rock, J.R.; Gao, X.; Xue, Y.; Randell, S.H.; Kong, Y.Y.; Hogan, B.L. Notch-dependent differentiation of adult airway basal stem cells. Cell Stem Cell 2011, 8, 639–648. [Google Scholar] [CrossRef] [PubMed]
- Reynolds, S.D.; Reynolds, P.R.; Pryhuber, G.S.; Finder, J.D.; Stripp, B.R. Secretoglobins SCGB3A1 and SCGB3A2 define secretory cell subsets in mouse and human airways. Am. J. Respir. Crit. Care Med. 2002, 166, 1498–1509. [Google Scholar] [CrossRef] [PubMed]
- Song, H.; Yao, E.; Lin, C.; Gacayan, R.; Chen, M.-H.; Chuang, P.-T. Functional characterization of pulmonary neuroendocrine cells in lung development, injury, and tumorigenesis. Proc. Natl. Acad. Sci. USA 2012, 109, 17531–17536. [Google Scholar] [CrossRef] [PubMed]
- Giangreco, A.; Reynolds, S.D.; Stripp, B.R. Terminal bronchioles harbor a unique airway stem cell population that localizes to the bronchoalveolar duct junction. Am. J. Pathol. 2002, 161, 173–182. [Google Scholar] [CrossRef]
- Rawlins, E.L.; Okubo, T.; Xue, Y.; Brass, D.M.; Auten, R.L.; Hasegawa, H.; Wang, F.; Hogan, B.L. The role of Scgb1a1+ Clara cells in the long-term maintenance and repair of lung airway, but not alveolar, epithelium. Cell Stem Cell 2009, 4, 525–534. [Google Scholar] [CrossRef] [PubMed]
- Hong, K.U.; Reynolds, S.D.; Giangreco, A.; Hurley, C.M.; Stripp, B.R. Clara cell secretory protein-expressing cells of the airway neuroepithelial body microenvironment include a label-retaining subset and are critical for epithelial renewal after progenitor cell depletion. Am. J. Respir. Cell Mol. Biol. 2001, 24, 671–681. [Google Scholar] [CrossRef] [PubMed]
- Guha, A.; Vasconcelos, M.; Cai, Y.; Yoneda, M.; Hinds, A.; Qian, J.; Li, G.; Dickel, L.; Johnson, J.E.; Kimura, S.; et al. Neuroepithelial body microenvironment is a niche for a distinct subset of Clara-like precursors in the developing airways. Proc. Natl. Acad. Sci. USA 2012, 109, 12592–12597. [Google Scholar] [CrossRef] [PubMed]
- Morimoto, M.; Nishinakamura, R.; Saga, Y.; Kopan, R. Different assemblies of Notch receptors coordinate the distribution of the major bronchial Clara, ciliated and neuroendocrine cells. Development 2012, 139, 4365–4373. [Google Scholar] [CrossRef] [PubMed]
- Volckaert, T.; Dill, E.; Campbell, A.; Tiozzo, C.; Majka, S.; Bellusci, S.; de Langhe, S.P. Parabronchial smooth muscle constitutes an airway epithelial stem cell niche in the mouse lung after injury. J. Clin. Investig. 2011, 121, 4409–4419. [Google Scholar] [CrossRef] [PubMed]
- Giangreco, A.; Arwert, E.N.; Rosewell, I.R.; Snyder, J.; Watt, F.M.; Stripp, B.R. Stem cells are dispensable for lung homeostasis but restore airways after injury. Proc. Natl. Acad. Sci. USA 2009, 106, 9286–9291. [Google Scholar] [CrossRef] [PubMed]
- Bertalanffy, F.D.; Leblond, C.P. Structure of respiratory tissue. Lancet 1955, 266, 1365–1368. [Google Scholar] [CrossRef]
- Mason, R.J.; Williams, M.C. Type II alveolar cell. Defender of the alveolus. Am. Rev. Respir. Dis. 1977, 115, 81–91. [Google Scholar] [PubMed]
- Kauffman, S.L. Alterations in cell proliferation in mouse lung following urethane exposure. 3. Effects of chronic exposure on type 2 alveolar epithelial cell. Am. J. Pathol. 1972, 68, 317–326. [Google Scholar] [PubMed]
- Kauffman, S.L. Kinetics of alveolar epithelial hyperplasia in lungs of mice exposed to urethane. I. quantitative analysis of cell populations. Lab. Investig. 1974, 30, 170–175. [Google Scholar] [PubMed]
- Kalina, M.; Riklis, S.; Blau, H. Pulmonary epithelial cell proliferation in primary culture of alveolar type II cells. Exp. Lung Res. 1993, 19, 153–175. [Google Scholar] [CrossRef] [PubMed]
- Witschi, H. Proliferation of type II alveolar cells: A review of common responses in toxic lung injury. Toxicology 1976, 5, 267–277. [Google Scholar] [CrossRef]
- Bitterman, P.B.; Polunovsky, V.A.; Ingbar, D.H. Repair after acute lung injury. Chest 1994, 105, 118S–121S. [Google Scholar] [CrossRef] [PubMed]
- Barkauskas, C.E.; Cronce, M.J.; Rackley, C.R.; Bowie, E.J.; Keene, D.R.; Stripp, B.R.; Randell, S.H.; Noble, P.W.; Hogan, B.L. Type 2 alveolar cells are stem cells in adult lung. J. Clin. Investig. 2013, 123, 3025–3036. [Google Scholar] [CrossRef] [PubMed]
- Desai, T.J.; Brownfield, D.G.; Krasnow, M.A. Alveolar progenitor and stem cells in lung development, renewal and cancer. Nature 2014, 507, 190–194. [Google Scholar] [CrossRef] [PubMed]
- Kim, C.F.; Jackson, E.L.; Woolfenden, A.E.; Lawrence, S.; Babar, I.; Vogel, S.; Crowley, D.; Bronson, R.T.; Jacks, T. Identification of bronchioalveolar stem cells in normal lung and lung cancer. Cell 2005, 121, 823–835. [Google Scholar] [CrossRef] [PubMed]
- Rock, J.R.; Barkauskas, C.E.; Cronce, M.J.; Xue, Y.; Harris, J.R.; Liang, J.; Noble, P.W.; Hogan, B.L. Multiple stromal populations contribute to pulmonary fibrosis without evidence for epithelial to mesenchymal transition. Proc. Natl. Acad. Sci. USA 2011, 108, E1475–E1483. [Google Scholar] [CrossRef] [PubMed]
- Zheng, D.; Limmon, G.V.; Yin, L.; Leung, N.H.; Yu, H.; Chow, V.T.; Chen, J. Regeneration of alveolar type I and II cells from Scgb1a1-expressing cells following severe pulmonary damage induced by bleomycin and influenza. PLoS ONE 2012, 7, e48451. [Google Scholar] [CrossRef] [PubMed]
- Da Silva Meirelles, L.; Chagastelles, P.C.; Nardi, N.B. Mesenchymal stem cells reside in virtually all post-natal organs and tissues. J. Cell Sci. 2006, 119, 2204–2213. [Google Scholar] [CrossRef] [PubMed]
- McQualter, J.L.; Brouard, N.; Williams, B.N.; Baird, B.N.; Sims-Lucas, S.; Yuen, K.; Nilsson, S.K.; Simmons, P.J.; Bertoncello, I. Endogenous fibroblastic progenitor cells in the adult mouse lung are highly enriched in the SCA-1 positive cell fraction. Stem Cells 2009, 27, 623–633. [Google Scholar] [CrossRef] [PubMed]
- Summer, R.; Fitzsimmons, K.; Dwyer, D.; Murphy, J.; Fine, A. Isolation of an adult mouse lung mesenchymal progenitor cell population. Am. J. Respir. Cell Mol. Biol. 2007, 37, 152–159. [Google Scholar] [CrossRef] [PubMed]
- Hegab, A.E.; Kubo, H.; Fujino, N.; Suzuki, T.; He, M.; Kato, H.; Yamaya, M. Isolation and characterization of murine multipotent lung stem cells. Stem Cells Dev. 2010, 19, 523–536. [Google Scholar] [CrossRef] [PubMed]
- Reynolds, S.D.; Shen, H.; Reynolds, P.R.; Betsuyaku, T.; Pilewski, J.M.; Gambelli, F.; di Giuseppe, M.; Ortiz, L.A.; Stripp, B.R. Molecular and functional properties of lung SP cells. Am. J. Physiol. Lung Cell. Mol. Physiol. 2007, 292, L972–L983. [Google Scholar] [CrossRef] [PubMed]
- Martin, J.; Helm, K.; Ruegg, P.; Varella-Garcia, M.; Burnham, E.; Majka, S. Adult lung side population cells have mesenchymal stem cell potential. Cytotherapy 2008, 10, 140–151. [Google Scholar] [CrossRef] [PubMed]
- Jarvinen, L.; Badri, L.; Wettlaufer, S.; Ohtsuka, T.; Standiford, T.J.; Toews, G.B.; Pinsky, D.J.; Peters-Golden, M.; Lama, V.N. Lung resident mesenchymal stem cells isolated from human lung allografts inhibit T cell proliferation via a soluble mediator. J. Immunol. 2008, 181, 4389–4396. [Google Scholar] [CrossRef] [PubMed]
- Lama, V.N.; Smith, L.; Badri, L.; Flint, A.; Andrei, A.C.; Murray, S.; Wang, Z.; Liao, H.; Toews, G.B.; Krebsbach, P.H.; et al. Evidence for tissueresident mesenchymal stem cells in human adult lung from studies of transplanted allografts. J. Clin. Investig. 2007, 117, 989–996. [Google Scholar] [CrossRef] [PubMed]
- Hennrick, K.T.; Keeton, A.G.; Nanua, S.; Kijek, T.G.; Goldsmith, A.M.; Sajjan, U.S.; Bentley, J.K.; Lama, V.N.; Moore, B.B.; Schumacher, R.E.; et al. Lung cells from neonates show a mesenchymal stem cell phenotype. Am. J. Respir. Crit. Care Med. 2007, 175, 1158–1164. [Google Scholar] [CrossRef] [PubMed]
- Ingenito, E.P.; Sen, E.; Tsai, L.W. Design and testing of biological scaffolds for delivering reparative cells to target sites in the lung. J. Tissue Eng. Regen. Med. 2010, 4, 259–272. [Google Scholar] [CrossRef] [PubMed]
- Ingenito, E.P.; Tsai, L.; Murthy, S. Autologous lung-derived mesenchymal stem cell transplantation in experimental emphysema. Cell Transplant. 2011, 21, 175–189. [Google Scholar] [CrossRef] [PubMed]
- Dominici, M.; le Blanc, K.; Mueller, I.; Slaper-Cortenbach, I.; Marini, F.; Krause, D.; Deans, R.; Keating, A.; Prockop, D.; Horwitz, E. Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy 2006, 8, 315–317. [Google Scholar] [CrossRef] [PubMed]
- Karoubi, G.; Cortes-Dericks, L.; Breyer, I.; Schmid, R.; Dutly, A. Identification of mesenchymal stromal cells in human lung parenchyma capable of differentiating into aquaporin 5-expressing cells. Lab. Investig. 2009, 89, 1100–1114. [Google Scholar] [CrossRef] [PubMed]
- Hegab, A.; Ha, V.; Gilbert, J.; Zhang, K.X.; Malkoski, S.P.; Chon, A.T.; Darmawan, D.O.; Bisht, B.; Ooi, A.T.; Pellegrini, M.; et al. Novel stem/progenitor cell population from murine tracheal submucosal gland ducts with multipotent regenerative potential. Stem Cells 2011, 29, 1283–1293. [Google Scholar] [CrossRef] [PubMed]
- Morrisey, E.; Hogan, B. Preparing for the first breath: Genetic and cellular mechanisms in lung development. Dev. Cell 2010, 18, 8–23. [Google Scholar] [CrossRef] [PubMed]
- McQualter, J.; Yuen, K.; Williams, B.; Bertoncello, I. Evidence of an epithelial stem/progenitor cell hierarchy in the adult mouse lung. Proc. Natl. Acad. Sci. USA 2010, 107, 1414–1419. [Google Scholar] [CrossRef] [PubMed]
- Hoffman, A.; Paxson, J.; Mazan, M.; Davis, A.M.; Tyagi, S.; Murthy, S.; Ingenito, E.P. Lung-derived mesenchymal stromal cell post-transplantation survival, persistence, paracrine expression, and repair of elastase injured lung. Stem Cells Dev. 2011, 20, 1779–1792. [Google Scholar] [CrossRef] [PubMed]
- Hogan, B.L.; Barkauskas, C.E.; Chapman, H.A.; Epstein, J.A.; Jain, R.; Hsia, C.C.; Niklason, L.; Calle, E.; Le, A.; Randell, S.H.; et al. Repair and regeneration of the respiratory system: Complexity, plasticity, and mechanisms of lung stem cell function. Cell Stem Cell 2014, 15, 123–138. [Google Scholar] [CrossRef] [PubMed]
- Dane, D.M.; Yilmaz, C.; Estrera, A.S.; Hsia, C.C. Separating in vivo mechanical stimuli for postpneumonectomy compensation: Physiological assessment. J. Appl. Physiol. 2013, 114, 99–106. [Google Scholar] [CrossRef] [PubMed]
- Ravikumar, P.; Yilmaz, C.; Bellotto, D.J.; Dane, D.M.; Estrera, A.S.; Hsia, C.C. Separating in vivo mechanical stimuli for postpneumonectomy compensation: Imaging and ultrastructural assessment. J. Appl. Physiol. 2013, 114, 961–970. [Google Scholar] [CrossRef] [PubMed]
- Ravikumar, P.; Yilmaz, C.; Dane, D.M.; Bellotto, D.J.; Estrera, A.S.; Hsia, C.C. Defining a stimuli-response relationship in compensatory lung growth following major resection. J. Appl. Physiol. 2014, 116, 816–824. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Acciani, T.; le Cras, T.; Lutzko, C.; Perl, A.K. Dynamic regulation of platelet-derived growth factor receptor a expression in alveolar fibroblasts during realveolarization. Am. J. Respir. Cell Mol. Biol. 2012, 47, 517–527. [Google Scholar] [CrossRef] [PubMed]
- Eisenhauer, P.; Earle, B.; Loi, R.; Sueblinvong, V.; Goodwin, M.; Allen, G.B.; Lundblad, L.; Mazan, M.R.; Hoffman, A.M.; Weiss, D.J. Endogenous distal airway progenitor cells, lung mechanics, and disproportionate lobar growth following long-term postpneumonectomy in mice. Stem Cells 2013, 31, 1330–1339. [Google Scholar] [CrossRef] [PubMed]
- Hoffman, A.M.; Shifren, A.; Mazan, M.R.; Gruntman, A.M.; Lascola, K.M.; Nolen-Walston, R.D.; Kim, C.F.; Tsai, L.; Pierce, R.A.; Mecham, R.P.; et al. Matrix modulation of compensatory lung regrowth and progenitor cell proliferation in mice. Am. J. Physiol. Lung Cell. Mol. Physiol. 2010, 298, L158–L168. [Google Scholar] [CrossRef] [PubMed]
- Hsia, C.C. Signals and mechanisms of compensatory lung growth. J. Appl. Physiol. 2004, 97, 1992–1998. [Google Scholar] [CrossRef] [PubMed]
- Thane, K.; Ingenito, E.P.; Hoffman, A.M. Lung regeneration and translational implications of the postpneumonectomy model. Transl. Res. 2014, 163, 363–376. [Google Scholar] [CrossRef] [PubMed]
- Voswinckel, R.; Motejl, V.; Fehrenbach, A.; Wegmann, M.; Mehling, T.; Fehrenbach, H.; Seeger, W. Characterisation of post-pneumonectomy lung growth in adult mice. Eur. Respir. J. 2004, 24, 524–532. [Google Scholar] [CrossRef] [PubMed]
- Kaza, A.K.; Laubach, V.E.; Kern, J.A.; Long, S.M.; Fiser, S.M.; Tepper, J.A.; Nguyen, R.P.; Shockey, K.S.; Tribble, C.G.; Kron, I.L. Epidermal growth factor augments postpneumonectomy lung growth. J. Thorac. Cardiovasc. Surg. 2000, 120, 916–921. [Google Scholar] [CrossRef] [PubMed]
- Kaza, A.K.; Kron, I.L.; Leuwerke, S.M.; Tribble, C.G.; Laubach, V.E. Keratinocyte growth factor enhances post-pneumonectomy lung growth by alveolar proliferation. Circulation 2002, 106, I120–I124. [Google Scholar] [PubMed]
- Al Alam, D.; Green, M.; Tabatabai Irani, R.; Parsa, S.; Danopoulos, S.; Sala, F.G.; Branch, J.; El Agha, E.; Tiozzo, C.; Voswinckel, R.; et al. Contrasting expression of canonical Wnt signaling reporters TOPGAL, BATGAL and Axin2(LacZ) during murine lung development and repair. PLoS ONE 2011, 6, e23139. [Google Scholar] [CrossRef] [PubMed]
- Aumiller, V.; Balsara, N.; Wilhelm, J.; Günther, A.; Königshoff, M. WNT/β-catenin signaling induces IL-1B expression by alveolar epithelial cells in pulmonary fibrosis. Am. J. Respir. Cell Mol. Biol. 2013, 49, 96–104. [Google Scholar] [CrossRef] [PubMed]
- Flozak, A.S.; Lam, A.P.; Russell, S.; Jain, M.; Peled, O.N.; Sheppard, K.A.; Beri, R.; Mutlu, G.M.; Budinger, G.R.; Gottardi, C.J. β-Catenin/T-cell factor signaling is activated during lung injury and promotes the survival and migration of alveolar epithelial cells. J. Biol. Chem. 2010, 285, 3157–3167. [Google Scholar] [CrossRef] [PubMed]
- Hashimoto, S.; Chen, H.; Que, J.; Brockway, B.L.; Drake, J.A.; Snyder, J.C.; Randell, S.H.; Stripp, B.R. β-Catenin-SOX2 signaling regulates the fate of developing airway epithelium. J. Cell Sci. 2012, 125, 932–942. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Goss, A.M.; Cohen, E.D.; Kadzik, R.; Lepore, J.J.; Muthukumaraswamy, K.; Yang, J.; DeMayo, F.J.; Whitsett, J.A.; Parmacek, M.S.; et al. A GATA6-Wnt pathway required for epithelial stem cell development and airway regeneration. Nat. Genet. 2008, 40, 862–870. [Google Scholar] [CrossRef] [PubMed]
- Zemke, A.C.; Teisanu, R.M.; Giangreco, A.; Drake, J.A.; Brockway, B.L.; Reynolds, S.D.; Stripp, B.R. β-Catenin is not necessary for maintenance or repair of the bronchiolar epithelium. Am. J. Respir. Cell Mol. Biol. 2009, 41, 535–543. [Google Scholar] [CrossRef] [PubMed]
- Tanjore, H.; Degryse, A.L.; Crossno, P.F.; Xu, X.C.; McConaha, M.E.; Jones, B.R.; Polosukhin, V.V.; Bryant, A.J.; Cheng, D.S.; Newcomb, D.C.; et al. β-Catenin in the alveolar epithelium protects from lung fibrosis after intratracheal bleomycin. Am. J. Respir. Crit. Care Med. 2013, 187, 630–639. [Google Scholar] [CrossRef] [PubMed]
- Henderson, W.R., Jr.; Chi, E.Y.; Ye, X.; Tien, Y.T.; Zhou, B.; Borok, Z.; Knight, D.A.; Kahn, M. Inhibition of Wnt/β-catenin/CREB binding protein (CBP) signaling reverses pulmonary fibrosis. Proc. Natl. Acad. Sci. USA 2010, 107, 14309–14314. [Google Scholar] [CrossRef] [PubMed]
- Chilosi, M.; Poletti, V.; Zamo, A.; Lestani, M.; Montagna, L.; Piccoli, P.; Pedron, S.; Bertaso, M.; Scarpa, A.; Murer, B.; et al. Aberrant Wnt/β-catenin pathway activation in idiopathic pulmonary fibrosis. Am. J. Pathol. 2003, 162, 1495–1502. [Google Scholar] [CrossRef]
- Morimoto, M.; Liu, Z.; Cheng, H.T.; Winters, N.; Bader, D.; Kopan, R. Canonical Notch signaling in the developing lung is required for determination of arterial smooth muscle cells and selection of Clara vs. ciliated cell fate. J. Cell Sci. 2010, 123, 213–224. [Google Scholar] [CrossRef] [PubMed]
- Tsao, P.N.; Vasconcelos, M.; Izvolsky, K.I.; Qian, J.; Lu, J.; Cardoso, W.V. Notch signaling controls the balance of ciliated and secretory cell fates in developing airways. Development 2009, 136, 2297–2307. [Google Scholar] [CrossRef] [PubMed]
- Xing, Y.; Li, A.; Borok, Z.; Li, C.; Minoo, P. NOTCH1 is required for regeneration of Clara cells during repair of airway injury. Stem Cells 2012, 30, 946–955. [Google Scholar] [CrossRef] [PubMed]
- Paul, M.K.; Bisht, B.; Darmawan, D.O.; Chiou, R.; Ha, V.L.; Wallace, W.D.; Chon, A.T.; Hegab, A.E.; Grogan, T.; Elashoff, D.A.; et al. Dynamic changes in intracellular ROS levels regulate airway basal stem cell homeostasis through Nrf2-dependent Notch signaling. Cell Stem Cell 2014, 15, 199–214. [Google Scholar] [CrossRef] [PubMed]
- Yin, Z.; Gonzales, L.; Kolla, V.; Rath, N.; Zhang, Y.; Lu, M.M.; Kimura, S.; Ballard, P.L.; Beers, M.F.; Epstein, J.A.; et al. Hop functions downstream of Nkx2.1 and GATA6 to mediate HDAC-dependent negative regulation of pulmonary gene expression. Am. J. Physiol. Lung Cell. Mol. Physiol. 2006, 291, L191–L199. [Google Scholar] [CrossRef] [PubMed]
- Ito, K.; Ito, M.; Elliott, W.M.; Cosio, B.; Caramori, G.; Kon, O.M.; Barczyk, A.; Hayashi, S.; Adcock, I.M.; Hogg, J.C.; et al. Decreased histone deacetylase activity in chronic obstructive pulmonary disease. N. Engl. J. Med. 2005, 352, 1967–1976. [Google Scholar] [CrossRef] [PubMed]
- Gunawardhana, L.P.; Gibson, P.G.; Simpson, J.L.; Powell, H.; Baines, K.J. Activity and expression of histone acetylases and deacetylases in inflammatory phenotypes of asthma. Clin. Exp. Allergy 2014, 44, 47–57. [Google Scholar] [CrossRef] [PubMed]
- Tian, Y.; Zhang, Y.; Hurd, L.; Hannenhalli, S.; Liu, F.; Lu, M.M.; Morrisey, E.E. Regulation of lung endoderm progenitor cell behavior by miR302/367. Development 2011, 138, 1235–1245. [Google Scholar] [CrossRef] [PubMed]
- Ventura, A.; Young, A.G.; Winslow, M.M.; Lintault, L.; Meissner, A.; Erkeland, S.J.; Newman, J.; Bronson, R.T.; Crowley, D.; Stone, J.R.; et al. Targeted deletion reveals essential and overlapping functions of the miR-17 through 92 family of miRNA clusters. Cell 2008, 132, 875–886. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.; Thomson, J.M.; Wong, H.Y.; Hammond, S.M.; Hogan, B.L. Transgenic over-expression of the microRNA miR-17–92 cluster promotes proliferation and inhibits differentiation of lung epithelial progenitor cells. Dev. Biol. 2007, 310, 442–453. [Google Scholar] [CrossRef] [PubMed]
- Herriges, M.J.; Swarr, D.T.; Morley, M.P.; Rathi, K.S.; Peng, T.; Stewart, K.M.; Morrisey, E.E. Long noncoding RNAs are spatially correlated with transcription factors and regulate lung development. Genes Dev. 2014, 28, 1363–1379. [Google Scholar] [CrossRef] [PubMed]
- Davidson, J.M. Biochemistry and turnover of lung interstitium. Eur. Respir. J. 1990, 3, 1048–1063. [Google Scholar] [PubMed]
- Lane, S.; Rippon, H.J.; Bishop, A.E. Stem cells in lung repair and regeneration. Regen. Med. 2007, 2, 407–415. [Google Scholar] [CrossRef] [PubMed]
- Dunsmore, S.E.; Rannels, D.E. Extracellular matrix biology in the lung. Am. J. Physiol. 1996, 270, L3–L27. [Google Scholar] [PubMed]
- Suki, B.; Ito, S.; Stamenovic, D.; Lutchen, K.R.; Ingenito, E.P. Biomechanics of the lung parenchyma: Critical roles of collagen and mechanical forces. J. Appl. Physiol. 2005, 98, 1892–1899. [Google Scholar] [CrossRef] [PubMed]
- Kreis, T.; Vale, R. Guidebook to the Extracellular Matrix, Anchor, and Adhesion Proteins, 2nd ed.; Oxford University Press: Oxford, UK, 1999; p. 422. [Google Scholar]
- Olsen, C.O.; Isakson, B.E.; Seedorf, G.J.; Lubman, R.L.; Boitano, S. Extracellular matrix-driven alveolar epithelial cell differentiation in vitro. Exp. Lung Res. 2005, 31, 461–482. [Google Scholar] [CrossRef] [PubMed]
- Isakson, B.E.; Lubman, R.L.; Seedorf, G.J.; Boitano, S. Modulation of pulmonary alveolar type II cell phenotype and communication by extracellular matrix and KGF. Am. J. Physiol. Cell Physiol. 2001, 281, C1291–C1299. [Google Scholar] [PubMed]
- Lin, Y.M.; Zhang, A.; Rippon, H.J.; Bismarck, A.; Bishop, A.E. Tissue engineering of lung: The effect of extracellular matrix on the differentiation of embryonic stem cells to pneumocytes. Tissue Eng. Part A 2010, 16, 1515–1526. [Google Scholar] [CrossRef] [PubMed]
- Petersena, T.H.; Calle, E.A.; Niklason, L.E. Strategies for lung regeneration. Mater. Today 2011, 14, 196–201. [Google Scholar] [CrossRef]
- Daley, W.P.; Peters, S.B.; Larsen, M. Extracellular matrix dynamics in development and regenerative medicine. J. Cell Sci. 2008, 121, 255–264. [Google Scholar] [CrossRef] [PubMed]
- Odorico, J.; Kaufman, D.; Thomson, J. Multilineage differentiation from human embryonic stem cell lines. Stem Cells 2001, 19, 193–204. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, K.; Tanabe, K.; Ohnuki, M.; Narita, M.; Ichisaka, T.; Tomoda, K.; Yamanaka, S. Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell 2007, 131, 861–872. [Google Scholar] [PubMed]
- Takahashi, K.; Yamanaka, S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell 2006, 126, 663–676. [Google Scholar] [CrossRef] [PubMed]
- Yu, J.; Vodyanik, M.; Smuga-Otto, K.; Antosiewicz-Bourget, J.; Frane, J.L.; Tian, S.; Nie, J.; Jonsdottir, G.A.; Ruotti, V.; Stewart, R.; et al. Induced pluripotent stem cell lines derived from human somatic cells. Science 2007, 318, 1917–1920. [Google Scholar] [CrossRef] [PubMed]
- Perl, A.; Kist, R.; Shan, Z.; Scherer, G.; Whitsett, J. Normal lung development and function after Sox9 inactivation in the respiratory epithelium. Genesis 2005, 41, 23–32. [Google Scholar] [CrossRef] [PubMed]
- Shu, W.; Lu, M.; Zhang, Y.; Tucker, P.; Zhou, D.; Morrisey, E. Foxp2 and Foxp1 cooperatively regulate lung and esophagus development. Development 2007, 134, 1991–2000. [Google Scholar] [CrossRef] [PubMed]
- Van Haute, L.; de Block, G.; Liebaers, I.; Sermon, K.; DeRycke, M. Generation of lung epithelial-like tissue from human embryonic stem cells. Respir. Res. 2009, 5. [Google Scholar] [CrossRef] [PubMed]
- Green, M.; Chen, A.; Nostro, M.; d’Souza, S.; Lemischka, I.; Gouon-Evans, V.; Keller, G.; Snoeck, H.W. Generation of anterior foregut endoderm from human embryonic and induced pluripotent stem cells. Nat. Biotechnol. 2011, 29, 267–272. [Google Scholar] [CrossRef] [PubMed]
- Mou, H.; Zhao, R.; Sherwood, R.; Ahfeldt, T.; Lapey, A.; Wain, J.; Sicilian, L.; Izvolsky, K.; Musunuru, K.; Cowan, C.; et al. Generation of multipotent lung and airway progenitors from mouse ESCs and patient-specific cystic fibrosis iPSCs. Cell Stem Cell 2012, 10, 385–397. [Google Scholar] [CrossRef] [PubMed]
- Longmire, T.A.; Ikonomou, L.; Hawkins, F.; Christodoulou, C.; Cao, Y.; Jean, J.C.; Kwok, L.W.; Mou, H.; Rajagopal, J.; Shen, S.S.; et al. Efficient derivation of purified lung and thyroid progenitors from embryonic stem cells. Cell Stem Cell 2012, 10, 398–411. [Google Scholar] [CrossRef] [PubMed]
- Wong, A.; Bear, C.; Chin, S.; Pasceri, P.; Thompson, T.O.; Huan, L.J.; Ratjen, F.; Ellis, J.; Rossant, J. Directed differentiation of human pluripotent stem cells into mature airway epithelia expressing functional CFTR protein. Nat. Biotechnol. 2012, 9, 876–882. [Google Scholar] [CrossRef] [PubMed]
- Huang, S.X.; Islam, M.N.; O’Neill, J.; Hu, Z.; Yang, Y.G.; Chen, Y.W.; Mumau, M.; Green, M.D.; Vunjak-Novakovic, G.; Bhattacharya, J.; et al. Efficient generation of lung and airway epithelial cells from human pluripotent stem cells. Nat. Biotechnol. 2014, 32, 84–91. [Google Scholar] [CrossRef] [PubMed]
- Berry, M.F.; Engler, A.J.; Woo, Y.J.; Pirolli, T.J.; Bish, L.T.; Jayasankar, V.; Morine, K.J.; Gardner, T.J.; Discher, D.E.; Sweeney, H.L. Mesenchymal stem cell injection after myocardial infarction improves myocardial compliance. Am. J. Physiol. Heart Circ. Physiol. 2006, 290, 196–203. [Google Scholar] [CrossRef] [PubMed]
- Kotton, D.N.; Ma, B.Y.; Cardoso, W.V.; Sanderson, E.A.; Summer, R.S.; Williams, M.C.; Fine, A. Bone marrow-derived cells as progenitors of lung alveolar epithelium. Development 2001, 128, 5181–5188. [Google Scholar] [PubMed]
- Morigi, M.; Imberti, B.; Zoja, C.; Corna, D.; Tomasoni, S.; Abbate, M.; Rottoli, D.; Angioletti, S.; Benigni, A.; Perico, N.; et al. Mesenchymal stem cells are renotropic, helping to repair the kidney and improve function in acute renal failure. J. Am. Soc. Nephrol. 2004, 15, 1794–1804. [Google Scholar] [CrossRef] [PubMed]
- Rojas, M.; Xu, J.; Woods, C.R.; Mora, A.L.; Spears, W.; Roman, J.; Brigham, K.L. Bone marrow-derived mesenchymal stem cells in repair of the injured lung. Am. J. Respir. Cell Mol. Biol. 2005, 33, 145–152. [Google Scholar] [CrossRef] [PubMed]
- Baber, S.R.; Deng, W.; Master, R.G.; Bunnell, B.A.; Taylor, B.K.; Murthy, S.N.; Hyman, A.L.; Kadowitz, P.J. Intratracheal mesenchymal stem cell administration attenuates monocrotaline-induced pulmonary hypertension and endothelial dysfunction. Am. J. Physiol. Heart Circ. Physiol. 2007, 292, 120–128. [Google Scholar]
- Burdon, T.J.; Paul, A.; Noiseux, N.; Prakash, S.; Shum-Tim, D. Bone marrow stem cell derived paracrine factors for regenerative medicine: Current perspectives and therapeutic potential. Bone Marrow Res. 2011, 2011, 207326. [Google Scholar] [CrossRef] [PubMed]
- Nemeth, K.; Leelahavanichkul, A.; Yuen, P.S.; Mayer, B.; Parmelee, A.; Doi, K.; Robey, P.G.; Leelahavanichkul, K.; Koller, B.H.; Brown, J.M.; et al. Bone marrow stromal cells attenuate sepsis via prostaglandin E(2)-dependent reprogramming of host macrophages to increase their interleukin-10 production. Nat. Med. 2009, 15, 42–49. [Google Scholar] [CrossRef] [PubMed]
- Ortiz, L.A.; Dutreil, M.; Fattman, C.; Pandey, A.C.; Torres, G.; Go, K.; Phinney, D.G. Interleukin 1 receptor antagonist mediates the anti-inflammatory and anti-fibrotic effect of mesenchymal stem cells during lung injury. Proc. Natl. Acad. Sci. USA 2007, 104, 11002–11007. [Google Scholar] [CrossRef] [PubMed]
- Zhen, G.; Liu, H.; Gu, N.; Zhang, H.; Xu, Y.; Zhang, Z. Mesenchymal stem cells transplantation protects against rat pulmonary emphysema. Front. Biosci. 2008, 13, 3415–3422. [Google Scholar] [CrossRef] [PubMed]
- Akram, K.M.; Samad, S.; Spiteri, M.A.; Forsyth, N.R. Mesenchymal stem cells promote alveolar epithelial cell wound repair in vitro through distinct migratory and paracrine mechanisms. Respir. Res. 2013, 14. [Google Scholar] [CrossRef] [PubMed]
- Gnecchi, M.; Zhang, Z.; Ni, A.; Dzau, V.J. Paracrine mechanisms in adult stem cell signalling and therapy. Circ. Res. 2008, 103, 1204–1219. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, G.; Iyer, V.; Lee, T.C.; Canty, J.M., Jr. Autologous mesenchymal stem cells mobilize cKit+ and CD133+ bone marrow progenitor cells and improve regional function in hibernating myocardium. Circ. Res. 2011, 109, 1044–1054. [Google Scholar] [CrossRef] [PubMed]
- Islam, M.; Das, S.; Emin, M.; Wei, M.; Sun, L.; Westphalen, K.; Rowlands, D.J.; Quadri, S.K.; Bhattacharya, S.; Bhattacharya, J. Mitochondrial transfer from bone marrow derived stromal cells to pulmonary alveoli protects against acute lung injury. Nat. Med. 2012, 18, 759–765. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.; Mitsialis, S.; Aslam, M.; Vitali, S.; Vergadi, E.; Konstantinou, G.; Sdrimas, K.; Fernandez-Gonzalez, A.; Kourembanas, S. Exosomes mediate the cytoprotective action of mesenchymal stromal cells on hypoxia-induced pulmonary hypertension. Circulation 2012, 126, 2601–2611. [Google Scholar] [CrossRef] [PubMed]
- Akram, K.M.; Samad, S.; Spiteri, M.; Forsyth, N.R. Mesenchymal stem cell therapy and lung diseases. Adv. Biochem. Eng. Biotechnol. 2013, 130, 105–129. [Google Scholar] [PubMed]
- Ware, L.B.; Matthay, M.A. The acute respiratory distress syndrome. N. Engl. J. Med. 2000, 342, 1334–1349. [Google Scholar] [CrossRef] [PubMed]
- Danchuk, S.; Joni, H.; Hossain, F.F.; Sorge, R.F.; Ramsey, A.; Ryan, W.; Sullivan, D.E. Human multipotent stromal cells attenuate lipopolysaccharide-induced acute lung injury in mice via secretion of tumor necrosis factor-α-induced protein 6. Stem Cell Res. Ther. 2010, 2, 27–42. [Google Scholar]
- Gupta, N.; Su, X.; Popov, B.; Lee, J.W.; Serikov, V.; Matthay, M.A. Intrapulmonary delivery of bone marrow-derived mesenchymal stem cells improves survival and attenuates endotoxin-induced acute lung injury in mice. J. Immunol. 2007, 179, 855–1863. [Google Scholar] [CrossRef]
- Lee, J.W.; Fang, X.; Gupta, N.; Serikov, V.; Matthay, M.A. Allogeneic human mesenchymal stem cells for treatment of E. coli endotoxin-induced acute lung injury in the ex vivo perfused human lung. Proc. Natl. Acad. Sci. USA 2009, 106, 16357–16362. [Google Scholar] [CrossRef] [PubMed]
- Mei, S.H.J.; McCarter, S.D.; Deng, Y.; Parker, C.H.; Liles, W.C.; Stewart, D.J. Prevention of LPS-induced acute lung injury in mice by mesenchymal stem cells overexpressing angiopoietin 1. PLoS Med. 2007, 4, e269. [Google Scholar] [CrossRef] [PubMed]
- Chang, Y.S.; Oh, W.; Choi, S.J.; Sung, D.K.; Kim, S.Y.; Choi, E.Y.; Kang, S.; Jin, H.J.; Yang, Y.S.; Park, W.S. Human umbilical cord blood-derived mesenchymal stem cells attenuate hyperoxia-induced lung injury in neonatal rats. Cell Transplant. 2009, 18, 869–886. [Google Scholar] [CrossRef] [PubMed]
- Krasnodembskaya, A.; Song, Y.; Fang, X.; Gupta, N.; Serikov, V.; Lee, J.W.; Matthay, M.A. Antibacterial effect of human mesenchymal stem cells is mediated in part from secretion of the antimicrobial peptide LL-37. Stem Cells 2010, 28, 2229–2238. [Google Scholar] [CrossRef] [PubMed]
- Wilson, J.G.; Liu, K.D.; Zhuo, H.; Caballero, L.; McMillan, M.; Fang, X.; Cosgrove, K.; Vojnik, R.; Calfee, C.S.; Lee, J.W.; et al. Mesenchymal stem (stromal) cells for treatment of ARDS: A phase 1 clinical trial. Lancet Respir. Med. 2015, 3, 24–32. [Google Scholar] [CrossRef]
- Kanki-Horimoto, S.; Horimoto, H.; Mieno, S.; Kishida, K.; Watanabe, F.; Furuya, E.; Katsumata, T. Implantation of mesenchymal stem cells overexpressing endothelial nitric oxide synthase improves right ventricular impairments caused by pulmonary hypertension. Circulation 2006, 114, I181–I185. [Google Scholar] [CrossRef] [PubMed]
- Liang, O.; Mitsialis, S.; Chang, M.; Vergadi, E.; Lee, C.; Aslam, M.; Fernandez-Gonzalez, A.; Liu, X.; Baveja, R.; Kourembanas, S. Mesenchymal stromal cells expressing heme oxygenase-1 reverse pulmonary hypertension. Stem Cells 2011, 29, 99–107. [Google Scholar] [CrossRef] [PubMed]
- Lomas, N.J.; Watts, K.L.; Akram, K.M.; Forsyth, N.R.; Spiteri, M.A. Idiopathic pulmonary fibrosis: Immunohistochemical analysis provides fresh insights into lung tissue remodelling with implications for novel prognostic markers. Int. J. Clin. Exp. Pathol. 2012, 5, 58–71. [Google Scholar] [PubMed]
- Akram, K.M.; Lomas, N.J.; Spiteri, M.A.; Forsyth, N.R. Club cells inhibit alveolar epithelial wound repair via TRAIL-dependent apoptosis. Eur. Respir. J. 2013, 41, 683–694. [Google Scholar] [CrossRef] [PubMed]
- Akram, K.M.; Lomas, N.J.; Forsyth, N.R.; Spiteri, M.A. Alveolar epithelial cells in idiopathic pulmonary fibrosis display upregulation of TRAIL, DR4 and DR5 expression with simultaneous preferential over-expression of pro-apoptotic marker p53. Int. J. Clin. Exp. Pathol. 2014, 7, 552–564. [Google Scholar] [PubMed]
- Aguilar, S.; Scotton, C.; McNutty, K.; Nye, E.; Stamp, G.; Laurent, G.; Bonnet, D.; Janes, S.M. Bone marrow stem cells expressing keratinocyte growth factor via an inducible lentivirus protects against bleomycin induced pulmonary fibrosis. PLoS ONE 2009, 4, e8013. [Google Scholar] [CrossRef] [PubMed]
- Kumamoto, M.; Nishiwaki, T.; Matsuo, N.; Kimura, H.; Matsushima, K. Minimally cultured bone marrow mesenchymal stem cells ameliorate fibrotic lung injury. Eur. Respir. J. 2009, 34, 740–748. [Google Scholar] [CrossRef] [PubMed]
- Ortiz, L.; Gambelli, F.; McBride, C.; Gaupp, D.; Baddoo, M.; Kaminski, N.; Phinney, D.G. Mesenchymal stem cell engraftment in lung is enhanced in response to bleomycin exposure and ameliorates its fibrotic effects. Proc. Natl. Acad. Sci. USA 2003, 100, 8407–8411. [Google Scholar] [CrossRef] [PubMed]
- Guo, J.; Yi, E.S.; Havill, A.M.; Sarosi, I.; Whitcomb, L.; Yin, S.; Middleton, S.C.; Piguet, P.; Ulich, T.R. Intravenous keratinocyte growth factor protects against experimental pulmonary injury. Am. J. Physiol. 1998, 275, 800–805. [Google Scholar]
- Panos, R.J.; Rubin, J.S.; Csaky, K.G.; Aaronson, S.A.; Mason, R.J. Keratinocyte growth factor and hepatocyte growth factor/scatter factor are heparin-binding growth factors for alveolar type II cells in fibroblast-conditioned medium. J. Clin. Investig. 1993, 92, 969–977. [Google Scholar] [CrossRef] [PubMed]
- Yano, T.; Mason, R.J.; Pan, T.; Deterding, R.R.; Nielsen, L.D.; Shannon, J.M. KGF regulates pulmonary epithelial proliferation and surfactant protein gene expression in adult rat lung. Am. J. Physiol. Lung Cell. Mol. Physiol. 2000, 279, 1146–1158. [Google Scholar]
- Chapman, H.A.; Allen, C.L.; Stone, O.L. Abnormalities in pathways of alveolar fibrin turnover among patients with interstitial lung disease. Am. Rev. Respir. Dis. 1986, 133, 437–443. [Google Scholar] [PubMed]
- Abreu, S.C.; Antunes, M.A.; Pelosi, P.; Morales, M.M.; Rocco, P.R. Mechanisms of cellular therapy in respiratory diseases. Intensive Care Med. 2011, 37, 1421–1431. [Google Scholar] [CrossRef] [PubMed]
- Yuhgetsu, H.; Ohno, Y.; Funaguchi, N.; Asai, T.; Sawada, M.; Takemura, G.; Minatoguchi, S.; Fujiwara, H.; Fujiwara, T. Beneficial effects of autologous bone marrow mononuclear cell transplantation against elastase-induced emphysema in rabbits. Exp. Lung Res. 2006, 32, 413–426. [Google Scholar] [CrossRef] [PubMed]
- Weiss, D.; Casaburi, R.; Flannery, R.; Leroux-Williams, M.; Tashkin, D. A placebo-controlled, randomized trial of mesenchymal stem cells in COPD. Chest 2013, 143, 1590–1598. [Google Scholar] [CrossRef] [PubMed]
- Nichols, J.E.; Cortiella, J. Engineering of a complex organ: Progress toward development of a tissue-engineered lung. Proc. Am. Thorac. Soc. 2008, 5, 723–730. [Google Scholar] [CrossRef] [PubMed]
- Tomei, A.A.; Boschetti, F.; Gervaso, F.; Swartz, M.A. 3D collagen cultures under welldefined dynamic strain: A novel strain device with a porous elastomeric support. Biotechnol. Bioeng. 2009, 103, 217–225. [Google Scholar] [CrossRef] [PubMed]
- Omori, K.; Tada, Y.; Suzuki, T.; Nomoto, Y.; Matsuzuka, T.; Kobayashi, K.; Nakamura, T.; Kanemaru, S.; Yamashita, M.; Asato, R. Clinical application of in situ tissue engineering using a scaffolding technique for reconstruction of the larynx and trachea. Ann. Otol. Rhinol. Laryngol. 2008, 117, 673–678. [Google Scholar] [CrossRef] [PubMed]
- Omori, K.; Nakamura, T.; Kanemaru, S.; Magrufov, A.; Yamashita, M.; Shimizu, Y. In situ tissue engineering of the cricoid and trachea in a canine model. Ann. Otol. Rhinol. Laryngol. 2008, 117, 609–613. [Google Scholar] [CrossRef] [PubMed]
- Macchiarini, P.; Jungebluth, P.; Go, T.; Asnaghi, M.A.; Rees, L.E.; Cogan, T.A.; Dodson, A.; Martorell, J.; Bellini, S.; Parnigotto, P.P.; et al. Clinical transplantation of a tissue-engineered airway. Lancet 2008, 372, 2023–2030. [Google Scholar] [CrossRef]
- Ott, H.C.; Clippinger, B.; Conrad, C.; Schuetz, C.; Pomerantseva, I.; Ikonomou, L.; Kotton, D.; Vacanti, J.P. Regeneration and orthotopic transplantation of a bioartificial lung. Nat. Med. 2010, 16, 927–933. [Google Scholar] [CrossRef] [PubMed]
- Petersen, T.H.; Calle, E.A.; Zhao, L.; Lee, E.J.; Gui, L.; Raredon, M.B.; Gavrilov, K.; Yi, T.; Zhuang, Z.W.; Breuer, C.; et al. Tissue-engineered lungs for in vivo implantation. Science 2010, 329, 538–541. [Google Scholar] [CrossRef] [PubMed]
- Price, A.P.; England, K.A.; Matson, A.M.; Blazar, B.R.; Panoskaltsis-Mortari, A. Development of a decellularized lung bioreactor system for bioengineering the lung: The matrix reloaded. Tissue Eng. Part A 2010, 16, 2581–2591. [Google Scholar] [CrossRef] [PubMed]
- Daly, A.B.; Wallis, J.M.; Borg, Z.D.; Bonvillain, R.W.; Deng, B.; Ballif, B.A.; Jaworski, D.M.; Allen, G.B.; Weiss, D.J. Initial binding and recellularization of decellularized mouse lung scaffolds with bone marrow–derived mesenchymal stromal cells. Tissue Eng. Part A 2012, 18, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Wallis, J.M.; Borg, Z.D.; Daly, A.B.; Deng, B.; Ballif, B.A.; Allen, G.B.; Jaworski, D.M.; Weiss, D.J. Comparative assessment of detergent-based protocols for mouse lung de-cellularization and re-cellularization. Tissue Eng. Part C Methods 2012, 18, 420–432. [Google Scholar] [CrossRef] [PubMed]
- Cortiella, J.; Niles, J.; Cantu, A.; Brettler, A.; Pham, A.; Vargas, G.; Winston, S.; Wang, J.; Walls, S.; Nichols, J.E. Influence of acellular natural lung matrix on murine embryonic stem cell differentiation and tissue formation. Tissue Eng. Part A 2010, 16, 2565–2580. [Google Scholar] [CrossRef] [PubMed]
- Ghaedi, M.; Calle, E.A.; Mendez, J.J.; Gard, A.L.; Balestrini, J.; Booth, A.; Bove, P.F.; Gui, L.; White, E.S.; Niklason, L.E. Human IPS cell-derived alveolar epithelium repopulates lung extracellular matrix. J. Clin. Investig. 2013, 123, 4950–4962. [Google Scholar] [CrossRef] [PubMed]
- Bonvillain, R.W.; Scarritt, M.E.; Pashos, N.C.; Mayeux, J.P.; Meshberger, C.L.; Betancourt, A.M.; Sullivan, D.E.; Bunnell, B.A. Nonhuman primate lung decellularization and recellularization using a specialized large-organ bioreactor. J. Vis. Exp. 2013, 82. [Google Scholar] [CrossRef] [PubMed]
- Booth, A.J.; Hadley, R.; Cornett, A.M.; Dreffs, A.A.; Matthes, S.A.; Tsui, J.L.; Weiss, K.; Horowitz, J.C.; Fiore, V.F.; Barker, T.H.; et al. Acellular normal and fibrotic human lung matrices as a culture system for in vitro investigation. Am. J. Respir. Crit. Care Med. 2012, 186, 866–876. [Google Scholar] [CrossRef] [PubMed]
- Gilpin, S.E.; Guyette, J.P.; Gonzalez, G.; Ren, X.; Asara, J.M.; Mathisen, D.J.; Vacanti, J.P.; Ott, H.C. Perfusion decellularization of human and porcine lungs: Bringing the matrix to clinical scale. J. Heart Lung Transplant. 2013, 33, 298–308. [Google Scholar] [CrossRef] [PubMed]
- Nichols, J.E.E.; Niles, J.; Riddle, M.; Vargas, G.; Schilagard, T.; Ma, L.; Edward, K.; La Francesca, S.; Sakamoto, J.; Vega, S.; et al. Production and assessment of decellularized pig and human lung scaffolds. Tissue Eng. Part A 2013, 19, 2045–2062. [Google Scholar] [CrossRef] [PubMed]
- Wagner, D.E.; Bonenfant, N.R.; Sokocevic, D.; Desarno, M.J.; Borg, Z.D.; Parsons, C.S.; Brooks, E.M.; Platz, J.J.; Khalpey, Z.I.; Hoganson, D.M.; et al. Three-dimensional scaffolds of acellular human and porcine lungs for high throughput studies of lung disease and regeneration. Biomaterials 2014, 35, 2664–2679. [Google Scholar] [CrossRef] [PubMed]
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Akram, K.M.; Patel, N.; Spiteri, M.A.; Forsyth, N.R. Lung Regeneration: Endogenous and Exogenous Stem Cell Mediated Therapeutic Approaches. Int. J. Mol. Sci. 2016, 17, 128. https://doi.org/10.3390/ijms17010128
Akram KM, Patel N, Spiteri MA, Forsyth NR. Lung Regeneration: Endogenous and Exogenous Stem Cell Mediated Therapeutic Approaches. International Journal of Molecular Sciences. 2016; 17(1):128. https://doi.org/10.3390/ijms17010128
Chicago/Turabian StyleAkram, Khondoker M., Neil Patel, Monica A. Spiteri, and Nicholas R. Forsyth. 2016. "Lung Regeneration: Endogenous and Exogenous Stem Cell Mediated Therapeutic Approaches" International Journal of Molecular Sciences 17, no. 1: 128. https://doi.org/10.3390/ijms17010128
APA StyleAkram, K. M., Patel, N., Spiteri, M. A., & Forsyth, N. R. (2016). Lung Regeneration: Endogenous and Exogenous Stem Cell Mediated Therapeutic Approaches. International Journal of Molecular Sciences, 17(1), 128. https://doi.org/10.3390/ijms17010128