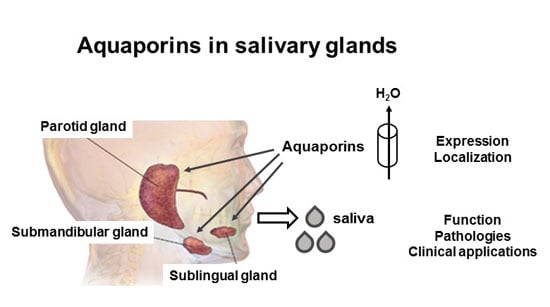
Aquaporins in Salivary Glands: From Basic Research to Clinical Applications
Abstract
:1. Introduction
2. Morphology of Salivary Glands
2.1. Epithelial Cell Types

2.2. Salivary Glands Types
3. Expression and Localization of AQPs in Salivary Glands
3.1. Human
3.2. Rat
3.3. Mouse
| AQP | Human | Rat | Mouse |
|---|---|---|---|
| AQP1 | EC, MEC | EC | EC, MEC |
| AQP3 | AC (BM) | AC | AC (BM), DC |
| AQP4 | N.D. (mRNA) | AC (controversial) | AC (BM), DC |
| AQP5 | AC (AM) | AC, DC (controversial) | AC |
| AQP6 | N.D. (mRNA) | AC | N.D. |
| AQP7 | N.D. (mRNA) | N.D. | EC. |
| AQP8 | N.D. | MEC | AC (BM), DC |
| AQP9 | N.D. | N.D. | N.D. (mRNA) |
| AQP11 | N.D. | N.D. | DC |
4. Physiology of Saliva Secretion

5. Role of Aquaporins in Saliva Secretion
6. Aquaporins and Pathophysiological Conditions
6.1. Sjögren’s Syndrome
6.2. Radiation Therapy
6.3. Diabetes
6.4. Senescence
7. Aquaporins and Clinical Applications
7.1. Medications
7.2. Gene Therapy
7.3. Stem/Progenitor Cells and Tissue Regeneration Therapy
8. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Agre, P. Aquaporin water channels (Nobel lecture). Angew. Chem. Int. Ed. 2004, 43, 4278–4290. [Google Scholar] [CrossRef] [PubMed]
- Verkman, A.S. Novel roles of aquaporins revealed by phenotype analysis of knockout mice. Rev. Physiol. Biochem. Pharmacol. 2005, 155, 31–55. [Google Scholar] [PubMed]
- Musa-Aziz, R.; Chen, L.M.; Pelletier, M.F.; Boron, W.F. Relative Co2/NH3 selectivities of AQP1, AQP4, AQP5, amtB, and RhAG. Proc. Natl. Acad. Sci. USA 2009, 106, 5406–5411. [Google Scholar] [CrossRef] [PubMed]
- Yool, A.J. Functional domains of aquaporin-1: Keys to physiology, and targets for drug discovery. Curr. Pharm. Des. 2007, 13, 3212–3221. [Google Scholar] [CrossRef] [PubMed]
- Hara-Chikuma, M.; Verkman, A.S. Physiological roles of glycerol-transporting aquaporins: The aquaglyceroporins. Cell. Mol. Life Sci. 2006, 63, 1386–1392. [Google Scholar] [CrossRef] [PubMed]
- Rojek, A.; Praetorius, J.; Frokiaer, J.; Nielsen, S.; Fenton, R.A. A current view of the mammalian aquaglyceroporins. Annu. Rev. Physiol. 2008, 70, 301–327. [Google Scholar] [CrossRef] [PubMed]
- Ishibashi, K.; Tanaka, Y.; Morishita, Y. The role of mammalian superaquaporins inside the cell. Biochim. Biophys. Acta 2014, 1840, 1507–1512. [Google Scholar] [CrossRef] [PubMed]
- Denny, P.C.; Ball, W.D.; Redman, R.S. Salivary glands: A paradigm for diversity of gland development. Crit. Rev. Oral Biol. Med. 1997, 8, 51–75. [Google Scholar] [CrossRef] [PubMed]
- Young, J.A.; van Lennep, E.W. The Morphology of Salivary Glands; Academic Press: New York, NY, USA, 1978. [Google Scholar]
- Redman, R.S. Development of salivary glands. In The Salivary System; Sreebny, L.M., Ed.; CRC Press: Boca Raton, FL, USA, 1987; pp. 1–20. [Google Scholar]
- Amano, O.; Mizobe, K.; Bando, Y.; Sakiyama, K. Anatomy and histology of rodent and human major salivary glands: Overview of the Japan salivary gland society-sponsored workshop. Acta Histochem. Cytochem. 2012, 45, 241–250. [Google Scholar] [CrossRef] [PubMed]
- Hand, A.R.; Pathmanathan, D.; Field, R.B. Morphological features of the minor salivary glands. Arch. Oral Biol. 1999, 44, S3–S10. [Google Scholar] [CrossRef]
- Mobasheri, A.; Marples, D. Expression of the AQP-1 water channel in normal human tissues: A semiquantitative study using tissue microarray technology. Am. J. Physiol. Cell Physiol. 2004, 286, C529–C537. [Google Scholar] [CrossRef] [PubMed]
- Gresz, V.; Burghardt, B.; Ferguson, C.J.; Hurley, P.T.; Takacs, M.; Nielsen, S.; Varga, G.; Zelles, T.; Case, R.M.; Steward, M.C. Expression of aquaporin 1 (AQP1) water channels in human labial salivary glands. Arch. Oral Biol. 1999, 44, S53–S57. [Google Scholar] [CrossRef]
- Gresz, V.; Kwon, T.H.; Hurley, P.T.; Varga, G.; Zelles, T.; Nielsen, S.; Case, R.M.; Steward, M.C. Identification and localization of aquaporin water channels in human salivary glands. Am. J. Physiol. Gastrointest. Liver Physiol. 2001, 281, G247–G254. [Google Scholar] [PubMed]
- Wang, W.; Hart, P.S.; Piesco, N.P.; Lu, X.; Gorry, M.C.; Hart, T.C. Aquaporin expression in developing human teeth and selected orofacial tissues. Calcif. Tissue Int. 2003, 72, 222–227. [Google Scholar] [CrossRef] [PubMed]
- Beroukas, D.; Hiscock, J.; Gannon, B.J.; Jonsson, R.; Gordon, T.P.; Waterman, S.A. Selective down-regulation of aquaporin-1 in salivary glands in primary Sjögren’s syndrome. Lab. Investig. 2002, 82, 1547–1552. [Google Scholar] [CrossRef] [PubMed]
- Steinfeld, S.; Cogan, E.; King, L.S.; Agre, P.; Kiss, R.; Delporte, C. Abnormal distribution of aquaporin-5 water channel protein in salivary glands from Sjögren’s syndrome patients. Lab. Investig. 2001, 81, 143–148. [Google Scholar] [CrossRef] [PubMed]
- Akamatsu, T.; Parvin, M.N.; Murdiastuti, K.; Kosugi-Tanaka, C.; Yao, C.; Miki, O.; Kanamori, N.; Hosoi, K. Expression and localization of aquaporins, members of the water channel family, during development of the rat submandibular gland. Pflug. Arch. 2003, 446, 641–651. [Google Scholar] [CrossRef] [PubMed]
- He, X.; Tse, C.M.; Donowitz, M.; Alper, S.L.; Gabriel, S.E.; Baum, B.J. Polarized distribution of key membrane transport proteins in the rat submandibular gland. Pflug. Arch. 1997, 433, 260–268. [Google Scholar] [CrossRef]
- Li, J.; Nielsen, S.; Dai, Y.; Lazowski, K.W.; Christensen, E.I.; Tabak, L.A.; Baum, B.J. Examination of rat salivary glands for the presence of the aquaporin chip. Pflug. Arch. 1994, 428, 455–460. [Google Scholar] [CrossRef]
- Nielsen, S.; Smith, B.L.; Christensen, E.I.; Agre, P. Distribution of the aquaporin chip in secretory and resorptive epithelia and capillary endothelia. Proc. Natl. Acad. Sci. USA 1993, 90, 7275–7279. [Google Scholar] [CrossRef] [PubMed]
- King, L.S.; Nielsen, S.; Agre, P. Aquaporins in complex tissues. I. Developmental patterns in respiratory and glandular tissues of rat. Am. J. Physiol. 1997, 273, C1541–C1548. [Google Scholar] [PubMed]
- Nielsen, S.; King, L.S.; Christensen, B.M.; Agre, P. Aquaporins in complex tissues. II. Subcellular distribution in respiratory and glandular tissues of rat. Am. J. Physiol. 1997, 273, C1549–C1561. [Google Scholar] [PubMed]
- Frigeri, A.; Gropper, M.A.; Turck, C.W.; Verkman, A.S. Immunolocalization of the mercurial-insensitive water channel and glycerol intrinsic protein in epithelial cell plasma membranes. Proc. Natl. Acad. Sci. USA 1995, 92, 4328–4331. [Google Scholar] [CrossRef] [PubMed]
- Matsuzaki, T.; Suzuki, T.; Koyama, H.; Tanaka, S.; Takata, K. Aquaporin-5 (AQP5), a water channel protein, in the rat salivary and lacrimal glands: Immunolocalization and effect of secretory stimulation. Cell Tissue Res. 1999, 295, 513–521. [Google Scholar] [CrossRef] [PubMed]
- Funaki, H.; Yamamoto, T.; Koyama, Y.; Kondo, D.; Yaoita, E.; Kawasaki, K.; Kobayashi, H.; Sawaguchi, S.; Abe, H.; Kihara, I. Localization and expression of AQP5 in cornea, serous salivary glands, and pulmonary epithelial cells. Am. J. Physiol. 1998, 275, C1151–C1157. [Google Scholar] [PubMed]
- Murdiastuti, K.; Miki, O.; Yao, C.; Parvin, M.N.; Kosugi-Tanaka, C.; Akamatsu, T.; Kanamori, N.; Hosoi, K. Divergent expression and localization of aquaporin 5, an exocrine-type water channel, in the submandibular gland of Sprague-Dawley rats. Pflug. Arch. 2002, 445, 405–412. [Google Scholar] [CrossRef] [PubMed]
- Delporte, C.; O’Connell, B.C.; He, X.; Lancaster, H.E.; O’Connell, A.C.; Agre, P.; Baum, B.J. Increased fluid secretion after adenoviral-mediated transfer of the aquaporin-1 cDNA to irradiated rat salivary glands. Proc. Natl. Acad. Sci. USA 1997, 94, 3268–3273. [Google Scholar] [CrossRef] [PubMed]
- Raina, S.; Preston, G.M.; Guggino, W.B.; Agre, P. Molecular cloning and characterization of an aquaporin cDNA from salivary, lacrimal, and respiratory tissues. J. Biol. Chem. 1995, 270, 1908–1912. [Google Scholar] [PubMed]
- Matsuki-Fukushima, M.; Fujita-Yoshigaki, J.; Murakami, M.; Katsumata-Kato, O.; Yokoyama, M.; Sugiya, H. Involvement of AQP6 in the mercury-sensitive osmotic lysis of rat parotid secretory granules. J. Membr. Biol. 2013, 246, 209–214. [Google Scholar] [CrossRef] [PubMed]
- Koyama, Y.; Yamamoto, T.; Kondo, D.; Funaki, H.; Yaoita, E.; Kawasaki, K.; Sato, N.; Hatakeyama, K.; Kihara, I. Molecular cloning of a new aquaporin from rat pancreas and liver. J. Biol. Chem. 1997, 272, 30329–30333. [Google Scholar] [CrossRef] [PubMed]
- Elkjaer, M.L.; Nejsum, L.N.; Gresz, V.; Kwon, T.H.; Jensen, U.B.; Frokiaer, J.; Nielsen, S. Immunolocalization of aquaporin-8 in rat kidney, gastrointestinal tract, testis, and airways. Am. J. Physiol. Ren. Physiol. 2001, 281, F1047–F1057. [Google Scholar] [CrossRef]
- Wellner, R.B.; Redman, R.S.; Swaim, W.D.; Baum, B.J. Further evidence for AQP8 expression in the myoepithelium of rat submandibular and parotid glands. Pflug. Arch. 2006, 451, 642–645. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, M.; Saga, T.; Watanabe, K.; Takahashi, N.; Tabira, Y.; Kusukawa, J.; Yamaki, K. An immunohistochemistry-based study on aquaporin (AQP)-1, 3, 4, 5 and 8 in the parotid glands, submandibular glands and sublingual glands of Sjögren’s syndrome mouse models chronically administered cevimeline. Kurume Med. J. 2013, 60, 7–19. [Google Scholar] [CrossRef] [PubMed]
- Larsen, H.S.; Aure, M.H.; Peters, S.B.; Larsen, M.; Messelt, E.B.; Kanli Galtung, H. Localization of AQP5 during development of the mouse submandibular salivary gland. J. Mol. Histol. 2011, 42, 71–81. [Google Scholar] [CrossRef] [PubMed]
- Matsuzaki, T.; Ablimit, A.; Suzuki, T.; Aoki, T.; Hagiwara, H.; Takata, K. Changes of aquaporin 5-distribution during release and reaccumulation of secretory granules in isoproterenol-treated mouse parotid gland. J. Electron Microsc. 2006, 55, 183–189. [Google Scholar] [CrossRef] [PubMed]
- Aure, M.H.; Ruus, A.K.; Galtung, H.K. Aquaporins in the adult mouse submandibular and sublingual salivary glands. J. Mol. Histol. 2014, 45, 69–80. [Google Scholar] [CrossRef] [PubMed]
- Delporte, C. Aquaporins in salivary glands and pancreas. Biochim. Biophys. Acta 2014, 1840, 1524–1532. [Google Scholar] [CrossRef] [PubMed]
- Carpenter, G.H. The secretion, components, and properties of saliva. Annu. Rev. Food Sci. Technol. 2013, 4, 267–276. [Google Scholar] [CrossRef] [PubMed]
- Ship, J.A. Diagnosing, managing, and preventing salivary gland disorders. Oral Dis. 2002, 8, 77–89. [Google Scholar] [CrossRef] [PubMed]
- Melvin, J.E.; Yule, D.; Shuttleworth, T.; Begenisich, T. Regulation of fluid and electrolyte secretion in salivary gland acinar cells. Annu. Rev. Physiol. 2005, 67, 445–469. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.G.; Ohana, E.; Park, H.W.; Yang, D.; Muallem, S. Molecular mechanism of pancreatic and salivary gland fluid and HCO3 secretion. Physiol. Rev. 2012, 92, 39–74. [Google Scholar] [CrossRef] [PubMed]
- Murakami, M.; Murdiastuti, K.; Hosoi, K.; Hill, A.E. AQP and the control of fluid transport in a salivary gland. J. Membr. Biol. 2006, 210, 91–103. [Google Scholar] [CrossRef] [PubMed]
- Mangos, J.A.; McSherry, N.R. Micropuncture study of urea excretion in parotid saliva of the rat. Am. J. Physiol. 1970, 218, 1329–1332. [Google Scholar] [PubMed]
- Proctor, G.B.; Carpenter, G.H. Regulation of salivary gland function by autonomic nerves. Auton. Neurosci. 2007, 133, 3–18. [Google Scholar] [CrossRef] [PubMed]
- Nezu, A.; Morita, T.; Tanimura, A.; Tojyo, Y. Comparison of agonist-induced Ca2+ responses in rat submandibular acini and ducts. Arch. Oral Biol. 2005, 50, 585–592. [Google Scholar] [CrossRef] [PubMed]
- Ambudkar, I.S. Ca2+ signaling and regulation of fluid secretion in salivary gland acinar cells. Cell Calcium 2014, 55, 297–305. [Google Scholar] [CrossRef] [PubMed]
- Hosoi, K. Physiological role of aquaporin-5 in salivary glands. Pflüg. Arch. Eur. J. Physiol. 2015. [Google Scholar] [CrossRef] [PubMed]
- Ekstrom, J. Gustatory-salivary reflexes induce non-adrenergic, non-cholinergic acinar degranulation in the rat parotid gland. Exp. Physiol. 2001, 86, 475–480. [Google Scholar] [CrossRef] [PubMed]
- Nakamoto, T.; Brown, D.A.; Catalan, M.A.; Gonzalez-Begne, M.; Romanenko, V.G.; Melvin, J.E. Purinergic P2X7 receptors mediate ATP-induced saliva secretion by the mouse submandibular gland. J. Biol. Chem. 2009, 284, 4815–4822. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Luo, X.; Zeng, W.; Muallem, S. Cell-specific behavior of P2X7 receptors in mouse parotid acinar and duct cells. J. Biol. Chem. 2003, 278, 47554–47561. [Google Scholar] [CrossRef] [PubMed]
- Bhattacharya, S.; Imbery, J.F.; Ampem, P.T.; Giovannucci, D.R. Crosstalk between purinergic receptors and canonical signaling pathways in the mouse salivary gland. Cell Calcium 2015, 58, 589–597. [Google Scholar] [CrossRef] [PubMed]
- Mirfendereski, S.; Tobin, G.; Hakanson, R.; Ekstrom, J. Pituitary Adenylate Cyclase Activating Peptide (PACAP) in salivary glands of the rat: Origin, and secretory and vascular effects. Acta Physiol. Scand. 1997, 160, 15–22. [Google Scholar] [CrossRef] [PubMed]
- Ma, T.; Song, Y.; Gillespie, A.; Carlson, E.J.; Epstein, C.J.; Verkman, A.S. Defective secretion of saliva in transgenic mice lacking aquaporin-5 water channels. J. Biol. Chem. 1999, 274, 20071–20074. [Google Scholar] [CrossRef] [PubMed]
- Krane, C.M.; Melvin, J.E.; Nguyen, H.V.; Richardson, L.; Towne, J.E.; Doetschman, T.; Menon, A.G. Salivary acinar cells from aquaporin 5-deficient mice have decreased membrane water permeability and altered cell volume regulation. J. Biol. Chem. 2001, 276, 23413–23420. [Google Scholar] [CrossRef] [PubMed]
- Verkman, A.S.; Yang, B.; Song, Y.; Manley, G.T.; Ma, T. Role of water channels in fluid transport studied by phenotype analysis of aquaporin knockout mice. Exp. Physiol. 2000, 85, 233s–241s. [Google Scholar] [CrossRef] [PubMed]
- Yang, B.; Song, Y.; Zhao, D.; Verkman, A.S. Phenotype analysis of aquaporin-8 null mice. Am. J. Physiol. Cell Physiol. 2005, 288, C1161–C1170. [Google Scholar] [CrossRef] [PubMed]
- Hill, A.E.; Shachar-Hill, B. A new approach to epithelial isotonic fluid transport: An osmosensor feedback model. J. Membr. Biol. 2006, 210, 77–90. [Google Scholar] [CrossRef] [PubMed]
- Maclaren, O.J.; Sneyd, J.; Crampin, E.J. Efficiency of primary saliva secretion: An analysis of parameter dependence in dynamic single-cell and acinus models, with application to aquaporin knockout studies. J. Membr. Biol. 2012, 245, 29–50. [Google Scholar] [CrossRef] [PubMed]
- Maclaren, O.J.; Sneyd, J.; Crampin, E.J. What do aquaporin knockout studies tell us about fluid transport in epithelia? J. Membr. Biol. 2013, 246, 297–305. [Google Scholar] [CrossRef] [PubMed]
- Sneyd, J.; Crampin, E.; Yule, D. Multiscale modelling of saliva secretion. Math. Biosci. 2014, 257, 69–79. [Google Scholar] [CrossRef] [PubMed]
- Ishikawa, Y.; Eguchi, T.; Skowronski, M.T.; Ishida, H. Acetylcholine acts on M3 muscarinic receptors and induces the translocation of aquaporin5 water channel via cytosolic Ca2+ elevation in rat parotid glands. Biochem. Biophys. Res. Commun. 1998, 245, 835–840. [Google Scholar] [CrossRef] [PubMed]
- Cho, G.; Bragiel, A.M.; Wang, D.; Pieczonka, T.D.; Skowronski, M.T.; Shono, M.; Nielsen, S.; Ishikawa, Y. Activation of muscarinic receptors in rat parotid acinar cells induces AQP5 trafficking to nuclei and apical plasma membrane. Biochim. Biophys. Acta 2015, 1850, 784–793. [Google Scholar] [CrossRef] [PubMed]
- Matsuki, M.; Hashimoto, S.; Shimono, M.; Murakami, M.; Fujita-Yoshigaki, J.; Furuyama, S.; Sugiya, H. Involvement of aquaporin-5 water channel in osmoregulation in parotid secretory granules. J. Membr. Biol. 2005, 203, 119–126. [Google Scholar] [CrossRef] [PubMed]
- Sjögren, H. Zur kentniss der keratoconjonctivitis sicca. Acta Ophthalmol. 1933, 2, 1–151. [Google Scholar]
- Manoussakis, M.N.; Kapsogeorgou, E.K. The role of intrinsic epithelial activation in the pathogenesis of Sjögren’s syndrome. J. Autoimmun. 2010, 35, 219–224. [Google Scholar] [CrossRef] [PubMed]
- Pers, J.O.; Lahiri, A.; Tobon, G.J.; Youinou, P. Pathophysiological cytokine network in primary Sjögren’s syndrome. Presse Med. 2012, 41, e467–e474. [Google Scholar] [CrossRef] [PubMed]
- Delporte, C.; Perret, J.; Soyfoo, M.S. Primary Sjögren’s syndrome: Current pathophysiological, diagnostic and therapeutic advances. In Autoimmune Disorders; Huang, F.P., Ed.; Intech Publishers Inc.: Rijeka, Croatia, 2011; pp. 41–66. [Google Scholar]
- Barrera, M.J.; Bahamondes, V.; Sepulveda, D.; Quest, A.F.; Castro, I.; Cortes, J.; Aguilera, S.; Urzua, U.; Molina, C.; Perez, P.; et al. Sjögren’s syndrome and the epithelial target: A comprehensive review. J. Autoimmun. 2013, 42, 7–18. [Google Scholar] [CrossRef] [PubMed]
- Ludwig, H.; Schernthaner, G.; Scherak, O.; Kolarz, G. Antibodies to pancreatic duct cells in Sjögren’s syndrome and rheumatoid arthritis. Gut 1977, 18, 311–315. [Google Scholar] [CrossRef] [PubMed]
- Safadi, R.; Ligumsky, M.; Goldin, E.; Ilan, Y.; Haviv, Y.S.; Nusair, S. Increased serum CA 19-9 antibodies in Sjögren’s syndrome. Postgrad. Med. J. 1998, 74, 543–544. [Google Scholar] [CrossRef] [PubMed]
- Kim, N.; Shin, Y.; Choi, S.; Namkoong, E.; Kim, M.; Lee, J.; Song, Y.; Park, K. Effect of antimuscarinic autoantibodies in primary Sjögren’s syndrome. J. Dent. Res. 2015, 94, 722–728. [Google Scholar] [CrossRef] [PubMed]
- Kyriakidis, N.C.; Kapsogeorgou, E.K.; Tzioufas, A.G. A comprehensive review of autoantibodies in primary Sjögren’s syndrome: Clinical phenotypes and regulatory mechanisms. J. Autoimmun. 2014, 51, 67–74. [Google Scholar] [CrossRef] [PubMed]
- Enger, T.B.; Aure, M.H.; Jensen, J.L.; Galtung, H.K. Calcium signaling and cell volume regulation are altered in Sjögren’s syndrome. Acta Odontol. Scand. 2014, 72, 549–556. [Google Scholar] [CrossRef] [PubMed]
- Yoshimura, S.; Nakamura, H.; Horai, Y.; Nakajima, H.; Shiraishi, H.; Hayashi, T.; Takahashi, T.; Kawakami, A. Abnormal distribution of AQP5 in labial salivary glands is associated with poor saliva secretion in patients with Sjögren’s syndrome including neuromyelitis optica complicated patients. Mod. Rheumatol. 2015. [Google Scholar] [CrossRef] [PubMed]
- Beroukas, D.; Hiscock, J.; Jonsson, R.; Waterman, S.A.; Gordon, T.P. Subcellular distribution of aquaporin 5 in salivary glands in primary Sjögren’s syndrome. Lancet 2001, 358, 1875–1876. [Google Scholar] [CrossRef]
- Gresz, V.; Horvath, A.; Gera, I.; Nielsen, S.; Zelles, T. Immunolocalization of AQP5 in resting and stimulated normal labial glands and in Sjögren’s syndrome. Oral Dis. 2015, 21, e114–e120. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Teos, L.Y.; Zhang, Y.; Cotrim, A.P.; Swaim, W.; Won, J.H.; Ambrus, J.; Shen, L.; Bebris, L.; Grisius, M.; Jang, S.I.; et al. IP3R deficit underlies loss of salivary fluid secretion in Sjögren’s syndrome. Sci. Rep. 2015, 5, 13953. [Google Scholar] [CrossRef] [PubMed]
- Konttinen, Y.T.; Tensing, E.K.; Laine, M.; Porola, P.; Tornwall, J.; Hukkanen, M. Abnormal distribution of aquaporin-5 in salivary glands in the nod mouse model for Sjögren’s syndrome. J. Rheumatol. 2005, 32, 1071–1075. [Google Scholar] [PubMed]
- Soyfoo, M.S.; de Vriese, C.; Debaix, H.; Martin-Martinez, M.D.; Mathieu, C.; Devuyst, O.; Steinfeld, S.D.; Delporte, C. Modified aquaporin 5 expression and distribution in submandibular glands from nod mice displaying autoimmune exocrinopathy. Arthritis Rheum. 2007, 56, 2566–2574. [Google Scholar] [CrossRef] [PubMed]
- Soyfoo, M.S.; Konno, A.; Bolaky, N.; Oak, J.S.; Fruman, D.; Nicaise, C.; Takiguchi, M.; Delporte, C. Link between inflammation and aquaporin-5 distribution in submandibular gland in Sjögren’s syndrome? Oral Dis. 2012, 18, 568–574. [Google Scholar] [CrossRef] [PubMed]
- Lin, X.; Shaw, P.C.; Sze, S.C.; Tong, Y.; Zhang, Y. Dendrobium officinale polysaccharides ameliorate the abnormality of aquaporin 5, pro-inflammatory cytokines and inhibit apoptosis in the experimental Sjögren’s syndrome mice. Int. Immunopharmacol. 2011, 11, 2025–2032. [Google Scholar] [CrossRef] [PubMed]
- Satoh, K.; Narita, T.; Matsuki-Fukushima, M.; Okabayashi, K.; Ito, T.; Senpuku, H.; Sugiya, H. E2f1-deficient nod/scid mice have dry mouth due to a change of acinar/duct structure and the down-regulation of AQP5 in the salivary gland. Pflug. Arch. 2013, 465, 271–281. [Google Scholar] [CrossRef] [PubMed]
- Yamamura, Y.; Motegi, K.; Kani, K.; Takano, H.; Momota, Y.; Aota, K.; Yamanoi, T.; Azuma, M. TNF-α inhibits aquaporin 5 expression in human salivary gland acinar cells via suppression of histone H4 acetylation. J. Cell. Mol. Med. 2012, 16, 1766–1775. [Google Scholar] [CrossRef] [PubMed]
- Yao, C.; Purwanti, N.; Karabasil, M.R.; Azlina, A.; Javkhlan, P.; Hasegawa, T.; Akamatsu, T.; Hosoi, T.; Ozawa, K.; Hosoi, K. Potential down-regulation of salivary gland AQP5 by LPS via cross-coupling of NF-κb and P-C-Jun/c-Fos. Am. J. Pathol. 2010, 177, 724–734. [Google Scholar] [CrossRef] [PubMed]
- Shiozawa, S.; Cummins, J.M.; Fox, P.C. Opening the flood gates: Interferon-α treatment for Sjögren’s syndrome. BioDrugs 2000, 13, 305–311. [Google Scholar] [CrossRef] [PubMed]
- Ship, J.A.; Fox, P.C.; Michalek, J.E.; Cummins, M.J.; Richards, A.B. Treatment of primary Sjögren’s syndrome with low-dose natural human interferon-alpha administered by the oral mucosal route: A phase II clinical trial. IFN protocol study group. J. Interferon Cytokine Res. 1999, 19, 943–951. [Google Scholar] [CrossRef] [PubMed]
- Smith, J.K.; Siddiqui, A.A.; Modica, L.A.; Dykes, R.; Simmons, C.; Schmidt, J.; Krishnaswamy, G.A.; Berk, S.L. Interferon-α upregulates gene expression of aquaporin-5 in human parotid glands. J. Interferon Cytokine Res. 1999, 19, 929–935. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Ha, Y.M.; Ku, N.Y.; Choi, S.Y.; Lee, S.J.; Oh, S.B.; Kim, J.S.; Lee, J.H.; Lee, E.B.; Song, Y.W.; et al. Inhibitory effects of autoantibodies on the muscarinic receptors in Sjögren’s syndrome. Lab. Investig. 2004, 84, 1430–1438. [Google Scholar] [CrossRef] [PubMed]
- Lee, B.H.; Gauna, A.E.; Perez, G.; Park, Y.J.; Pauley, K.M.; Kawai, T.; Cha, S. Autoantibodies against muscarinic type 3 receptor in Sjögren’s syndrome inhibit aquaporin 5 trafficking. PLoS ONE 2013. [Google Scholar] [CrossRef] [PubMed]
- Thanou-Stavraki, A.; James, J.A. Primary Sjögren’s syndrome: Current and prospective therapies. Semin. Arthritis Rheum. 2008, 37, 273–292. [Google Scholar] [CrossRef] [PubMed]
- Ramos-Casals, M.; Brito-Zeron, P. Emerging biological therapies in primary Sjögren’s syndrome. Rheumatology 2007, 46, 1389–1396. [Google Scholar] [CrossRef] [PubMed]
- Ring, T.; Kallenbach, M.; Praetorius, J.; Nielsen, S.; Melgaard, B. Successful treatment of a patient with primary Sjögren’s syndrome with rituximab. Clin. Rheumatol. 2006, 25, 891–894. [Google Scholar] [CrossRef] [PubMed]
- De Paoli, S.; de Souza da Fonseca, A.; de Paoli, F.; Geller, M.; Presta, G.A.; Santos-Filho, S.D.; Bernardo-Filho, M. A review of scientific papers about head and neck cancers. Braz. Arch. Biol. Technol. 2008, 51, 63–69. [Google Scholar] [CrossRef]
- Johnson, J.T.; Ferretti, G.A.; Nethery, W.J.; Valdez, I.H.; Fox, P.C.; Ng, D.; Muscoplat, C.C.; Gallagher, S.C. Oral pilocarpine for post-irradiation xerostomia in patients with head and neck cancer. N. Engl. J. Med. 1993, 329, 390–395. [Google Scholar] [CrossRef] [PubMed]
- Takagi, K.; Yamaguchi, K.; Sakurai, T.; Asari, T.; Hashimoto, K.; Terakawa, S. Secretion of saliva in X-irradiated rat submandibular glands. Radiat. Res. 2003, 159, 351–360. [Google Scholar] [CrossRef]
- Choi, J.H.; Wu, H.G.; Jung, K.C.; Lee, S.H.; Kwon, E.K. Apoptosis and expression of AQP5 and TGF-β in the irradiated rat submandibular gland. Cancer Res. Treat. 2009, 41, 145–154. [Google Scholar] [CrossRef] [PubMed]
- Asari, T.; Maruyama, K.; Kusama, H. Salivation triggered by pilocarpine involves aquaporin-5 in normal rats but not in irradiated rats. Clin. Exp. Pharmacol. Physiol. 2009, 36, 531–538. [Google Scholar] [CrossRef] [PubMed]
- Wild, S.; Roglic, G.; Green, A.; Sicree, R.; King, H. Global prevalence of diabetes: Estimates for the year 2000 and projections for 2030. Diabetes Care 2004, 27, 1047–1053. [Google Scholar] [CrossRef] [PubMed]
- Moore, P.A.; Guggenheimer, J.; Etzel, K.R.; Weyant, R.J.; Orchard, T. Type 1 diabetes mellitus, xerostomia, and salivary flow rates. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endod. 2001, 92, 281–291. [Google Scholar] [CrossRef] [PubMed]
- Visvanathan, V.; Nix, P. Managing the patient presenting with xerostomia: A review. Int. J. Clin. Pract. 2010, 64, 404–407. [Google Scholar] [CrossRef] [PubMed]
- Soyfoo, M.S.; Bolaky, N.; Depoortere, I.; Delporte, C. Relationship between aquaporin-5 expression and saliva flow in streptozotocin-induced diabetic mice? Oral Dis. 2012, 18, 501–505. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Yuan, Z.; Inoue, N.; Cho, G.; Shono, M.; Ishikawa, Y. Abnormal subcellular localization of AQP5 and downregulated AQP5 protein in parotid glands of streptozotocin-induced diabetic rats. Biochim. Biophys. Acta 2011, 1810, 543–554. [Google Scholar] [CrossRef] [PubMed]
- Affoo, R.H.; Foley, N.; Garrick, R.; Siqueira, W.L.; Martin, R.E. Meta-analysis of salivary flow rates in young and older adults. J. Am. Geriatr. Soc. 2015, 63, 2142–2151. [Google Scholar] [CrossRef] [PubMed]
- Inoue, N.; Iida, H.; Yuan, Z.; Ishikawa, Y.; Ishida, H. Age-related decreases in the response of aquaporin-5 to acetylcholine in rat parotid glands. J. Dent. Res. 2003, 82, 476–480. [Google Scholar] [CrossRef] [PubMed]
- Ishikawa, Y.; Iida, H.; Ishida, H. The muscarinic acetylcholine receptor-stimulated increase in aquaporin-5 levels in the apical plasma membrane in rat parotid acinar cells is coupled with activation of nitric oxide/cGMP signal transduction. Mol. Pharmacol. 2002, 61, 1423–1434. [Google Scholar] [CrossRef] [PubMed]
- Yamamura, Y.; Aota, K.; Yamanoi, T.; Kani, K.; Takano, H.; Momota, Y.; Motegi, K.; Azuma, M. DNA demethylating agent decitabine increases AQP5 expression and restores salivary function. J. Dent. Res. 2012, 91, 612–617. [Google Scholar] [CrossRef] [PubMed]
- Baum, B.J.; Zheng, C.; Cotrim, A.P.; Goldsmith, C.M.; Atkinson, J.C.; Brahim, J.S.; Chiorini, J.A.; Voutetakis, A.; Leakan, R.A.; van Waes, C.; et al. Transfer of the AQP1 cDNA for the correction of radiation-induced salivary hypofunction. Biochim. Biophys. Acta 2006, 1758, 1071–1077. [Google Scholar] [CrossRef] [PubMed]
- Delporte, C.; Hoque, A.T.; Kulakusky, J.A.; Braddon, V.R.; Goldsmith, C.M.; Wellner, R.B.; Baum, B.J. Relationship between adenovirus-mediated aquaporin 1 expression and fluid movement across epithelial cells. Biochem. Biophys. Res. Commun. 1998, 246, 584–588. [Google Scholar] [CrossRef] [PubMed]
- He, X.; Kuijpers, G.A.; Goping, G.; Kulakusky, J.A.; Zheng, C.; Delporte, C.; Tse, C.M.; Redman, R.S.; Donowitz, M.; Pollard, H.B.; et al. A polarized salivary cell monolayer useful for studying transepithelial fluid movement in vitro. Pflug. Arch. 1998, 435, 375–381. [Google Scholar] [CrossRef] [PubMed]
- Shan, Z.; Li, J.; Zheng, C.; Liu, X.; Fan, Z.; Zhang, C.; Goldsmith, C.M.; Wellner, R.B.; Baum, B.J.; Wang, S. Increased fluid secretion after adenoviral-mediated transfer of the human aquaporin-1 cDNA to irradiated miniature pig parotid glands. Mol. Ther. 2005, 11, 444–451. [Google Scholar] [CrossRef] [PubMed]
- O’Connell, A.C.; Baccaglini, L.; Fox, P.C.; O’Connell, B.C.; Kenshalo, D.; Oweisy, H.; Hoque, A.T.; Sun, D.; Herscher, L.L.; Braddon, V.R.; et al. Safety and efficacy of adenovirus-mediated transfer of the human aquaporin-1 cDNA to irradiated parotid glands of non-human primates. Cancer Gene Ther. 1999, 6, 505–513. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Zourelias, L.; Wu, C.; Edwards, P.C.; Trombetta, M.; Passineau, M.J. Ultrasound-assisted nonviral gene transfer of AQP1 to the irradiated minipig parotid gland restores fluid secretion. Gene Ther. 2015, 22, 739–749. [Google Scholar] [CrossRef] [PubMed]
- Baum, B.J.; Alevizos, I.; Zheng, C.; Cotrim, A.P.; Liu, S.; McCullagh, L.; Goldsmith, C.M.; Burbelo, P.D.; Citrin, D.E.; Mitchell, J.B.; et al. Early responses to adenoviral-mediated transfer of the aquaporin-1 cDNA for radiation-induced salivary hypofunction. Proc. Natl. Acad. Sci. USA 2012, 109, 19403–19407. [Google Scholar] [CrossRef] [PubMed]
- Zheng, C.; Baum, B.J.; Liu, X.; Goldsmith, C.M.; Perez, P.; Jang, S.I.; Cotrim, A.P.; McCullagh, L.; Ambudkar, I.S.; Alevizos, I. Persistence of hAQP1 expression in human salivary gland cells following AdhAQP1 transduction is associated with a lack of methylation of hCMV promoter. Gene Ther. 2015, 22, 758–766. [Google Scholar] [CrossRef] [PubMed]
- Pringle, S.; van Os, R.; Coppes, R.P. Concise review: Adult salivary gland stem cells and a potential therapy for xerostomia. Stem Cells 2013, 31, 613–619. [Google Scholar] [CrossRef] [PubMed]
- Maria, O.M.; Maria, A.M.; Cai, Y.; Tran, S.D. Cell surface markers CD44 and CD166 localized specific populations of salivary acinar cells. Oral Dis. 2012, 18, 162–168. [Google Scholar] [CrossRef] [PubMed]
- Lim, J.Y.; Yi, T.G.; Lee, S.; Kim, J.; Kim, S.; Song, S.U.; Kim, Y.M. Establishment and Characterization of mesenchymal stem cell-like clonal stem cells from mouse salivary glands. Tissue Eng. C Methods 2015, 21, 447–457. [Google Scholar] [CrossRef] [PubMed]
- Aure, M.H.; Arany, S.; Ovitt, C.E. Salivary glands: Stem cells, self-duplication, or both? J. Dent. Res. 2015, 94, 1502–1507. [Google Scholar] [CrossRef] [PubMed]
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Delporte, C.; Bryla, A.; Perret, J. Aquaporins in Salivary Glands: From Basic Research to Clinical Applications. Int. J. Mol. Sci. 2016, 17, 166. https://doi.org/10.3390/ijms17020166
Delporte C, Bryla A, Perret J. Aquaporins in Salivary Glands: From Basic Research to Clinical Applications. International Journal of Molecular Sciences. 2016; 17(2):166. https://doi.org/10.3390/ijms17020166
Chicago/Turabian StyleDelporte, Christine, Angélic Bryla, and Jason Perret. 2016. "Aquaporins in Salivary Glands: From Basic Research to Clinical Applications" International Journal of Molecular Sciences 17, no. 2: 166. https://doi.org/10.3390/ijms17020166
APA StyleDelporte, C., Bryla, A., & Perret, J. (2016). Aquaporins in Salivary Glands: From Basic Research to Clinical Applications. International Journal of Molecular Sciences, 17(2), 166. https://doi.org/10.3390/ijms17020166