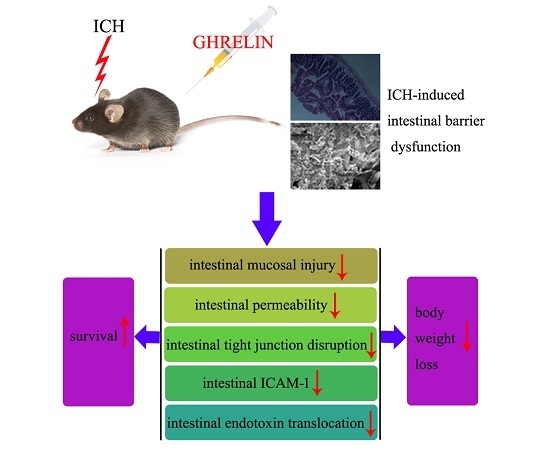
Ghrelin Attenuates Intestinal Barrier Dysfunction Following Intracerebral Hemorrhage in Mice
Abstract
:1. Introduction
2. Results
2.1. Ghrelin Improved Histological Changes in the Intestinal Mucosa after ICH
2.2. Ghrelin Attenuated Intestinal Permeability after ICH
2.3. Ghrelin Upregulated Tight Junction Protein Expression after ICH
2.4. Ghrelin Downregulated ICAM-1 Expression after ICH
2.5. Ghrelin Suppressed the Translocation of Intestinal Endotoxin after ICH
2.6. Effect of GHrelin on Survival and Body Weight after ICH
3. Discussion
4. Experimental Section
4.1. Experimental Groups and Drugs
4.2. ICH Model
4.3. Histopathology
4.4. Intestinal Permeability
4.5. Serum Endotoxin Level
4.6. Quantitative RT-PCR
4.7. Western Blot Analysis
4.8. Immunohistochemistry
4.9. Statistical Analysis
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Keep, R.F.; Hua, Y.; Xi, G. Intracerebral haemorrhage: Mechanisms of injury and therapeutic targets. Lancet Neurol. 2012, 11, 720–731. [Google Scholar] [CrossRef]
- Yang, T.C.; Li, J.G.; Shi, H.M.; Yu, D.M.; Shan, K.; Li, L.X.; Dong, X.Y.; Ren, T.H. Gastrointestinal bleeding after intracerebral hemorrhage: A retrospective review of 808 cases. Am. J. Med. Sci. 2013, 346, 279–282. [Google Scholar] [CrossRef] [PubMed]
- Lu, W.Y.; Rhoney, D.H.; Boling, W.B.; Johnson, J.D.; Smith, T.C. A review of stress ulcer prophylaxis in the neurosurgical intensive care unit. Neurosurgery 1997, 41, 416–426. [Google Scholar] [CrossRef]
- Bansal, V.; Costantini, T.; Kroll, L.; Peterson, C.; Loomis, W.; Eliceiri, B.; Baird, A.; Wolf, P.; Coimbra, R. Traumatic brain injury and intestinal dysfunction: Uncovering the neuro-enteric axis. J. Neurotrauma 2009, 26, 1353–1359. [Google Scholar] [CrossRef] [PubMed]
- Bansal, V.; Ryu, S.Y.; Blow, C.; Costantini, T.; Loomis, W.; Eliceiri, B.; Baird, A.; Wolf, P.; Coimbra, R. The hormone ghrelin prevents traumatic brain injury induced intestinal dysfunction. J. Neurotrauma 2010, 27, 2255–2260. [Google Scholar] [CrossRef] [PubMed]
- Misra, U.K.; Kalita, J.; Pandey, S.; Mandal, S.K. Predictors of gastrointestinal bleeding in acute intracerebral haemorrhage. J. Neurol. Sci. 2003, 208, 25–29. [Google Scholar] [CrossRef]
- Misra, U.K.; Kalita, J.; Pandey, S.; Mandal, S.K.; Srivastava, M. A randomized placebo controlled trial of ranitidine versus sucralfate in patients with spontaneous intracerebral hemorrhage for prevention of gastric hemorrhage. J. Neurol. Sci. 2005, 239, 5–10. [Google Scholar] [CrossRef] [PubMed]
- Kojima, M.; Hosoda, H.; Date, Y.; Nakazato, M.; Matsuo, H.; Kangawa, K. Ghrelin is a growth-hormone-releasing acylated peptide from stomach. Nature 1999, 402, 656–660. [Google Scholar] [CrossRef] [PubMed]
- Date, Y.; Kojima, M.; Hosoda, H.; Sawaguchi, A.; Mondal, M.S.; Suganuma, T.; Matsukura, S.; Kangawa, K.; Nakazato, M. Ghrelin, a novel growth hormone-releasing acylated peptide, is synthesized in a distinct endocrine cell type in the gastrointestinal tracts of rats and humans. Endocrinology 2000, 141, 4255–4261. [Google Scholar] [CrossRef] [PubMed]
- Peeters, T.L. Ghrelin and the gut. Endocr. Dev. 2013, 25, 41–48. [Google Scholar] [PubMed]
- Wu, R.; Dong, W.; Qiang, X.; Wang, H.; Blau, S.A.; Ravikumar, T.S.; Wang, P. Orexigenic hormone ghrelin ameliorates gut barrier dysfunction in sepsis in rats. Crit. Care Med. 2009, 37, 2421–2426. [Google Scholar] [CrossRef] [PubMed]
- Lang, Y.; Fu, F.; Sun, D.; Xi, C.; Chen, F. Labetalol prevents intestinal dysfunction induced by traumatic brain injury. PLoS ONE 2015, 10, e0133215. [Google Scholar] [CrossRef] [PubMed]
- Sumaqin, R.; Robin, A.Z.; Nusrat, A.; Parkos, C.A. Transmigrated neutrophils in the intestinal lumen engage ICAM-1 to regulate the epithelial barrier and neutrophil recruitment. Mucosal. Immunol. 2014, 7, 905–915. [Google Scholar] [CrossRef] [PubMed]
- Hang, C.H.; Shi, J.X.; Li, J.S.; Wu, W.; Yin, H.X. Alterations of intestinal mucosa structure and barrier function following traumatic brain injury in rats. World J. Gastroenterol. 2003, 9, 2776–2781. [Google Scholar] [CrossRef] [PubMed]
- Jin, W.; Wang, H.; Ji, Y.; Hu, Q.; Yan, W.; Chen, G.; Yin, H. Increased intestinal inflammatory response and gut barrier dysfunctionin Nrf2-deficient mice after traumatic brain injury. Cytokine 2008, 44, 135–140. [Google Scholar] [CrossRef] [PubMed]
- Hang, C.H.; Shi, J.X.; Li, J.S.; Li, W.Q.; Yin, H.X. Up-regulation of intestinal nuclear factor κB and intercellular adhesion molecule-1 following traumatic brain injury in rats. World J. Gastroenterol. 2005, 11, 1149–1154. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.D.; Zhou, Y.T. Effects of progesterone on intestinal inflammatory response and mucosa structure alterations following SAH in male rats. J. Surg. Res. 2011, 171, e47–e53. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Zhu, Y.; Chuai, J. Changes in serum ghrelin and small intestinal motility in rats with ischemic stroke. Anat. Rec. 2012, 295, 307–312. [Google Scholar] [CrossRef] [PubMed]
- Hosoda, H.; Kangawa, K. Standard sample collections for blood ghrelin measurements. Methods Enzymol. 2012, 514, 113–126. [Google Scholar] [PubMed]
- Sun, D.A.; Deshpande, L.S.; Sombati, S.; Baranova, A.; Wilson, M.S.; Hamm, R.J.; DeLorenzo, R.J. Traumatic brain injury causes a long-lasting calcium (Ca2+)-plateau of elevated intracellular Ca levels and altered Ca2+ homeostatic mechanisms in hippocampal neurons surviving brain injury. Eur. J. Neurosci. 2008, 27, 1659–1672. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Jiang, X. Effects of enteral nutrition on the barrier function of the intestinal mucosa and dopamine receptor expression in rats with traumatic brain injury. JPEN J. Parenter. Enter. Nutr. 2015, 39, 114–123. [Google Scholar] [CrossRef] [PubMed]
- Diao, L.; Mei, Q.; Xu, J.M.; Liu, X.C.; Hu, J.; Jin, J.; Yao, Q.; Chen, M. Rebamipide suppresses diclofenac-induced intestinal permeability via mitochondrial protection in mice. World J. Gastroenterol. 2012, 18, 1059–1066. [Google Scholar] [CrossRef] [PubMed]
- Jin, W.; Wang, H.D.; Hu, Z.G.; Yan, W.; Chen, G.; Yin, H.X. Transcription factor Nrf2 plays a pivotal role in protection against traumatic brain injury-induced acute intestinal mucosal injury in mice. J. Surg. Res. 2009, 157, 251–260. [Google Scholar] [CrossRef] [PubMed]
- Lippai, D.; Bala, S.; Catalano, D.; Kodys, K.; Szabo, G. Micro-RNA-155 deficiency prevents alcohol-induced serum endotoxin increase and small bowel inflammation in mice. Alcohol. Clin. Exp. Res. 2014, 38, 2217–2224. [Google Scholar] [CrossRef] [PubMed]
- Doig, C.J.; Sutherland, L.R.; Sandham, J.D.; Fick, G.H.; Verhoef, M.; Meddings, J.B. Increased intestinal permeability is associated with the development of multiple organ dysfunction syndrome in critically ill ICU patients. Am. J. Respir. Crit. Care Med. 1998, 158, 444–451. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, S.H.; He, Y.Y.; Nassief, A.; Xu, J.; Xu, X.M.; Hsu, C.Y.; Faraci, F.M. Effects of lipopolysaccharide priming on acute ischemic brain injury. Stroke 2000, 31, 193–199. [Google Scholar] [CrossRef] [PubMed]
- Morganti-Kossmann, M.C.; Rancan, M.; Stahel, P.F.; Kossmann, T. Inflammatory response in acute traumatic brain injury: A double-edged sword. Curr. Opin. Crit. Care 2002, 8, 101–105. [Google Scholar] [CrossRef] [PubMed]
- Gumbiner, B.M. Cell adhesion: The molecular basis of tissue architecture and morphogenesis. Cell 1996, 84, 345–357. [Google Scholar] [CrossRef]
- Furuse, M.; Fujita, K.; Hiiragi, T.; Fujimoto, K.; Tsukita, S. Claudin-1 and -2: Novel integral membrane proteins localizing at tight junctions with no sequence similarity to occludin. J. Cell Biol. 1998, 141, 1539–1550. [Google Scholar] [CrossRef] [PubMed]
- Pan, L.; Qin, G.; Zhao, Y.; Wang, J.; Liu, F.; Che, D. Effects of soybean agglutinin on mechanical barrier function and tight junction protein expression in intestinal epithelial cells from piglets. Int. J. Mol. Sci. 2013, 14, 21689–21704. [Google Scholar] [CrossRef] [PubMed]
- Gumbiner, B.; Lowenkopf, T.; Apatira, D. Identification of a 160-kDa polypeptide that binds to the tight junction protein ZO-1. Proc. Natl. Acad. Sci. USA 1991, 88, 3460–3464. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Qin, G.; Han, R.; Wang, J.; Zhang, X.; Liu, D. β-conglycinin reduces the tight junction occludin and ZO-1 expression in IPEC-J2. Int. J. Mol. Sci. 2014, 15, 1915–1926. [Google Scholar] [CrossRef] [PubMed]
- Ikenouchi, J.; Furuse, M.; Furuse, K.; Sasaki, H.; Tsukita, S.; Tsukita, S. Tricellulin constitutes a novel barrier at tricellular contacts of epithelial cells. J. Cell Biol. 2005, 171, 939–945. [Google Scholar] [CrossRef] [PubMed]
- Zacharia, J.; Mauban, J.R.; Raina, H.; Fisher, S.A.; Wier, W.G. High vascular tone of mouse femoral arteries in vivo is determined by sympathetic nerve activity via α1A- and α1D-adrenoceptor subtypes. PLoS ONE 2013, 8, e65969. [Google Scholar] [CrossRef] [PubMed]
- Gong, Y.; Li, H.; Li, Y. Effects of Bacillus subtilis on epithelial tight junctions of mice with inflammatory bowel disease. J. Interferon Cytokine Res. 2016, 36, 75–85. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Tang, G.; Li, Y.; Wang, Y.; Chen, X.; Gu, X.; Zhang, Z.; Wang, Y.; Yang, G.Y. Metformin attenuates blood-brain barrier disruption in mice following middle cerebral artery occlusion. J. Neuroinflamm. 2014, 11, 177. [Google Scholar] [CrossRef] [PubMed]
- Jones, S.C.; Banks, R.E.; Haidar, A.; Gearing, A.J.; Hemingway, I.K.; Ibbotson, S.H.; Dixon, M.F.; Axon, A.T. Adhesion molecules in inflammatory bowel disease. Gut 1995, 36, 724–730. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, S.; Ohtani, H.; Watanabe, Y.; Fukushima, K.; Matsumoto, T.; Kitano, A.; Kobayashi, K.; Nagura, H. In situ expression of the cell adhesion molecules in inflammatory bowel disease: Evidence of immunologic activation of vascular endothelial cells. Lab. Investig. 1993, 69, 257–264. [Google Scholar] [PubMed]
- Feng, D.; Xu, W.; Chen, G.; Hang, C.; Gao, H.; Yin, H. Influence of glutamine on intestinal inflammatory response, mucosa structure alterations and apoptosis following traumatic brain injury in rats. J. Int. Med. Res. 2007, 35, 644–656. [Google Scholar] [CrossRef] [PubMed]
- Zhou, M.L.; Zhu, L.; Wang, J.; Hang, C.H.; Shi, J.X. The inflammation in the gut after experimental subarachnoid hemorrhage. J. Surg. Res. 2007, 137, 103–108. [Google Scholar] [CrossRef] [PubMed]
- Sun, Z.; Wang, X.; Lasson, A.; Bojesson, A.; Annborn, M.; Andersson, R. Effects of inhibition of PAF, ICAM-1 and PECAM-1 on gut barrier failure caused by intestinal ischemia and reperfusion. Scand. J. Gastroenterol. 2001, 36, 55–65. [Google Scholar] [CrossRef] [PubMed]
- Shibahara, T.; Miyazaki, K.; Sato, D.; Matsui, H.; Yanaka, A.; Nakahara, A.; Tanaka, N. Alteration of intestinal epithelial function by intraepithelial lymphocyte homing. J. Gastroenterol. 2005, 40, 878–886. [Google Scholar] [CrossRef] [PubMed]
- Kalff, J.C.; Hierholzer, C.; Tsukada, K.; Billiar, T.R.; Bauer, A.J. Hemorrhagic shock results in intestinal muscularis intercellular adhesion molecule (ICAM-1) expression, neutrophil infiltration, and smooth muscle dysfunction. Arch. Orthop. Trauma Surg. 1999, 119, 89–93. [Google Scholar] [CrossRef] [PubMed]
- Kellokoski, E.; Kunnari, A.; Jokela, M.; Makela, S.; Kesaniemi, Y.A.; Horkko, S. Ghrelin and obestatin modulate early atherogenic processes on cells: Enhancement of monocyte adhesion and oxidized low-density lipoprotein binding. Metabolism 2009, 58, 1572–1580. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Qu, X.; Yuan, F.; Yang, Y.; Xu, L.; Dai, J.; Wang, W.; Fei, J.; Hou, X.; Fang, W. Ghrelin receptor deficiency aggravates atherosclerotic plaque instability and vascular inflammation. Front. Biosci. 2015, 20, 604–613. [Google Scholar]
- Tschop, M.; Smiley, D.L.; Heiman, M.L. Ghrelin induces adiposity in rodents. Nature 2000, 407, 908–913. [Google Scholar] [CrossRef] [PubMed]
- Qi, L.; Cui, X.; Dong, W.; Barrera, R.; Nicastro, J.; Coppa, G.F.; Wang, P.; Wu, R. Ghrelin attenuates brain injury after traumatic brain injury and uncontrolled hemorrhagic shock in rats. Mol. Med. 2012, 18, 186–193. [Google Scholar] [CrossRef] [PubMed]
- Sangiao-Alvarellos, S.; Cordido, F. Effect of ghrelin on glucose-insulin homeostasis: Therapeutic implications. Int. J. Pept. 2010, 2010. [Google Scholar] [CrossRef] [PubMed]
- Nakazato, M.; Murakami, N.; Date, Y.; Kojima, M.; Matsuo, H.; Kangawa, K.; Matsukura, S. A role for ghrelin in the central regulation of feeding. Nature 2001, 409, 194–198. [Google Scholar] [CrossRef] [PubMed]
- Cowley, M.A.; Smith, R.G.; Diano, S.; Tschop, M.; Pronchuk, N.; Grove, K.L.; Strasburger, C.J.; Bidlingmaier, M.; Esterman, M.; Heiman, M.L.; et al. The distribution and mechanism of action of ghrelin in the CNS demonstrates a novel hypothalamic circuit regulating energy homeostasis. Neuron 2003, 37, 649–661. [Google Scholar] [CrossRef]
- Spencer, S.J.; Miller, A.A.; Andrews, Z.B. The Role of ghrelin in neuroprotection after is chemic brain injury. Brain Sci. 2013, 3, 344–359. [Google Scholar] [CrossRef] [PubMed]
- Hao, X.K.; Wu, W.; Wang, C.X.; Xie, G.B.; Li, T.; Wu, H.M.; Huang, L.T.; Zhou, M.L.; Hang, C.H.; Shi, J.X. Ghrelin alleviates early brain injury after subarachnoid hemorrhage via the PI3K/Akt signaling pathway. Brain Res. 2014, 1587, 15–22. [Google Scholar] [CrossRef] [PubMed]
- Lopez, N.E.; Gaston, L.; Lopez, K.R.; Coimbra, R.C.; Hageny, A.; Putnam, J.; Eliceiri, B.; Coimbra, R.; Bansal, V. Early ghrelin treatment attenuates disruption of the blood-brain barrier and apoptosis after traumatic brain injury through a UCP-2 mechanism. Brain Res. 2012, 1489, 140–148. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Costantini, Y.W.; D’Mello, R.; Eliceiri, B.P.; Coimbra, R.; Bansal, V. Altering leukocyte recruitment following traumatic brain injury with ghrelin therapy. J. Trauma Acute Care Surg. 2014, 77, 709–715. [Google Scholar] [CrossRef] [PubMed]
- Qiu, W.C.; Wang, Z.G.; Lv, R.; Wang, W.G.; Han, X.D.; Yan, J.; Wang, Y.; Zheng, Q.; Ai, K.X. Ghrelin improves delayed gastrointestinal transit in alloxan-induced diabetic mice. World J. Gastroenterol. 2008, 14, 2572–2577. [Google Scholar] [CrossRef] [PubMed]
- Wren, A.M.; Small, C.J.; Ward, H.L.; Murphy, K.G.; Dakin, C.L.; Taheri, S.; Kennedy, A.R.; Roberts, G.H.; Morgan, D.G.; Ghatei, M.A.; et al. The novel hypothalamic peptide ghrelin stimulates food intake and growth hormone secretion. Endocrinology 2000, 141, 4325–4328. [Google Scholar] [CrossRef] [PubMed]
- Rynkowski, M.A.; Kim, G.H.; Komotar, R.J.; Otten, M.L.; Ducruet, A.F.; Zacharia, B.E.; Kellner, C.P.; Hahn, D.K.; Merkow, M.B.; Garrett, M.C.; et al. A mouse model of intracerebral hemorrhage using autologous blood infusion. Nat. Protoc. 2008, 3, 122–128. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.W.; Hsu, C.M.; Cha, M.C.; Chen, J.S.; Chen, S.C. Changes in gut mucosal nitric oxide synthase (NOS) activity after thermal injury and its relation with barrier failure. Shock 1999, 11, 104–110. [Google Scholar] [CrossRef] [PubMed]
- Mei, Q.; Diao, L.; Xu, J.M.; Liu, X.C.; Jin, J. A protective effect of melatonin on intestinal permeability is induced by diclofenac via regulation of mitochondrial function in mice. Acta Pharmacol. Sin. 2011, 32, 495–502. [Google Scholar] [CrossRef] [PubMed]
- Lange, S.; Delbro, D.S.; Jennische, E. Evans blue permeation of intestinal mucosa in the rat. Scand. J. Gastroenterol. 1994, 29, 38–46. [Google Scholar] [CrossRef] [PubMed]







© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cheng, Y.; Wei, Y.; Yang, W.; Cai, Y.; Chen, B.; Yang, G.; Shang, H.; Zhao, W. Ghrelin Attenuates Intestinal Barrier Dysfunction Following Intracerebral Hemorrhage in Mice. Int. J. Mol. Sci. 2016, 17, 2032. https://doi.org/10.3390/ijms17122032
Cheng Y, Wei Y, Yang W, Cai Y, Chen B, Yang G, Shang H, Zhao W. Ghrelin Attenuates Intestinal Barrier Dysfunction Following Intracerebral Hemorrhage in Mice. International Journal of Molecular Sciences. 2016; 17(12):2032. https://doi.org/10.3390/ijms17122032
Chicago/Turabian StyleCheng, Yijun, Yongxu Wei, Wenlei Yang, Yu Cai, Bin Chen, Guoyuan Yang, Hanbing Shang, and Weiguo Zhao. 2016. "Ghrelin Attenuates Intestinal Barrier Dysfunction Following Intracerebral Hemorrhage in Mice" International Journal of Molecular Sciences 17, no. 12: 2032. https://doi.org/10.3390/ijms17122032
APA StyleCheng, Y., Wei, Y., Yang, W., Cai, Y., Chen, B., Yang, G., Shang, H., & Zhao, W. (2016). Ghrelin Attenuates Intestinal Barrier Dysfunction Following Intracerebral Hemorrhage in Mice. International Journal of Molecular Sciences, 17(12), 2032. https://doi.org/10.3390/ijms17122032