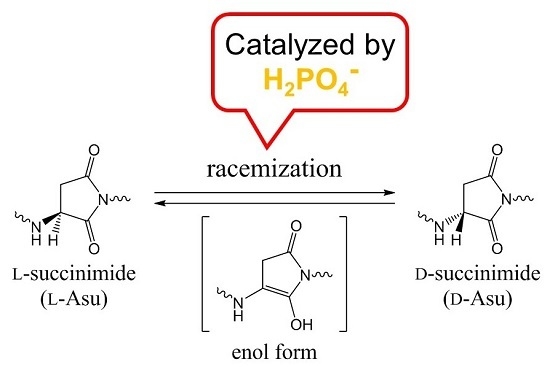
Racemization of the Succinimide Intermediate Formed in Proteins and Peptides: A Computational Study of the Mechanism Catalyzed by Dihydrogen Phosphate Ion
Abstract
Share and Cite
Takahashi, O.; Kirikoshi, R.; Manabe, N. Racemization of the Succinimide Intermediate Formed in Proteins and Peptides: A Computational Study of the Mechanism Catalyzed by Dihydrogen Phosphate Ion. Int. J. Mol. Sci. 2016, 17, 1698. https://doi.org/10.3390/ijms17101698
Takahashi O, Kirikoshi R, Manabe N. Racemization of the Succinimide Intermediate Formed in Proteins and Peptides: A Computational Study of the Mechanism Catalyzed by Dihydrogen Phosphate Ion. International Journal of Molecular Sciences. 2016; 17(10):1698. https://doi.org/10.3390/ijms17101698
Chicago/Turabian StyleTakahashi, Ohgi, Ryota Kirikoshi, and Noriyoshi Manabe. 2016. "Racemization of the Succinimide Intermediate Formed in Proteins and Peptides: A Computational Study of the Mechanism Catalyzed by Dihydrogen Phosphate Ion" International Journal of Molecular Sciences 17, no. 10: 1698. https://doi.org/10.3390/ijms17101698
APA StyleTakahashi, O., Kirikoshi, R., & Manabe, N. (2016). Racemization of the Succinimide Intermediate Formed in Proteins and Peptides: A Computational Study of the Mechanism Catalyzed by Dihydrogen Phosphate Ion. International Journal of Molecular Sciences, 17(10), 1698. https://doi.org/10.3390/ijms17101698