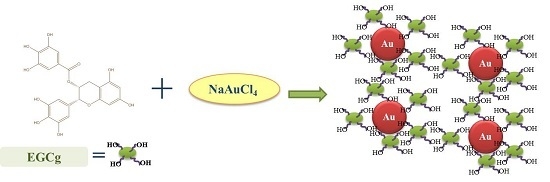
Laminin Receptor-Avid Nanotherapeutic EGCg-AuNPs as a Potential Alternative Therapeutic Approach to Prevent Restenosis
Abstract
:1. Introduction
2. Results
2.1. Synthesis and Characterization of Epigallocatechin-3-Gallate Conjugated Gold Nanoparticles (EGCg-AuNPs)
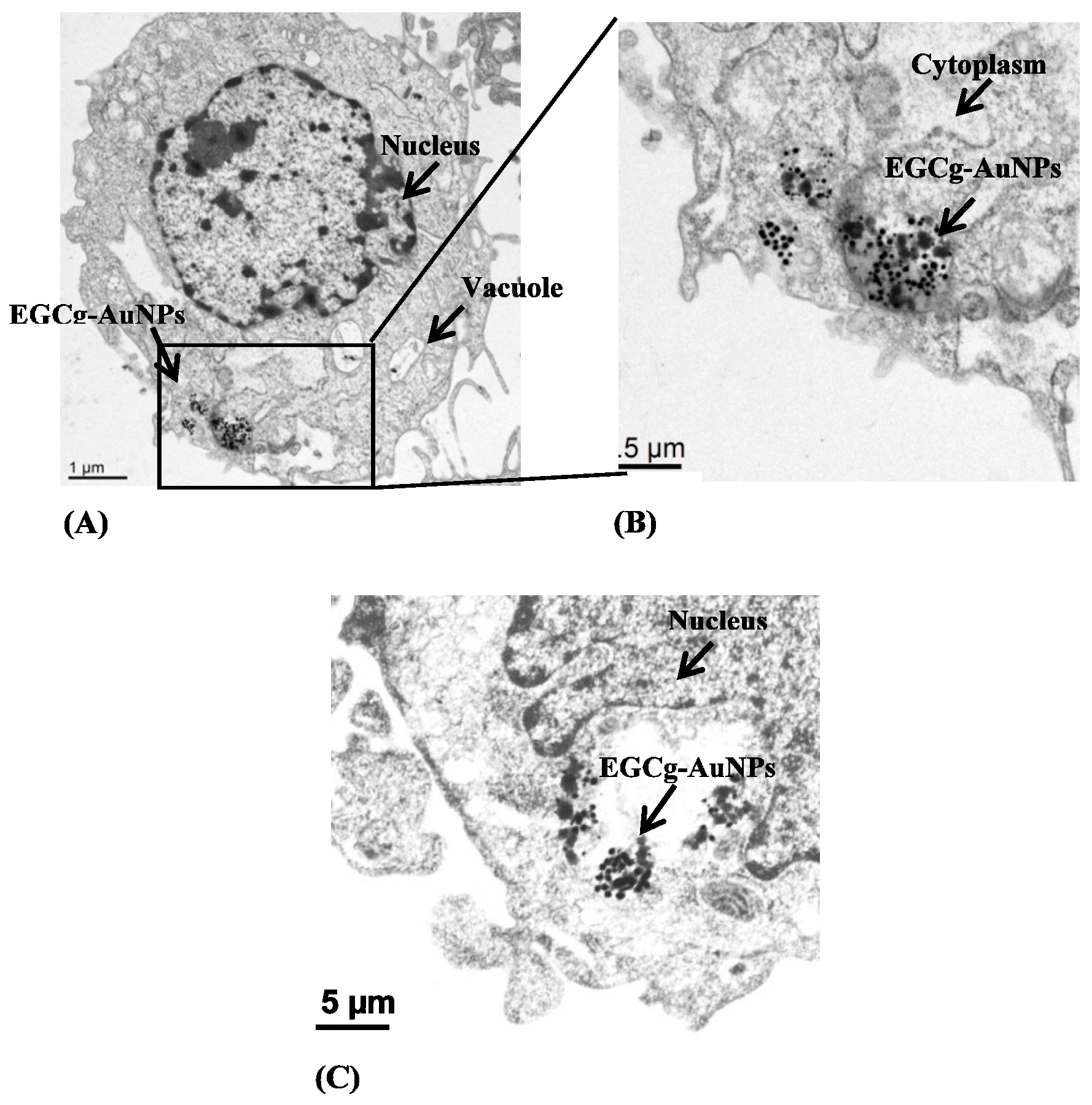
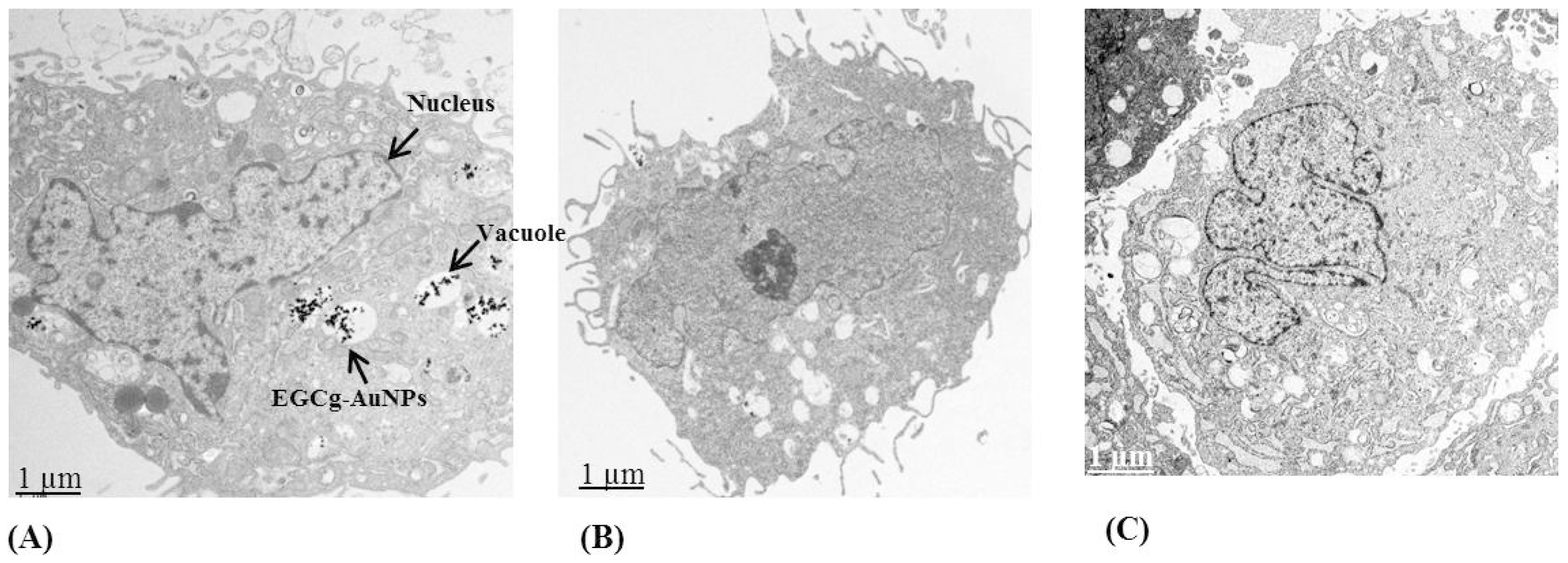
2.2. Cellular Internalization Study
2.3. Laminin Receptor Blocking Studies
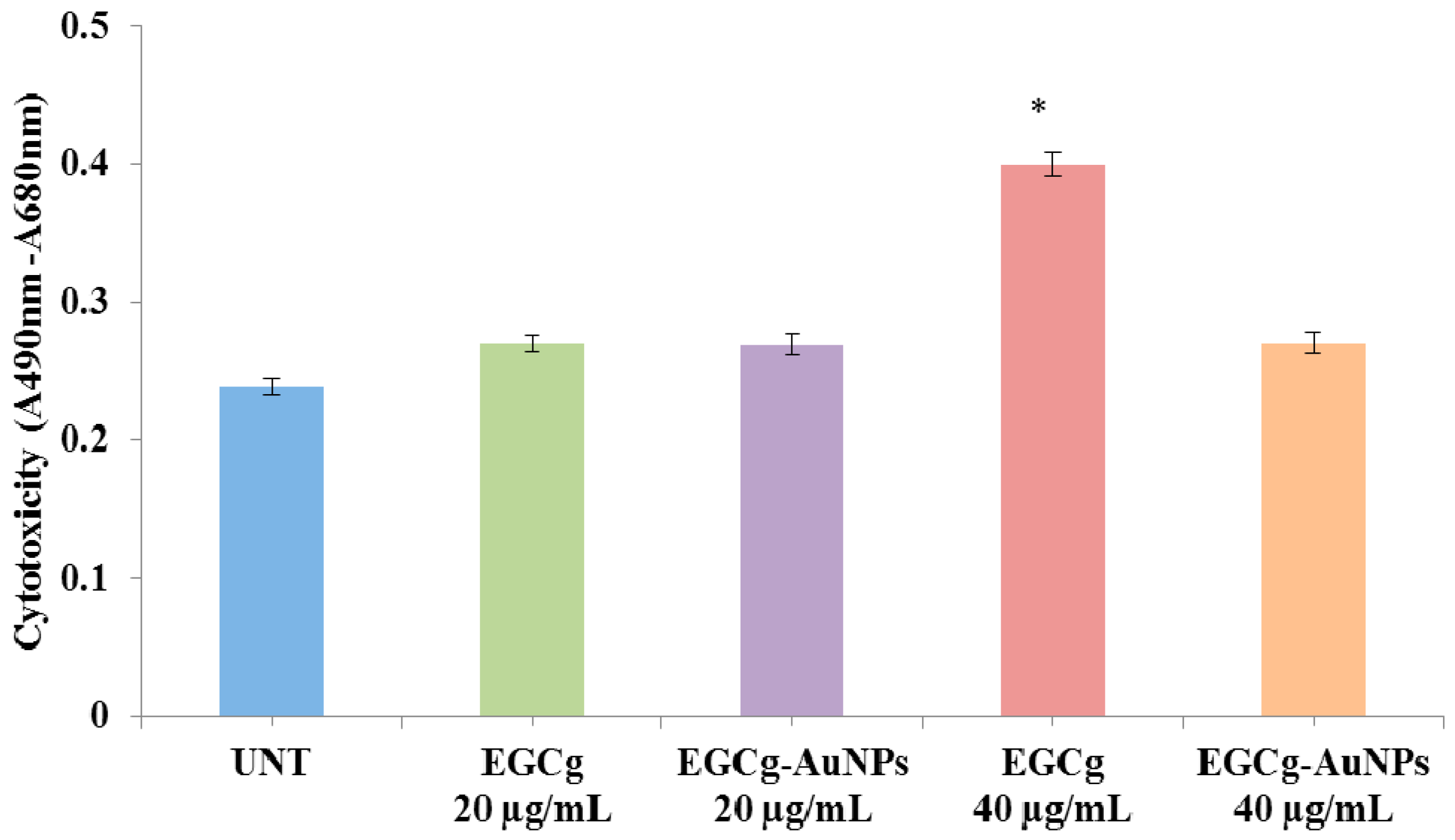
2.4. In Vitro Cell Viability and Cytotoxicity Profile of EGCg and EGCg-Coated Gold Nanoparticles
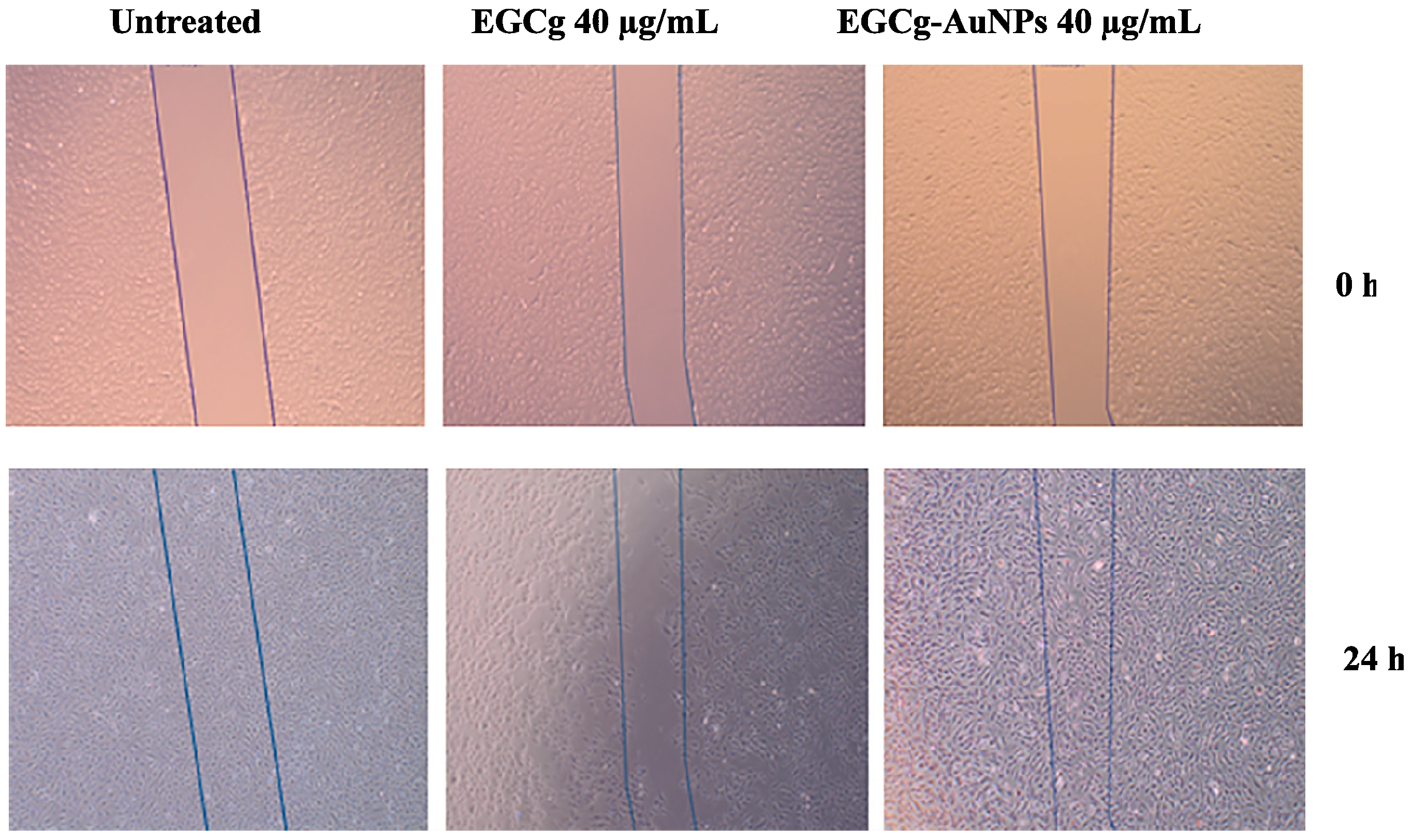
2.5. Effect of EGCg and EGCg-AuNPs on Endothelial and Smooth Muscle Cells Migration
3. Discussion
4. Experimental Section
4.1. Materials
4.2. Synthesis of EGCg Coated Gold Nanoparticles (EGCg-AuNPs)
4.3. Characterization of EGCg-AuNPs
4.4. Quantification of AuNPs by Furnace Atomic Absorption Spectroscopy (fAAS)
4.5. Endocytosis and Cellular Uptake Assay of EGCg-AuNPs by Dark Field Microscopy
4.6. Cellular Internalization of EGCg-AuNPs by Transmission Electron Microscopic (TEM)
4.7. Cell Viability Assay
4.8. Cytotoxicity Assay
4.9. Cell Migration Assay
Statistical Analysis
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Prabhakar, U.; Maeda, H.; Jain, R.K.; Sevick-Muraca, E.M.; Zamboni, W.; Farokhzad, O.C.; Barry, S.T.; Gabizon, A.; Grodzinski, P.; Blakey, D.C. Challenges and key considerations of the enhanced permeability and retention effect for nanomedicine drug delivery in oncology. Cancer Res. 2013, 73, 2412–2417. [Google Scholar] [CrossRef] [PubMed]
- Wen, R.; Banik, B.; Pathak, K.R.; Kumar, A.; Kolishetti, N.; Dhar, S. Nanotechnology inspired tools for mitochondrial dysfunction related diseases. Adv. Drug Deliv. Rev. 2016. [Google Scholar] [CrossRef] [PubMed]
- Sanna, V.; Pala, N.; Sechi, M. Targeted therapy using nanotechnology: Focus on cancer. Int. J. Nanomed. 2014, 9, 467–483. [Google Scholar]
- Etheridge, M.L.; Campbell, S.A.; Erdman, A.G.; Haynes, C.L.; Wolf, S.M.; McCullough, J. The big picture on nanomedicine: The state of investigational and approved nanomedicine products. Nanomed. Nanotechnol. 2013, 9, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Matsumura, Y. The drug discovery by nanomedicine and its clinical experience. Jpn. J. Clin. Oncol. 2014, 44, 515–525. [Google Scholar] [CrossRef] [PubMed]
- Nazir, S.; Hussain, T.; Ayub, A.; Rashid, U.; MacRobert, A.J. Nanomaterials in combating cancer: Therapeutic applications and developments. Nanomedicine 2014, 10, 19–34. [Google Scholar] [CrossRef] [PubMed]
- Sargent, J. Cardiovascular disease: New nanomedicines for treating atherosclerotic plaques. Nat. Rev. Endocrinol. 2015, 11, 256. [Google Scholar] [CrossRef] [PubMed]
- Godin, B.; Hu, Y.; Francesca, S.L.; Ferrari, M. Cardiovascular nanomedicine: Challenges and opportunities. In Molecular and Translational Vascular Medicine; Humana Press: New York, NY, USA, 2012; pp. 249–281. [Google Scholar]
- Chun, Y.W.; Crowder, S.W.; Mehl, S.C.; Wang, X.; Bae, H.; Sung, H.J. Therapeutic application of nanotechnology in cardiovascular and pulmonary regeneration. Comput. Struct. Biotechnol. J. 2013, 7, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Juliano, R. Nanomedicine: Is the wave cresting? Nat. Rev. Drug Discov. 2013, 12, 171–172. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Lin, B.; Ai, H. Theranostic nanoparticles for cancer and cardiovascular applications. Pharm. Res. 2014, 31, 1390–1406. [Google Scholar] [CrossRef] [PubMed]
- Chen, D.; Dougherty, C.A.; Zhu, K.; Hong, H. Theranostic applications of carbon nanomaterials in cancer: Focus on imaging and cargo delivery. J. Control. Release 2015, 210, 230–245. [Google Scholar] [CrossRef] [PubMed]
- Ballerini, C.; Baldi, G.; Aldinucci, A.; Maggi, P. Nanomaterial applications in multiple sclerosis inflamed brain. J. Neuroimmune Pharmacol. 2015, 10, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Austin, L.A.; Mackey, M.A.; Dreaden, E.C.; El-Sayed, M.A. The optical, photothermal, and facile surface chemical properties of gold and silver nanoparticles in biodiagnostics, therapy, and drug delivery. Arch. Toxicol. 2014, 88, 1391–1417. [Google Scholar] [CrossRef] [PubMed]
- Shang, L.; Nienhaus, K.; Nienhaus, G.U. Engineered nanoparticles interacting with cells: Size matters. J. Nanobiotechnol. 2014, 12. [Google Scholar] [CrossRef] [PubMed]
- Lane, L.A.; Qian, X.M.; Smith, A.M.; Nie, S.M. Physical chemistry of nanomedicine: Understanding the complex behaviors of nanoparticles in vivo. Annu. Rev. Phys. Chem. 2015, 66, 521–547. [Google Scholar] [CrossRef] [PubMed]
- Doane, T.L.; Burda, C. The unique role of nanoparticles in nanomedicine: Imaging, drug delivery and therapy. Chem. Soc. Rev. 2012, 41, 2885–2911. [Google Scholar] [CrossRef] [PubMed]
- Bao, G.; Mitragotri, S.; Tong, S. Multifunctional nanoparticles for drug delivery and molecular imaging. Annu. Rev. Biomed. Eng. 2013, 15, 253–282. [Google Scholar] [CrossRef] [PubMed]
- Anselmo, A.C.; Modery-Pawlowski, C.L.; Menegatti, S.; Kumar, S.; Vogus, D.R.; Tian, L.L.; Chen, M.; Squires, T.M.; Sen Gupta, A.; Mitragotri, S. Platelet-like nanoparticles: Mimicking shape, flexibility, and surface biology of platelets to target vascular injuries. Acs Nano 2014, 8, 11243–11253. [Google Scholar] [CrossRef] [PubMed]
- Saha, K.; Kim, S.T.; Yan, B.; Miranda, O.R.; Alfonso, F.S.; Shlosman, D.; Rotello, V.M. Surface functionality of nanoparticles determines cellular uptake mechanisms in mammalian cells. Small 2013, 9, 300–305. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.Y.; Ho, W.; Zhang, X.Q.; Bertrand, N.; Farokhzad, O. Cancer nanomedicine: From targeted delivery to combination therapy. Trends Mol. Med. 2015, 21, 223–232. [Google Scholar] [CrossRef] [PubMed]
- Nune, S.K.; Chanda, N.; Shukla, R.; Katti, K.; Kulkarni, R.R.; Thilakavathi, S.; Mekapothula, S.; Kannan, R.; Katti, K.V. Green nanotechnology from tea: Phytochemicals in tea as building blocks for production of biocompatible gold nanoparticles. J. Mater. Chem. 2009, 19, 2912–2920. [Google Scholar] [CrossRef] [PubMed]
- Axiak-Bechtel, S.M.; Upendran, A.; Lattimer, J.C.; Kelsey, J.; Cutler, C.S.; Selting, K.A.; Bryan, J.N.; Henry, C.J.; Boote, E.; Tate, D.J.; et al. Gum arabic-coated radioactive gold nanoparticles cause no short-term local or systemic toxicity in the clinically relevant canine model of prostate cancer. Int. J. Nanomed. 2014, 9, 5001–5011. [Google Scholar] [CrossRef] [PubMed]
- Chanda, N.; Shukla, R.; Zambre, A.; Mekapothula, S.; Kulkarni, R.R.; Katti, K.; Bhattacharyya, K.; Fent, G.M.; Casteel, S.W.; Boote, E.J.; et al. An effective strategy for the synthesis of biocompatible gold nanoparticles using cinnamon phytochemicals for phantom CT imaging and photoacoustic detection of cancerous cells. Pharm. Res. 2011, 28, 279–291. [Google Scholar] [CrossRef] [PubMed]
- Dreaden, E.C.; Alkilany, A.M.; Huang, X.H.; Murphy, C.J.; El-Sayed, M.A. The golden age: Gold nanoparticles for biomedicine. Chem. Soc. Rev. 2012, 41, 2740–2779. [Google Scholar] [CrossRef] [PubMed]
- Khoobchandani, M.; Zambre, A.; Katti, K.; Lin, C.H.; Katti, K.V. Green nanotechnology from brassicaceae: Development of broccoli phytochemicals-encapsulated gold nanoparticles and their applications in Nanomedicine. Int. J. Green Nanotechnol. 2013, 1, 1–15. [Google Scholar] [CrossRef]
- Dykman, L.; Khlebtsov, N. Gold nanoparticles in biomedical applications: Recent advances and perspectives. Chem. Soc. Rev. 2012, 41, 2256–2282. [Google Scholar] [CrossRef] [PubMed]
- Mieszawska, A.J.; Mulder, W.J.; Fayad, Z.A.; Cormode, D.P. Multifunctional gold nanoparticles for diagnosis and therapy of disease. Mol. Pharm. 2013, 10, 831–847. [Google Scholar] [CrossRef] [PubMed]
- Katti, K.; Chanda, N.; Shukla, R.; Zambre, A.; Suibramanian, T.; Kulkarni, R.R.; Kannan, R.; Katti, K.V. Green nanotechnology from cumin phytochemicals: Generation of biocompatible gold nanoparticles. Int. J. Green Nanotechnol. Biomed. 2009, 1, B39–B52. [Google Scholar] [CrossRef] [PubMed]
- Chanda, N.; Shukla, R.; Katti, K.V.; Kannan, R. Gastrin releasing protein receptor specific gold nanorods: Breast and prostate tumor avid nanovectors for molecular imaging. Nano Lett. 2009, 9, 1798–1805. [Google Scholar] [CrossRef] [PubMed]
- Donaldson, K.; Duffin, R.; Langrish, J.P.; Miller, M.R.; Mills, N.L.; Poland, C.A.; Raftis, J.; Shah, A.; Shaw, C.A.; Newby, D.E. Nanoparticles and the cardiovascular system: A critical review. Nanomedicine 2013, 8, 403–423. [Google Scholar] [CrossRef] [PubMed]
- Chanda, N.; Kattumuri, V.; Shukla, R.; Zambre, A.; Katti, K.; Upendran, A.; Kulkarni, R.R.; Kan, P.; Fent, G.M.; Casteel, S.W.; et al. Bombesin functionalized gold nanoparticles show in vitro and in vivo cancer receptor specificity. Proc. Natl. Acad. Sci. USA 2010, 107, 8760–8765. [Google Scholar] [CrossRef] [PubMed]
- Shukla, R.; Chanda, N.; Zambre, A.; Upendran, A.; Katti, K.; Kulkarni, R.R.; Nune, S.K.; Casteel, S.W.; Smith, C.J.; Vimal, J.; et al. Laminin receptor specific therapeutic gold nanoparticles (198AuNP-EGCg) show efficacy in treating prostate cancer. Proc. Natl. Acad. Sci. USA 2012, 109, 12426–12431. [Google Scholar] [CrossRef] [PubMed]
- Jain, P.K.; Huang, X.; El-Sayed, I.H.; El-Sayad, M.A. Review of some interesting surface plasmon resonance-enhanced properties of noble metal nanoparticles and their applications to biosystems. Plasmonics 2007, 2, 107–118. [Google Scholar] [CrossRef]
- Go, A.S.; Mozaffarian, D.; Roger, V.L.; Benjamin, E.J.; Berry, J.D.; Blaha, M.J.; Dai, S.; Ford, E.S.; Fox, C.S.; Franco, S.; et al. Heart disease and stroke statistics—2014 update: A report from the American Heart Association. Circulation 2014, 129. [Google Scholar] [CrossRef] [PubMed]
- Go, A.S.; Mozaffarian, D.; Roger, V.L.; Benjamin, E.J.; Berry, J.D.; Borden, W.B.; Bravata, D.M.; Dai, S.F.; Ford, E.S.; Fox, C.S.; et al. Heart disease and stroke statistics—2013 Update a report from the American Heart Association. Circulation 2013, 127. [Google Scholar] [CrossRef] [PubMed]
- Schillinger, M.; Minar, E. Restenosis after percutaneous angioplasty: The role of vascular inflammation. Vasc. Health Risk Manag. 2005, 1, 73–78. [Google Scholar] [CrossRef] [PubMed]
- Lim, G.B. Drug-eluting stents in ischaemic peripheral artery disease. Nat. Rev. Cardiol. 2013, 10, 61. [Google Scholar] [CrossRef] [PubMed]
- Amin, A.P.; Spertus, J.A.; Cohen, D.J.; Chhatriwalla, A.; Kennedy, K.F.; Vilain, K.; Salisbury, A.C.; Venkitachalam, L.; Lai, S.M.; Mauri, L.; et al. Use of drug-eluting stents as a function of predicted benefit: Clinical and economic implications of current practice. Arch. Int. Med. 2012, 172, 1145–1152. [Google Scholar] [CrossRef] [PubMed]
- Limacher, A.; Raber, L.; Laube, E.; Lauterburg, A.; Lotscher, S.; Hess, N.; Moschovitis, A.; Baldinger, S.H.; Wenaweser, P.; Meier, B.; et al. Clinical long-term outcome after implantation of titanium nitride-oxide coated stents compared with paclitaxel- or sirolimus-eluting stents: Propensity-score matched analysis. EuroIntervention 2012, 7, 1043–1050. [Google Scholar] [CrossRef] [PubMed]
- Alfonso, F.; Perez-Vizcayno, M.J.; Dutary, J.; Zueco, J.; Cequier, A.; Garcia-Touchard, A.; Marti, V.; Lozano, I.; Angel, J.; Hernandez, J.M.; et al. Implantation of a drug-eluting stent with a different drug (switch strategy) in patients with drug-eluting stent restenosis. Results from a prospective multicenter study (RIBS III [Restenosis Intra-Stent: Balloon Angioplasty Versus Drug-Eluting Stent]). JACC. Cardiovasc. Interv. 2012, 5, 728–737. [Google Scholar] [CrossRef] [PubMed]
- Kolandaivelu, K.; Swaminathan, R.; Gibson, W.J.; Kolachalama, V.B.; Nguyen-Ehrenreich, K.L.; Giddings, V.L.; Coleman, L.; Wong, G.K.; Edelman, E.R. Stent thrombogenicity early in high-risk interventional settings is driven by stent design and deployment and protected by polymer-drug coatings. Circulation 2011, 123, 1400–1409. [Google Scholar] [CrossRef] [PubMed]
- Maluenda, G.; Ben-Dor, I.; Gaglia, M.A., Jr.; Wakabayashi, K.; Mahmoudi, M.; Sardi, G.; Laynez-Carnicero, A.; Torguson, R.; Xue, Z.; Margulies, A.D.; et al. Clinical outcomes and treatment after drug-eluting stent failure: The absence of traditional risk factors for in-stent restenosis. Circ. Cardiovasc. Interv. 2012, 5, 12–19. [Google Scholar] [CrossRef] [PubMed]
- Windecker, S.; Meier, B. Late coronary stent thrombosis. Circulation 2007, 116, 1952–1965. [Google Scholar] [CrossRef] [PubMed]
- Stone, G.W.; Witzenbichler, B.; Weisz, G.; Rinaldi, M.J.; Neumann, F.J.; Metzger, D.C.; Henry, T.D.; Cox, D.A.; Duffy, P.L.; Mazzaferri, E.; et al. Platelet reactivity and clinical outcomes after coronary artery implantation of drug-eluting stents (ADAPT-DES): A prospective multicentre registry study. Lancet 2013, 382, 614–623. [Google Scholar] [CrossRef]
- Shukla, R.; Nune, S.K.; Chanda, N.; Katti, K.; Mekapothula, S.; Kulkarni, R.R.; Welshons, W.V.; Kannan, R.; Katti, K.V. Soybeans as a phytochemical reservoir for the production and stabilization of biocompatible gold nanoparticles. Small 2008, 4, 1425–1436. [Google Scholar] [CrossRef] [PubMed]
- Kannan, R.; Rahing, V.; Cutler, C.; Pandrapragada, R.; Katti, K.K.; Kattumuri, V.; Robertson, J.D.; Casteel, S.J.; Jurisson, S.; Smith, C.; et al. Nanocompatible chemistry toward fabrication of target-specific gold nanoparticles. J. Am. Chem. Soc. 2006, 128, 11342–11343. [Google Scholar] [CrossRef] [PubMed]
- Leu, J.G.; Chen, S.A.; Chen, H.M.; Wu, W.M.; Hung, C.F.; Yao, Y.D.; Tu, C.S.; Liang, Y.J. The effects of gold nanoparticles in wound healing with antioxidant epigallocatechin gallate and α-lipoic acid. Nanomedicine 2012, 8, 767–775. [Google Scholar] [CrossRef] [PubMed]
- Weakley, S.M.; Wang, X.; Mu, H.; Lu, J.; Lin, P.H.; Yao, Q.; Chen, C. Ginkgolide A-gold nanoparticles inhibit vascular smooth muscle proliferation and migration in vitro and reduce neointimal hyperplasia in a mouse model. J. Surg. Res. 2011, 171, 31–39. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.C.; Hsieh, D.S.; Huang, K.J.; Chan, Y.L.; Hong, P.D.; Yeh, M.K.; Wu, C.J. Improving anticancer efficacy of (−)-epigallocatechin-3-gallate gold nanoparticles in murine B16F10 melanoma cells. Drug Des. Dev. Ther. 2014, 8, 459–474. [Google Scholar]
- Holy, E.W.; Stampfli, S.F.; Akhmedov, A.; Holm, N.; Camici, G.G.; Luscher, T.F.; Tanner, F.C. Laminin receptor activation inhibits endothelial tissue factor expression. J. Mol. Cell. Cardiol. 2010, 48, 1138–1145. [Google Scholar] [CrossRef] [PubMed]
- Katti, K.V.; Kannan, R.; Kattesh, K.K.; Nune, S.; Cutler, C.S.; Caldwell, C.; Shukla, R.; Chanda, N. EGCG stabilized gold nanoparticles and method for making same. Publication number US 20130129618 A1, 23 May 2013. [Google Scholar]
- Tachibana, H.; Koga, K.; Fujimura, Y.; Yamada, K. A receptor for green tea polyphenol EGCG. Nat. Struct. Mol. Biol. 2004, 11, 380–381. [Google Scholar] [CrossRef] [PubMed]
- Barkalina, N.; Charalambous, C.; Jones, C.; Coward, K. Nanotechnology in reproductive medicine: Emerging applications of nanomaterials. Nanomed. Nanotechnol. Biol. Med. 2014, 10, 921–938. [Google Scholar] [CrossRef] [PubMed]
- Ma, L.; Kohli, M.; Smith, A. Nanoparticles for combination drug therapy. Acs Nano 2013, 7, 9518–9525. [Google Scholar] [CrossRef] [PubMed]
- Kanapathipillai, M.; Brock, A.; Ingber, D.E. Nanoparticle targeting of anti-cancer drugs that alter intracellular signaling or influence the tumor microenvironment. Adv. Drug Deliv. Rev. 2014, 79–80, 107–118. [Google Scholar] [CrossRef] [PubMed]
- Iverson, N.; Plourde, N.; Chnari, E.; Nackman, G.B.; Moghe, P.V. Convergence of nanotechnology and cardiovascular medicine: Progress and emerging prospects. BioDrug. Clin. Immunother. Biopharm. Gene Ther. 2008, 22, 1–10. [Google Scholar] [CrossRef]
- Marx, S.O.; Totary-Jain, H.; Marks, A.R. Vascular smooth muscle cell proliferation in restenosis. Circ. Cardiovasc. Interv. 2011, 4, 104–111. [Google Scholar] [CrossRef] [PubMed]
- Libby, P. Inflammation in atherosclerosis. Arterioscler. Thromb. Vasc. Biol. 2012, 32, 2045–2051. [Google Scholar] [CrossRef] [PubMed]
- Mika, M.; Kostogrys, R.B.; Franczyk-Zarow, M.; Wikiera, A.; Maslak, E. Anti-atherosclerotic activity of catechins depends on their stereoisomerism. Atherosclerosis 2015, 240, 125–130. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J.; Zhang, X.; Li, L.; Su, G. Protective effects of epigallocatechin-3 gallate on atrial electrical and structural remodeling in a rabbit rapid atrial pacing model. Cell Biochem. Biophys. 2015, 71, 897–903. [Google Scholar] [CrossRef] [PubMed]
- Zhong, W.; Huan, X.D.; Cao, Q.; Yang, J. Cardioprotective effect of epigallocatechin-3-gallate against myocardial infarction in hypercholesterolemic rats. Exp. Ther. Med. 2015, 9, 405–410. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.S.; Quon, M.J.; Kim, J.A. New insights into the mechanisms of polyphenols beyond antioxidant properties; lessons from the green tea polyphenol, epigallocatechin 3-gallate. Redox Biol. 2014, 2, 187–195. [Google Scholar] [CrossRef] [PubMed]
- Won, S.M.; Park, Y.H.; Kim, H.J.; Park, K.M.; Lee, W.J. Catechins inhibit angiotensin II-induced vascular smooth muscle cell proliferation via mitogen-activated protein kinase pathway. Exp. Mol. Med. 2006, 38, 525–534. [Google Scholar] [CrossRef] [PubMed]
- Han, S.G. Protective effects of EGCG through Inhibition of NADPH oxidase expression in endothelial cells. Food Sci. Biotechnol. 2014, 23, 1611–1614. [Google Scholar] [CrossRef]
- Han, D.-W.; Lee, M.H.; Kwon, B.-J.; Kim, H.-L.; Hyon, S.-H.; Park, J.-C. Selective inhibitory effect of epigallocatechin-3-gallate on migration of vascular smooth muscle cells. Molecules 2010, 15, 8488–8500. [Google Scholar]
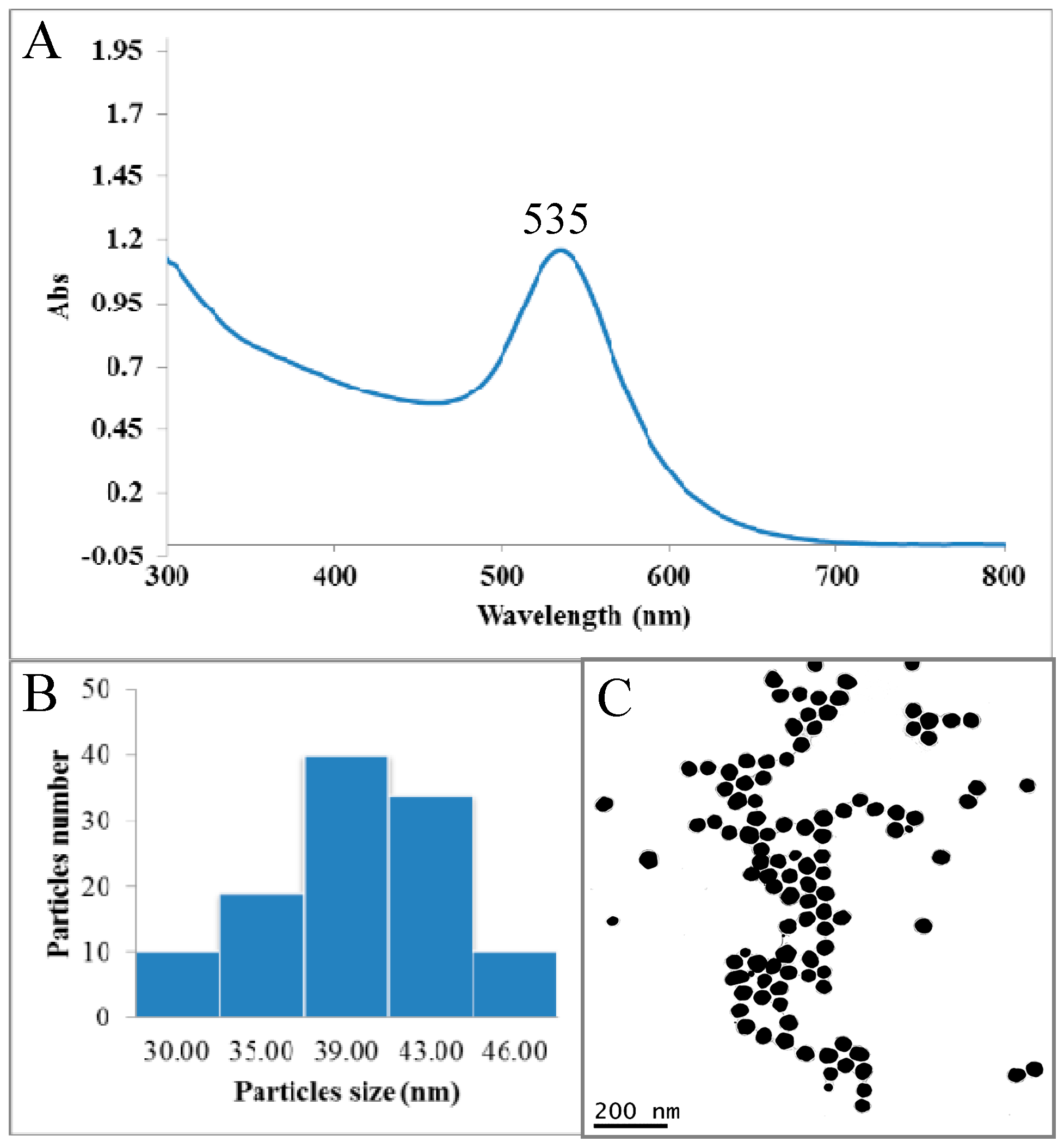






| UV Visible Spectrophotometry | DLS | ζ Potential | TEM | AAS |
|---|---|---|---|---|
| 535 nm | 65 ± 5 nm | −35.0 ± 2 mV | 40 ± 5 nm | 0.52 mg Au/mg of AuNPs |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Khoobchandani, M.; Katti, K.; Maxwell, A.; Fay, W.P.; Katti, K.V. Laminin Receptor-Avid Nanotherapeutic EGCg-AuNPs as a Potential Alternative Therapeutic Approach to Prevent Restenosis. Int. J. Mol. Sci. 2016, 17, 316. https://doi.org/10.3390/ijms17030316
Khoobchandani M, Katti K, Maxwell A, Fay WP, Katti KV. Laminin Receptor-Avid Nanotherapeutic EGCg-AuNPs as a Potential Alternative Therapeutic Approach to Prevent Restenosis. International Journal of Molecular Sciences. 2016; 17(3):316. https://doi.org/10.3390/ijms17030316
Chicago/Turabian StyleKhoobchandani, Menka, Kavita Katti, Adam Maxwell, William P. Fay, and Kattesh V. Katti. 2016. "Laminin Receptor-Avid Nanotherapeutic EGCg-AuNPs as a Potential Alternative Therapeutic Approach to Prevent Restenosis" International Journal of Molecular Sciences 17, no. 3: 316. https://doi.org/10.3390/ijms17030316
APA StyleKhoobchandani, M., Katti, K., Maxwell, A., Fay, W. P., & Katti, K. V. (2016). Laminin Receptor-Avid Nanotherapeutic EGCg-AuNPs as a Potential Alternative Therapeutic Approach to Prevent Restenosis. International Journal of Molecular Sciences, 17(3), 316. https://doi.org/10.3390/ijms17030316