Zebrafish as a Vertebrate Model System to Evaluate Effects of Environmental Toxicants on Cardiac Development and Function
Abstract
:1. Introduction
2. Comparison of Zebrafish and the Human Heart
3. Advantages of Zebrafish in Cardiotoxicity Study
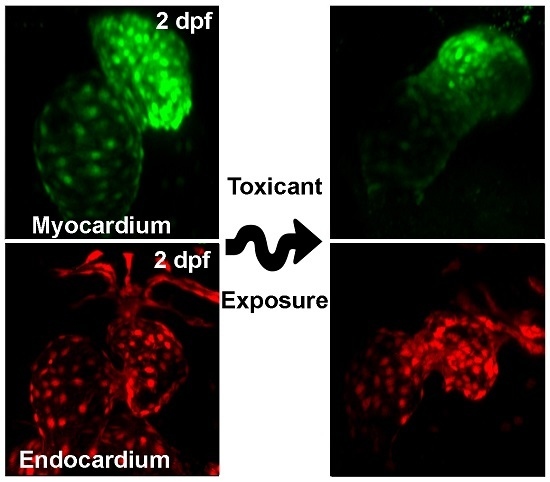
4. Toxic Substances Causing Heart Defects in Zebrafish
4.1. Substances Causing Cardiac Development Defects
4.2. Substances Causing Cardiac Function Defects in Larvae
4.3. Cardiotoxicity Due to Exposure to Adult
5. Conclusions
Acknowledgments
Conflicts of Interest
References
- Cardiovascular Diseases (CVDs). Available online: http://www.who.int/mediacentre/factsheets/fs317/en/ (accessed on 13 December 2016).
- Ellis, L.D.; Soo, E.C.; Achenbach, J.C.; Morash, M.G.; Soanes, K.H. Use of the zebrafish larvae as a model to study cigarette smoke condensate toxicity. PLoS ONE 2014, 9, e115305. [Google Scholar] [CrossRef] [PubMed]
- Go, A.S.; Mozaffarian, D.; Roger, V.L.; Benjamin, E.J.; Berry, J.D.; Blaha, M.J.; Dai, S.; Ford, E.S.; Fox, C.S.; Franco, S.; et al. Heart disease and stroke statistics—2014 update: A report from the american heart association. Circulation 2014, 129, e28–e292. [Google Scholar] [CrossRef] [PubMed]
- Chen, E.K.; Zmirou-Navier, D.; Padilla, C.; Deguen, S. Effects of air pollution on the risk of congenital anomalies: A systematic review and meta-analysis. Int. J. Environ. Res. Public Health 2014, 11, 7642–7668. [Google Scholar] [CrossRef] [PubMed]
- Bohnsack, J.P.; Assemi, S.; Miller, J.D.; Furgeson, D.Y. The primacy of physicochemical characterization of nanomaterials for reliable toxicity assessment: A review of the zebrafish nanotoxicology model. Methods Mol. Biol. 2012, 926, 261–316. [Google Scholar] [PubMed]
- Fang, M.; Guo, J.; Chen, D.; Li, A.; Hinton, D.E.; Dong, W. Halogenated carbazoles induce cardiotoxicity in developing zebrafish (Danio rerio) embryos. Environ. Toxicol. Chem. 2016, 35, 2523–2529. [Google Scholar] [CrossRef] [PubMed]
- Guo, J.; Chen, D.; Potter, D.; Rockne, K.J.; Sturchio, N.C.; Giesy, J.P.; Li, A. Polyhalogenated carbazoles in sediments of lake michigan: A new discovery. Environ. Sci. Technol. 2014, 48, 12807–12815. [Google Scholar] [CrossRef] [PubMed]
- Pena-Abaurrea, M.; Jobst, K.J.; Ruffolo, R.; Shen, L.; McCrindle, R.; Helm, P.A.; Reiner, E.J. Identification of potential novel bioaccumulative and persistent chemicals in sediments from ontario (Canada) using scripting approaches with GCXGC-TOF MS analysis. Environ. Sci. Technol. 2014, 48, 9591–9599. [Google Scholar] [CrossRef] [PubMed]
- Zhu, L.; Hites, R.A. Identification of brominated carbazoles in sediment cores from Lake Michigan. Environ. Sci. Technol. 2005, 39, 9446–9451. [Google Scholar] [CrossRef] [PubMed]
- Staudt, D.; Stainier, D. Uncovering the molecular and cellular mechanisms of heart development using the zebrafish. Annu. Rev. Genet. 2012, 46, 397–418. [Google Scholar] [CrossRef] [PubMed]
- Battle, H.I.; Hisaoka, K.K. Effects of ethyl carbamate (urethan) on the early development of the teleost Brachydanio rerio. Cancer Res. 1952, 12, 334–340. [Google Scholar] [PubMed]
- Jones, R.W.; Huffman, M.N. Fish embryos as bio-assay material in testing chemicals for effects on cell division and differentiation. Trans. Am. Microsc. Soc. 1957, 76, 177–183. [Google Scholar] [CrossRef]
- De Esch, C.; Slieker, R.; Wolterbeek, A.; Woutersen, R.; de Groot, D. Zebrafish as potential model for developmental neurotoxicity testing: A mini review. Neurotoxicol. Teratol. 2012, 34, 545–553. [Google Scholar] [CrossRef] [PubMed]
- Garcia, G.R.; Noyes, P.D.; Tanguay, R.L. Advancements in zebrafish applications for 21st century toxicology. Pharmacol. Ther. 2016, 161, 11–21. [Google Scholar] [CrossRef] [PubMed]
- Hill, A.J.; Teraoka, H.; Heideman, W.; Peterson, R.E. Zebrafish as a model vertebrate for investigating chemical toxicity. Toxicol. Sci. 2005, 86, 6–19. [Google Scholar] [CrossRef] [PubMed]
- Planchart, A.; Mattingly, C.J.; Allen, D.; Ceger, P.; Casey, W.; Hinton, D.; Kanungo, J.; Kullman, S.W.; Tal, T.; Bondesson, M.; et al. Advancing toxicology research using in vivo high throughput toxicology with small fish models. Altex 2016, 33, 435–452. [Google Scholar] [CrossRef] [PubMed]
- Chen, J. Impaired cardiovascular function caused by different stressors elicits a common pathological and transcriptional response in zebrafish embryos. Zebrafish 2013, 10, 389–400. [Google Scholar] [CrossRef] [PubMed]
- Miura, G.I.; Yelon, D. A guide to analysis of cardiac phenotypes in the zebrafish embryo. Methods Cell Biol. 2011, 101, 161–180. [Google Scholar] [PubMed]
- Asnani, A.; Peterson, R.T. The zebrafish as a tool to identify novel therapies for human cardiovascular disease. Dis. Model. Mech. 2014, 7, 763–767. [Google Scholar] [CrossRef] [PubMed]
- Buckingham, M.; Meilhac, S.; Zaffran, S. Building the mammalian heart from two sources of myocardial cells. Nat. Rev.Genet. 2005, 6, 826–835. [Google Scholar] [CrossRef] [PubMed]
- Tomita, Y.; Matsumura, K.; Wakamatsu, Y.; Matsuzaki, Y.; Shibuya, I.; Kawaguchi, H.; Ieda, M.; Kanakubo, S.; Shimazaki, T.; Ogawa, S.; et al. Cardiac neural crest cells contribute to the dormant multipotent stem cell in the mammalian heart. J. Cell Biol. 2005, 170, 1135–1146. [Google Scholar] [CrossRef] [PubMed]
- Cavanaugh, A.M.; Huang, J.; Chen, J.N. Two developmentally distinct populations of neural crest cells contribute to the zebrafish heart. Dev. Biol. 2015, 404, 103–112. [Google Scholar] [CrossRef] [PubMed]
- Lazic, S.; Scott, I.C. Mef2cb regulates late myocardial cell addition from a second heart field-like population of progenitors in zebrafish. Dev. Biol. 2011, 354, 123–133. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Cashman, T.J.; Nevis, K.R.; Obregon, P.; Carney, S.A.; Liu, Y.; Gu, A.; Mosimann, C.; Sondalle, S.; Peterson, R.E.; et al. Latent TGF-β binding protein 3 identifies a second heart field in zebrafish. Nature 2011, 474, 645–648. [Google Scholar] [CrossRef] [PubMed]
- Stainier, D.Y. Zebrafish genetics and vertebrate heart formation. Nat. Rev. Genet. 2001, 2, 39–48. [Google Scholar] [CrossRef] [PubMed]
- MacRae, C.A.; Peterson, R.T. Zebrafish as tools for drug discovery. Nat. Rev. Drug Discov. 2015, 14, 721–731. [Google Scholar] [CrossRef] [PubMed]
- Kopp, R.; Schwerte, T.; Pelster, B. Cardiac performance in the zebrafish breakdance mutant. J. Exp. Biol. 2005, 208, 2123–2134. [Google Scholar] [CrossRef] [PubMed]
- Milan, D.J.; Giokas, A.C.; Serluca, F.C.; Peterson, R.T.; MacRae, C.A. Notch1b and neuregulin are required for specification of central cardiac conduction tissue. Development 2006, 133, 1125–1132. [Google Scholar] [CrossRef] [PubMed]
- Sedmera, D.; Reckova, M.; deAlmeida, A.; Sedmerova, M.; Biermann, M.; Volejnik, J.; Sarre, A.; Raddatz, E.; McCarthy, R.A.; Gourdie, R.G.; et al. Functional and morphological evidence for a ventricular conduction system in zebrafish and xenopus hearts. Am. J. Physiol. Heart Circ. Physiol. 2003, 284, H1152–H1160. [Google Scholar] [CrossRef] [PubMed]
- Zhang, P.C.; Llach, A.; Sheng, X.Y.; Hove-Madsen, L.; Tibbits, G.F. Calcium handling in zebrafish ventricular myocytes. Am. J. Physiol. 2011, 300, R56–R66. [Google Scholar] [CrossRef] [PubMed]
- Luca, E.D.; Zaccaria, G.M.; Hadhoud, M.; Rizzo, G.; Ponzini, R.; Morbiducci, U.; Santoro, M.M. Zebrabeat: A flexible platform for the analysis of the cardiac rate in zebrafish embryos. Sci. Rep. 2014. [Google Scholar] [CrossRef]
- Foglia, M.J.; Poss, K.D. Building and re-building the heart by cardiomyocyte proliferation. Development 2016, 143, 729–740. [Google Scholar] [CrossRef] [PubMed]
- Milan, D.J.; Peterson, T.A.; Ruskin, J.N.; Peterson, R.T.; MacRae, C.A. Drugs that induce repolarization abnormalities cause bradycardia in zebrafish. Circulation 2003, 107, 1355–1358. [Google Scholar] [CrossRef] [PubMed]
- Sarmah, S.; Chism, G.W., III; Vaughan, M.A.; Muralidharan, P.; Marrs, J.A.; Marrs, K.A. Using zebrafish to implement a course-based undergraduate research experience to study teratogenesis in two biology laboratory courses. Zebrafish 2016, 13, 293–304. [Google Scholar] [CrossRef] [PubMed]
- Sarmah, S.; Marrs, J.A. Complex cardiac defects after ethanol exposure during discrete cardiogenic events in zebrafish: Prevention with folic acid. Dev. Dyn. 2013, 242, 1184–1201. [Google Scholar] [CrossRef] [PubMed]
- Sarmah, S.; Muralidharan, P.; Marrs, J.A. Embryonic ethanol exposure dysregulates Bmp and Notch signaling, leading to persistent atrio-ventricular valve defects in zebrafish. PLoS ONE 2016, 11, e0161205. [Google Scholar] [CrossRef] [PubMed]
- Choi, J.; Dong, L.; Ahn, J.; Dao, D.; Hammerschmidt, M.; Chen, J.N. Foxh1 negatively modulates flk1 gene expression and vascular formation in zebrafish. Dev. Biol. 2007, 304, 735–744. [Google Scholar] [CrossRef] [PubMed]
- D’Amico, L.; Scott, I.C.; Jungblut, B.; Stainier, D.Y. A mutation in zebrafish hmgcr1b reveals a role for isoprenoids in vertebrate heart-tube formation. Curr. Biol. 2007, 17, 252–259. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.J.; Tu, C.T.; Hsiao, C.D.; Hsieh, F.J.; Tsai, H.J. Germ-line transmission of a myocardium-specific GFP transgene reveals critical regulatory elements in the cardiac myosin light chain 2 promoter of zebrafish. Dev. Dyn. 2003, 228, 30–40. [Google Scholar] [CrossRef] [PubMed]
- Lawson, N.D.; Scheer, N.; Pham, V.N.; Kim, C.H.; Chitnis, A.B.; Campos-Ortega, J.A.; Weinstein, B.M. Notch signaling is required for arterial-venous differentiation during embryonic vascular development. Development 2001, 128, 3675–3683. [Google Scholar] [PubMed]
- Paffett-Lugassy, N.; Singh, R.; Nevis, K.R.; Guner-Ataman, B.; O’Loughlin, E.; Jahangiri, L.; Harvey, R.P.; Burns, C.G.; Burns, C.E. Heart field origin of great vessel precursors relies on nkx2.5-mediated vasculogenesis. Nat. Cell Biol. 2013, 15, 1362–1369. [Google Scholar] [CrossRef] [PubMed]
- Roman, B.L.; Pham, V.N.; Lawson, N.D.; Kulik, M.; Childs, S.; Lekven, A.C.; Garrity, D.M.; Moon, R.T.; Fishman, M.C.; Lechleider, R.J.; et al. Disruption of acvrl1 increases endothelial cell number in zebrafish cranial vessels. Development 2002, 129, 3009–3019. [Google Scholar] [PubMed]
- Rottbauer, W.; Saurin, A.J.; Lickert, H.; Shen, X.; Burns, C.G.; Wo, Z.G.; Kemler, R.; Kingston, R.; Wu, C.; Fishman, M. Reptin and pontin antagonistically regulate heart growth in zebrafish embryos. Cell 2002, 111, 661–672. [Google Scholar] [CrossRef]
- Traver, D.; Paw, B.H.; Poss, K.D.; Penberthy, W.T.; Lin, S.; Zon, L.I. Transplantation and in vivo imaging of multilineage engraftment in zebrafish bloodless mutants. Nat. Immunol. 2003, 4, 1238–1246. [Google Scholar] [CrossRef] [PubMed]
- Mickoleit, M.; Schmid, B.; Weber, M.; Fahrbach, F.O.; Hombach, S.; Reischauer, S.; Huisken, J. High-resolution reconstruction of the beating zebrafish heart. Nat. Methods 2014, 11, 919–922. [Google Scholar] [CrossRef] [PubMed]
- Pulak, R. Tools for automating the imaging of zebrafish larvae. Methods 2016, 96, 118–126. [Google Scholar] [CrossRef] [PubMed]
- Dhillon, S.S.; Doro, E.; Magyary, I.; Egginton, S.; Sik, A.; Muller, F. Optimisation of embryonic and larval ecg measurement in zebrafish for quantifying the effect of QT prolonging drugs. PLoS ONE 2013, 8, e60552. [Google Scholar] [CrossRef] [PubMed]
- Duan, J.; Yu, Y.; Li, Y.; Liu, H.; Jing, L.; Yang, M.; Wang, J.; Li, C.; Sun, Z. Low-dose exposure of silica nanoparticles induces cardiac dysfunction via neutrophil-mediated inflammation and cardiac contraction in zebrafish embryos. Nanotoxicology 2016, 10, 575–585. [Google Scholar] [CrossRef] [PubMed]
- Mosimann, C.; Panakova, D.; Werdich, A.A.; Musso, G.; Burger, A.; Lawson, K.L.; Carr, L.A.; Nevis, K.R.; Sabeh, M.K.; Zhou, Y.; et al. Chamber identity programs drive early functional partitioning of the heart. Nat. Commun. 2015, 6, 8146. [Google Scholar] [CrossRef] [PubMed]
- Musso, G.; Mosimann, C.; Panakova, D.; Burger, A.; Zhou, Y.; Zon, L.I.; MacRae, C.A. Generating and evaluating a ranked candidate gene list for potential vertebrate heart field regulators. Genom. Data 2015, 6, 199–201. [Google Scholar] [CrossRef] [PubMed]
- Nishiyama, T.; Kaneda, R.; Ono, T.; Tohyama, S.; Hashimoto, H.; Endo, J.; Tsuruta, H.; Yuasa, S.; Ieda, M.; Makino, S.; et al. miR-142–3p is essential for hematopoiesis and affects cardiac cell fate in zebrafish. Biochem. Biophys. Res. Commun. 2012, 425, 755–761. [Google Scholar] [CrossRef] [PubMed]
- Frese, K.S.; Meder, B.; Keller, A.; Just, S.; Haas, J.; Vogel, B.; Fischer, S.; Backes, C.; Matzas, M.; Kohler, D.; et al. RNA splicing regulated by RBFOX1 is essential for cardiac function in zebrafish. J. Cell Sci. 2015, 128, 3030–3040. [Google Scholar] [CrossRef] [PubMed]
- Kettleborough, R.N.; Busch-Nentwich, E.M.; Harvey, S.A.; Dooley, C.M.; de Bruijn, E.; van Eeden, F.; Sealy, I.; White, R.J.; Herd, C.; Nijman, I.J.; et al. A systematic genome-wide analysis of zebrafish protein-coding gene function. Nature 2013, 496, 494–497. [Google Scholar] [CrossRef] [PubMed]
- White, R.M. Genomic approaches to zebrafish cancer. Adv. Exp. Med. Biol. 2016, 916, 125–145. [Google Scholar] [PubMed]
- Froyset, A.K.; Khan, E.A.; Fladmark, K.E. Quantitative proteomics analysis of zebrafish exposed to sub-lethal dosages of β-methyl-amino-l-alanine (BMAA). Sci. Rep. 2016, 6, 29631. [Google Scholar] [CrossRef] [PubMed]
- Chakraborty, C.; Sharma, A.R.; Sharma, G.; Lee, S.S. Zebrafish: A complete animal model to enumerate the nanoparticle toxicity. J. Nanobiotechnol. 2016, 14, 65. [Google Scholar] [CrossRef] [PubMed]
- Asharani, P.V.; Lianwu, Y.; Gong, Z.; Valiyaveettil, S. Comparison of the toxicity of silver, gold and platinum nanoparticles in developing zebrafish embryos. Nanotoxicology 2011, 5, 43–54. [Google Scholar] [CrossRef] [PubMed]
- Dioxins and Their Effects on Human Health. Available online: http://www.who.int/mediacentre/factsheets/fs225/en/ (accessed on 13 December 2016).
- Belair, C.D.; Peterson, R.E.; Heideman, W. Disruption of erythropoiesis by dioxin in the zebrafish. Dev. Dyn. 2001, 222, 581–594. [Google Scholar] [CrossRef] [PubMed]
- Henry, T.R.; Spitsbergen, J.M.; Hornung, M.W.; Abnet, C.C.; Peterson, R.E. Early life stage toxicity of 2,3,7,8-tetrachlorodibenzo-p-dioxin in zebrafish (Danio rerio). Toxicol. Appl. Pharmacol. 1997, 142, 56–68. [Google Scholar] [CrossRef] [PubMed]
- Teraoka, H.; Dong, W.; Ogawa, S.; Tsukiyama, S.; Okuhara, Y.; Niiyama, M.; Ueno, N.; Peterson, R.E.; Hiraga, T. 2,3,7,8-Tetrachlorodibenzo-p-dioxin toxicity in the zebrafish embryo: Altered regional blood flow and impaired lower jaw development. Toxicol. Sci. 2002, 65, 192–199. [Google Scholar] [CrossRef] [PubMed]
- King-Heiden, T.C.; Mehta, V.; Xiong, K.M.; Lanham, K.A.; Antkiewicz, D.S.; Ganser, A.; Heideman, W.; Peterson, R.E. Reproductive and developmental toxicity of dioxin in fish. Mol. Cell. Endocrinol. 2012, 354, 121–138. [Google Scholar] [CrossRef] [PubMed]
- Stapleton, H.M.; Allen, J.G.; Kelly, S.M.; Konstantinov, A.; Klosterhaus, S.; Watkins, D.; McClean, M.D.; Webster, T.F. Alternate and new brominated flame retardants detected in U.S. House dust. Environ. Sci. Technol. 2008, 42, 6910–6916. [Google Scholar] [CrossRef] [PubMed]
- McGee, S.P.; Konstantinov, A.; Stapleton, H.M.; Volz, D.C. Aryl phosphate esters within a major pentabde replacement product induce cardiotoxicity in developing zebrafish embryos: Potential role of the aryl hydrocarbon receptor. Toxicol. Sci. 2013, 133, 144–156. [Google Scholar] [CrossRef] [PubMed]
- Gerlach, C.V.; Das, S.R.; Volz, D.C.; Bisson, W.H.; Kolluri, S.K.; Tanguay, R.L. Mono-substituted isopropylated triaryl phosphate, a major component of firemaster 550, is an AhR agonist that exhibits AhR-independent cardiotoxicity in zebrafish. Aquat. Toxicol. 2014, 154, 71–79. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Huang, L.; Wang, C.; Gao, D.; Zuo, Z. Phenanthrene exposure produces cardiac defects during embryo development of zebrafish (Danio rerio) through activation of MMP-9. Chemosphere 2013, 93, 1168–1175. [Google Scholar] [CrossRef] [PubMed]
- Brown, D.R.; Clark, B.W.; Garner, L.V.; Di Giulio, R.T. Zebrafish cardiotoxicity: The effects of CYP1A inhibition and AhR2 knockdown following exposure to weak aryl hydrocarbon receptor agonists. Environ. Sci. Pollut. Res. Int. 2015, 22, 8329–8338. [Google Scholar] [CrossRef] [PubMed]
- McIntyre, J.K.; Edmunds, R.C.; Anulacion, B.F.; Davis, J.W.; Incardona, J.P.; Stark, J.D.; Scholz, N.L. Severe coal tar sealcoat runoff toxicity to fish is prevented by bioretention filtration. Environ. Sci. Technol. 2016, 50, 1570–1578. [Google Scholar] [CrossRef] [PubMed]
- McIntyre, J.K.; Edmunds, R.C.; Redig, M.G.; Mudrock, E.M.; Davis, J.W.; Incardona, J.P.; Stark, J.D.; Scholz, N.L. Confirmation of stormwater bioretention treatment effectiveness using molecular indicators of cardiovascular toxicity in developing fish. Environ. Sci. Technol. 2016, 50, 1561–1569. [Google Scholar] [CrossRef] [PubMed]
- Incardona, J.P.; Carls, M.G.; Teraoka, H.; Sloan, C.A.; Collier, T.K.; Scholz, N.L. Aryl hydrocarbon receptor-independent toxicity of weathered crude oil during fish development. Environ. Health Perspect. 2005, 113, 1755–1762. [Google Scholar] [CrossRef] [PubMed]
- Incardona, J.P.; Linbo, T.L.; Scholz, N.L. Cardiac toxicity of 5-ring polycyclic aromatic hydrocarbons is differentially dependent on the aryl hydrocarbon receptor 2 isoform during zebrafish development. Toxicol. Appl. Pharmacol. 2011, 257, 242–249. [Google Scholar] [CrossRef] [PubMed]
- De Castro, V.L.; Goes, K.; Chiorato, S. Developmental toxicity potential of paclobutrazol in the rat. Int. J. Environ. Health Res. 2004, 14, 371–380. [Google Scholar] [CrossRef] [PubMed]
- Le, T.T.; Peijnenburg, W.J. Modeling toxicity of mixtures of perfluorooctanoic acid and triazoles (triadimefon and paclobutrazol) to the benthic cladoceran chydorus sphaericus. Environ. Sci. Technol. 2013, 47, 6621–6629. [Google Scholar] [PubMed]
- Li, J.; Sun, L.; Zuo, Z.; Chen, M.; Geng, H.; Wang, C. Exposure to paclobutrazol disrupts spermatogenesis in male sebastiscus marmoratus. Aquat. Toxicol. 2012, 122–123, 120–124. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Sun, L.; Zuo, Z.; Chen, M.; Wang, C. Effects of paclobutrazol exposure on antioxidant defense system in Sebastiscus marmoratus. Bull. Environ. Contam. Toxicol. 2012, 89, 723–726. [Google Scholar] [CrossRef] [PubMed]
- Sun, L.; Li, J.; Zuo, Z.; Chen, M.; Wang, C. Chronic exposure to paclobutrazol causes hepatic steatosis in male rockfish Sebastiscus marmoratus and the mechanism involved. Aquat. Toxicol. 2013, 126, 148–153. [Google Scholar] [CrossRef] [PubMed]
- Yekti, A.P.; Hsu, H.J.; Wang, W.D. The effect of paclobutrazol on the development of zebrafish (Danio rerio) embryos. Zebrafish 2014, 11, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Dlugos, C.A.; Rabin, R.A. Structural and functional effects of developmental exposure to ethanol on the zebrafish heart. Alcohol. Clin. Exp. Res. 2010, 34, 1013–1021. [Google Scholar] [CrossRef] [PubMed]
- Holbrook, B.D. The effects of nicotine on human fetal development. Birth Defects Res. Part C Embryo Today 2016, 108, 181–192. [Google Scholar] [CrossRef] [PubMed]
- Lee, L.J.; Lupo, P.J. Maternal smoking during pregnancy and the risk of congenital heart defects in offspring: A systematic review and metaanalysis. Pediatr. Cardiol. 2013, 34, 398–407. [Google Scholar] [CrossRef] [PubMed]
- Malik, S.; Cleves, M.A.; Honein, M.A.; Romitti, P.A.; Botto, L.D.; Yang, S.; Hobbs, C.A. Maternal smoking and congenital heart defects. Pediatrics 2008, 121, e810–e816. [Google Scholar] [CrossRef] [PubMed]
- Borgerding, M.; Klus, H. Analysis of complex mixtures—Cigarette smoke. Exp. Toxicol. Pathol. 2005, 57, 43–73. [Google Scholar] [CrossRef] [PubMed]
- Talhout, R.; Schulz, T.; Florek, E.; van Benthem, J.; Wester, P.; Opperhuizen, A. Hazardous compounds in tobacco smoke. Int. J. Environ. Res. Public Health 2011, 8, 613–628. [Google Scholar] [CrossRef] [PubMed]
- Palpant, N.J.; Hofsteen, P.; Pabon, L.; Reinecke, H.; Murry, C.E. Cardiac development in zebrafish and human embryonic stem cells is inhibited by exposure to tobacco cigarettes and e-cigarettes. PLoS ONE 2015, 10, e0126259. [Google Scholar] [CrossRef] [PubMed]
- Watson, F.L.; Schmidt, H.; Turman, Z.K.; Hole, N.; Garcia, H.; Gregg, J.; Tilghman, J.; Fradinger, E.A. Organophosphate pesticides induce morphological abnormalities and decrease locomotor activity and heart rate in Danio rerio and Xenopus laevis. Environ. Toxicol. Chem. 2014, 33, 1337–1345. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.C.; Hui, M.N.; Cheng, S.H. Toxicity and cardiac effects of carbaryl in early developing zebrafish (Danio rerio) embryos. Toxicol. Appl. Pharmacol. 2007, 222, 159–168. [Google Scholar] [CrossRef] [PubMed]
- Schock, E.N.; Ford, W.C.; Midgley, K.J.; Fader, J.G.; Giavasis, M.N.; McWhorter, M.L. The effects of carbaryl on the development of zebrafish (Danio rerio) embryos. Zebrafish 2012, 9, 169–178. [Google Scholar] [CrossRef] [PubMed]
- Meyer, K.D.; Zhang, L. Short- and long-term adverse effects of cocaine abuse during pregnancy on the heart development. Ther. Adv. Cardiovasc. Dis. 2009, 3, 7–16. [Google Scholar] [CrossRef] [PubMed]
- Mersereau, E.J.; Poitra, S.L.; Espinoza, A.; Crossley, D.A., II; Darland, T. The effects of cocaine on heart rate and electrocardiogram in zebrafish (Danio rerio). Comp. Biochem. Physiol. Toxicol. Pharmacol. 2015, 172–173, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Folkesson, M.; Sadowska, N.; Vikingsson, S.; Karlsson, M.; Carlhall, C.J.; Lanne, T.; Wagsater, D.; Jensen, L. Differences in cardiovascular toxicities associated with cigarette smoking and snuff use revealed using novel zebrafish models. Biol. Open 2016, 5, 970–978. [Google Scholar] [CrossRef] [PubMed]
- Feng, Y.; Caiping, M.; Li, C.; Can, R.; Feichao, X.; Li, Z.; Zhice, X. Fetal and offspring arrhythmia following exposure to nicotine during pregnancy. J. Appl. Toxicol. 2010, 30, 53–58. [Google Scholar] [CrossRef] [PubMed]
- Gerger, C.J.; Weber, L.P. Comparison of the acute effects of benzo-a-pyrene on adult zebrafish (Danio rerio) cardiorespiratory function following intraperitoneal injection versus aqueous exposure. Aquat. Toxicol. 2015, 165, 19–30. [Google Scholar] [CrossRef] [PubMed]


| Transgene Name | Cell Label | Description of the Expression | Reference |
|---|---|---|---|
| Tg(myl7:GFP) | Cardiomyocytes | GFP in the cytoplasm of differentiated cardiomyocytes. | [39] |
| Tg(myl7:nucDsred) | Cardiomyocytes | DsRed in the nuclei of differentiated cardiomyocytes. | [43] |
| Tg(myl7:ras-eGFP) | Cardiomyocytes | Enhanced GFP in the cell membrane of differentiated cardiomyocytes. | [38] |
| Tg(myl7:nlsKikGR) | Cardiomyocytes | KikGR in the nuclei of differentiated cardiomyocytes. UV light exposure photoconverts KikGR from green to a red fluorophore. | [23] |
| Tg(fli1:EGFP) | Endothelium and endocardium | Enhanced cytoplasmic GFP in the entire vasculature and in the endocardial cells. | [40] |
| Tg(fli1:nEGFP) | Endothelium and endocardium | Enhanced nuclear GFP in the entire vasculature and in the endocardial cells. | [42] |
| Tg(kdrl:GFP) | Endothelium and endocardial | GFP in the entire vasculature and in endocardial cells. | [37] |
| Tg(kdrl:nlsKikGR) | Endothelium and endocardial | KikGR in the nuclei of endothelia and endocardial cells. UV light exposure photoconverts KikGR from green to a red fluorophore. | [23] |
| Tg(gata1a:DsRed) | Red blood cells | DsRed in red blood cells | [44] |
| Tg(nkx2.5:nZsYellow) | nkx2.5 positive cells | ZsYellow in nkx2.5 positive cells allows lineage tracing of second heart field progenitors. | [41] |
| Tg(NC:mCherry) | Cardiac neural crest cells | A double transgenic for the sox10:GAL4-UAS-Cre and the ubi:Switch reporter. sox10 promoter drives the expression of Cre recombinase in neural crest cells which excises GFP and permanently labels cells of sox10 lineage with mCherry and allows lineage tracing of cardiac neural crest cells. | [22] |
| Tg(NC:NfsB-mCherry) | Cardiac neural crest cells | A double transgenic for sox10:GAL4-UAS-Cre and UAS:NfsB-mCherry in which the expression of Nitroreductase-mCherry fusion protein is controlled by sox10-driven GAL4 activity allowing lineage tracing of cardiac neural crest cells. | [22] |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sarmah, S.; Marrs, J.A. Zebrafish as a Vertebrate Model System to Evaluate Effects of Environmental Toxicants on Cardiac Development and Function. Int. J. Mol. Sci. 2016, 17, 2123. https://doi.org/10.3390/ijms17122123
Sarmah S, Marrs JA. Zebrafish as a Vertebrate Model System to Evaluate Effects of Environmental Toxicants on Cardiac Development and Function. International Journal of Molecular Sciences. 2016; 17(12):2123. https://doi.org/10.3390/ijms17122123
Chicago/Turabian StyleSarmah, Swapnalee, and James A. Marrs. 2016. "Zebrafish as a Vertebrate Model System to Evaluate Effects of Environmental Toxicants on Cardiac Development and Function" International Journal of Molecular Sciences 17, no. 12: 2123. https://doi.org/10.3390/ijms17122123
APA StyleSarmah, S., & Marrs, J. A. (2016). Zebrafish as a Vertebrate Model System to Evaluate Effects of Environmental Toxicants on Cardiac Development and Function. International Journal of Molecular Sciences, 17(12), 2123. https://doi.org/10.3390/ijms17122123