Ziyuglycoside I Inhibits the Proliferation of MDA-MB-231 Breast Carcinoma Cells through Inducing p53-Mediated G2/M Cell Cycle Arrest and Intrinsic/Extrinsic Apoptosis
Abstract
:1. Introduction
2. Results
2.1. Ziyuglycoside I Suppressed the Proliferation of MDA-MB-231 Cells
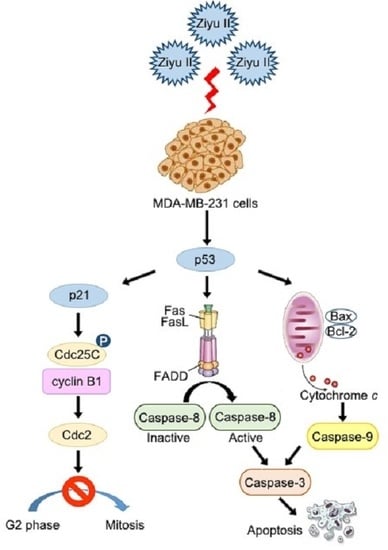
2.2. Ziyuglycoside I Induced G2/M Phase Arrest and Apoptosis on MDA-MB-231 Cells
2.3. Ziyuglycoside I Induced G2/M Phase Arrest in MDA-MB-231 Cells through Modulating Cell Cycle-Related Proteins
2.4. Ziyuglycoside I Induced Apoptosis in MDA-MB-231 Cells through Intrinsic and Extrinsic Pathways
2.5. Ziyuglycoside I-Induced Cell Cycle Arrest and Apoptosis in MDA-MB-231 Cells Were Partially Mediated by p53
3. Discussion
4. Materials and Methods
4.1. Materials
4.2. Methods
4.2.1. Cell Culture
4.2.2. Cell Viability Assay
4.2.3. Cell Cycle Distribution
4.2.4. Measurement of Cell Apoptosis
4.2.5. Caspase Activity Assay
4.2.6. Measurement of Mitochondrial Membrane Potential
4.2.7. Measurement of Cytochrome c Release
4.2.8. Measurement of Fas/APO-1 and FasL Levels
4.2.9. Transfection with Small Interfering RNA (siRNA)
4.2.10. Western Blot Analysis
4.2.11. Statistical Analysis
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Zeichner, S.B.; Terawaki, H.; Gogineni, K. A review of systemic treatment in metastatic triple-negative breast cancer. Breast Cancer 2016, 10, 25–36. [Google Scholar] [CrossRef] [PubMed]
- Mouh, F.Z.; Mzibri, M.E.; Slaoui, M.; Amrani, M. Recent progress in triple negative breast cancer research. Asian Pac. J. Cancer Prev. 2016, 17, 1595–1608. [Google Scholar] [CrossRef] [PubMed]
- Bianchini, G.; Balko, J.M.; Mayer, I.A.; Sanders, M.E.; Gianni, L. Triple-negative breast cancer: Challenges and opportunities of a heterogeneous disease. Nat. Rev. Clin. Oncol. 2016. [Google Scholar] [CrossRef] [PubMed]
- Collignon, J.; Lousberg, L.; Schroeder, H.; Jerusalem, G. Triple-negative breast cancer: Treatment challenges and solutions. Breast Cancer 2016, 8, 93–107. [Google Scholar] [PubMed]
- Choisy-Rossi, C.; Reisdorf, P.; Yonish-Rouach, E. Mechanisms of p53-induced apoptosis: In search of genes which are regulated during p53-mediated cell death. Toxicol. Lett. 1998, 102–103, 491–496. [Google Scholar] [CrossRef]
- Pflaum, J.; Schlosser, S.; Muller, M. p53 family and cellular stress responses in cancer. Front. Oncol. 2014, 4, 285. [Google Scholar] [CrossRef] [PubMed]
- Bates, S.; Vousden, K.H. p53 in signaling checkpoint arrest or apoptosis. Curr. Opin. Genet. Dev. 1996, 6, 12–18. [Google Scholar] [CrossRef]
- Pietenpol, J.A.; Stewart, Z.A. Cell cycle checkpoint signaling: Cell cycle arrest versus apoptosis. Toxicology 2002, 181–182, 475–481. [Google Scholar] [CrossRef]
- Waring, P.; Mullbacher, A. Cell death induced by the Fas/Fas ligand pathway and its role in pathology. Immunol. Cell Biol. 1999, 77, 312–317. [Google Scholar] [CrossRef] [PubMed]
- Callahan, R. p53 Mutations—Another breast cancer prognostic factor. J. Natl. Cancer Inst. 1992, 84, 826–827. [Google Scholar] [CrossRef] [PubMed]
- Hartmann, A.; Blaszyk, H.; Kovach, J.S.; Sommer, S.S. The molecular epidemiology of p53 gene mutations in human breast cancer. Trends Genet. 1997, 13, 27–33. [Google Scholar] [CrossRef]
- Zhu, L.; Li, L.; Li, Y.; Wang, J.; Wang, Q. Chinese herbal medicine as an adjunctive therapy for breast cancer: A systematic review and meta-analysis. Evid. Based Complement. Altern. Med. 2016, 2016, 9469276. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.H.; Chung, C.B.; Kim, J.G.; Ko, K.I.; Park, S.H.; Kim, J.H.; Eom, S.Y.; Kim, Y.S.; Hwang, Y.I.; Kim, K.H. Anti-wrinkle activity of ziyuglycoside I isolated from a Sanguisorba officinalis root extract and its application as a cosmeceutical ingredient. Biosci. Biotechnol. Biochem. 2008, 72, 303–311. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Loo, W.T.; Wang, N.; Chow, L.W.; Wang, D.; Han, F.; Zheng, X.; Chen, J.P. Effect of Sanguisorba officinalis L on breast cancer growth and angiogenesis. Expert. Opin. Ther. Targets 2012, 16, S79–S89. [Google Scholar] [CrossRef] [PubMed]
- Choi, E.S.; Kim, J.S.; Kwon, K.H.; Kim, H.S.; Cho, N.P.; Cho, S.D. Methanol extract of Sanguisorba officinalis L. with cytotoxic activity against PC3 human prostate cancer cells. Mol. Med. Rep. 2012, 6, 670–674. [Google Scholar] [CrossRef] [PubMed]
- Cai, Z.; Li, W.; Wang, H.; Yan, W.; Zhou, Y.; Wang, G.; Cui, J.; Wang, F. Anti-tumor and immunomodulating activities of a polysaccharide from the root of Sanguisorba officinalis L. Int. J. Biol. Macromol. 2012, 51, 484–488. [Google Scholar] [CrossRef] [PubMed]
- Zhu, X.; Wang, K.; Zhang, K.; Huang, B.; Zhang, J.; Zhang, Y.; Zhu, L.; Zhou, B.; Zhou, F. Ziyuglycoside II inhibits the growth of human breast carcinoma MDA-MB-435 cells via cell cycle arrest and induction of apoptosis through the mitochondria dependent pathway. Int. J. Mol. Sci. 2013, 14, 18041–18055. [Google Scholar] [CrossRef] [PubMed]
- Zhu, X.; Wang, K.; Zhang, K.; Zhu, L.; Zhou, F. Ziyuglycoside II induces cell cycle arrest and apoptosis through activation of ROS/JNK pathway in human breast cancer cells. Toxicol. Lett. 2014, 227, 65–73. [Google Scholar] [CrossRef] [PubMed]
- Lane, D.P. Cancer. p53, guardian of the genome. Nature 1992, 358, 15–16. [Google Scholar] [CrossRef] [PubMed]
- Cohen, G.M. Caspases: The executioners of apoptosis. Biochem. J. 1997, 326, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Blagosklonny, M.V.; Pardee, A.B. The restriction point of the cell cycle. Cell Cycle 2002, 1, 103–110. [Google Scholar] [CrossRef] [PubMed]
- Runnebaum, I.B.; Nagarajan, M.; Bowman, M.; Soto, D.; Sukumar, S. Mutations in p53 as potential molecular markers for human breast cancer. Proc. Natl. Acad. Sci. USA 1991, 88, 10657–10661. [Google Scholar] [CrossRef] [PubMed]
- Cahill, D.P.; Lengauer, C.; Yu, J.; Riggins, G.J.; Willson, J.K.; Markowitz, S.D.; Kinzler, K.W.; Vogelstein, B. Mutations of mitotic checkpoint genes in human cancers. Nature 1998, 392, 300–303. [Google Scholar] [PubMed]
- Turner, N.; Moretti, E.; Siclari, O.; Migliaccio, I.; Santarpia, L.; D′Incalci, M.; Piccolo, S.; Veronesi, A.; Zambelli, A.; del Sal, G.; et al. Targeting triple negative breast cancer: Is p53 the answer? Cancer Treat. Rev. 2013, 39, 541–550. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Zhu, H.; Xu, C.J.; Yuan, J. Cleavage of BID by caspase 8 mediates the mitochondrial damage in the Fas pathway of apoptosis. Cell 1998, 94, 491–501. [Google Scholar] [CrossRef]
- Koff, J.L.; Ramachandiran, S.; Bernal-Mizrachi, L. A time to kill: Targeting apoptosis in cancer. Int. J. Mol. Sci. 2015, 16, 2942–2955. [Google Scholar] [CrossRef] [PubMed]
- Cheng, E.H.; Kirsch, D.G.; Clem, R.J.; Ravi, R.; Kastan, M.B.; Bedi, A.; Ueno, K.; Hardwick, J.M. Conversion of Bcl-2 to a Bax-like death effector by caspases. Science 1997, 278, 1966–1968. [Google Scholar] [CrossRef] [PubMed]
- Porter, A.G.; Janicke, R.U. Emerging roles of caspase-3 in apoptosis. Cell Death Differ. 1999, 6, 99–104. [Google Scholar] [CrossRef] [PubMed]
- Speidel, D. Transcription-independent p53 apoptosis: An alternative route to death. Trends Cell Biol. 2010, 20, 14–24. [Google Scholar] [CrossRef] [PubMed]
- Muller, P.A.; Vousden, K.H. Mutant p53 in cancer: New functions and therapeutic opportunities. Cancer Cell 2014, 25, 304–317. [Google Scholar] [CrossRef] [PubMed]
- Kruger, N.J. The Bradford Method for Protein Quantitation. In Basic Protein and Peptide Protocols; Humana Press: Totowa, NJ, USA, 1994; pp. 9–15. [Google Scholar]











© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhu, X.; Wang, K.; Zhang, K.; Zhang, T.; Yin, Y.; Xu, F. Ziyuglycoside I Inhibits the Proliferation of MDA-MB-231 Breast Carcinoma Cells through Inducing p53-Mediated G2/M Cell Cycle Arrest and Intrinsic/Extrinsic Apoptosis. Int. J. Mol. Sci. 2016, 17, 1903. https://doi.org/10.3390/ijms17111903
Zhu X, Wang K, Zhang K, Zhang T, Yin Y, Xu F. Ziyuglycoside I Inhibits the Proliferation of MDA-MB-231 Breast Carcinoma Cells through Inducing p53-Mediated G2/M Cell Cycle Arrest and Intrinsic/Extrinsic Apoptosis. International Journal of Molecular Sciences. 2016; 17(11):1903. https://doi.org/10.3390/ijms17111903
Chicago/Turabian StyleZhu, Xue, Ke Wang, Kai Zhang, Ting Zhang, Yongxiang Yin, and Fei Xu. 2016. "Ziyuglycoside I Inhibits the Proliferation of MDA-MB-231 Breast Carcinoma Cells through Inducing p53-Mediated G2/M Cell Cycle Arrest and Intrinsic/Extrinsic Apoptosis" International Journal of Molecular Sciences 17, no. 11: 1903. https://doi.org/10.3390/ijms17111903
APA StyleZhu, X., Wang, K., Zhang, K., Zhang, T., Yin, Y., & Xu, F. (2016). Ziyuglycoside I Inhibits the Proliferation of MDA-MB-231 Breast Carcinoma Cells through Inducing p53-Mediated G2/M Cell Cycle Arrest and Intrinsic/Extrinsic Apoptosis. International Journal of Molecular Sciences, 17(11), 1903. https://doi.org/10.3390/ijms17111903