Evaluation of the Cytotoxic Behavior of Fungal Extracellular Synthesized Ag Nanoparticles Using Confocal Laser Scanning Microscope
Abstract
:1. Introduction
2. Results and Discussion
2.1. Extracellular Biosynthesis of Fungal Ag NPs
2.2. Characterization of Fungal Ag NPs
2.3. Cytotoxic Activity of Fungus Ag NPs
2.4. Confocal Laser Microscopic Mode of Action
3. Materials and Methods
3.1. Microorganisms
3.2. Biomass Production
3.3. Biosynthesis of Silver Nanoparticles
3.4. Characterization of Synthesized Silver Nanoparticles
3.5. Cytotoxic Activity
3.5.1. MCF-7 Cell Culture
3.5.2. WST-1 Assay
3.5.3. Confocal Laser Scanning Microscopy (CLSM)
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Salata, O. Applications of nanoparticles in biology and medicine. J. Nanobiotechnol. 2004, 6, 1–6. [Google Scholar]
- Mohanpuria, P.; Rana, N.K.; Yadav, S.K. Biosynthesis of nanoparticles: Technological concepts and future applications. J. Nanopart. Res. 2008, 10, 507–517. [Google Scholar] [CrossRef]
- Asmathunisha, N.; Kathiresan, K. A review on biosynthesis of nanoparticles by marine organisms. Coll. Surf. B Biointerfaces 2013, 103, 283–287. [Google Scholar] [CrossRef] [PubMed]
- Narayanan, K.B.; Sakthivel, N. Biological synthesis of metal nanoparticles by microbes. Adv. Colloid Interface Sci. 2010, 1, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Hassan, A.A.; Mansour, M.K.; Mahmoud, H.H. Biosynthesis of silver nanoparticles (Ag-Nps) (a model of metals) by Candida albicans and its antifungal activity on Some fungal pathogens (Trichophyton mentagrophytes and Candida albicans). N. Y. Sci. J. 2013, 6, 27–34. [Google Scholar]
- Mandal, D.; Molander, M.E.; Mukhopadhyay, D.; Sarkar, G.; Mukherjee, P. The use of microorganism for the formation of metal nanoparticles and their application. Appl. Microbiol. Biotechnol. 2006, 69, 485–492. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Xu, H.; Chen, Z.; Chen, G. Biosynthesis of nanoparticles by microorganisms and their applications. J. Nanomater. 2011. [Google Scholar] [CrossRef]
- Zhang, X.; Song, Y.; Tyagi, R.D.; Surampalli, R.Y. Synthesis of nanoparticles by microorganisms and their application in enhancing microbiological reaction rates. Chemosphere 2011, 82, 489–494. [Google Scholar] [CrossRef] [PubMed]
- Kalaiselvan, V.; Rajasekaran, A. Biosynthesis of silver nanoparticles from Aspergillus niger and evaluation of its wound healing activity in experimental rat model. Int. J. Pharm. Tech. Res. 2009, 4, 1523–1529. [Google Scholar]
- Valodkar, M.; Bhadorai, A.; Pohnerkar, J.; Mohan, M.; Thakore, S. Morphology and antibacterial activity of carbohydrate stabilized silver nanoparticles. Carbohydr. Res. 2010, 345, 1767–1773. [Google Scholar] [CrossRef] [PubMed]
- Prabhu, S.; Poulose, E.K. Silver nanoparticles: Mechanism of antimicrobial action, synthesis, medical applications, and toxicity effects. Int. Nano Lett. 2012, 2, 32. [Google Scholar] [CrossRef]
- Chitra, K.; Annadurai, G. Antibacterial activity of pH-dependent biosynthesized silver nanoparticles against clinical pathogen. BioMed. Res. Int. 2014. [Google Scholar] [CrossRef] [PubMed]
- Bhattacharya, R.; Mukherjee, P. Biological properties of “naked” metal nanoparticles. Adv. Drug Deliv. Rev. 2008, 60, 1289–1306. [Google Scholar] [CrossRef] [PubMed]
- Sriram, M.I.; Selvaraj, B.M.K.; Kalimuthu, K.; Sangiliyandi, G. Antitumor activity of silver nanoparticles in Dalton’s lymphoma ascites tumor model. Int. J. Nanomed. 2010, 5, 753–762. [Google Scholar]
- Sun, R.W.; Rong, C.; Chung, N.P.Y.; Ho, C.M.; Lin, C.L.S.; Che, C.M. Silver nanoparticles fabricated in Hepes buffer exhibit cytoprotective activities toward HIV-1 infected cells. Chem. Commun. (Camb.) 2005, 28, 5059–5061. [Google Scholar] [CrossRef] [PubMed]
- Lara, H.H.; Ayala-Nuñez, N.V.; Ixtepan-Turrent, L.; Rodriguez-Padilla, C. Mode of antiviral action of silver nanoparticles against HIV-1. J. Nanobiotechnol. 2010, 8, 1. [Google Scholar] [CrossRef] [PubMed]
- Arora, S.; Tyagi, N.; Bhardwaj, A.; Rusu, L.; Palanki, R.; Vig, K.; Singh, S.R.; Singh, A.P.; Palanki, S.; Miller, M.E.; et al. Silver nanoparticles protect human keratinocytes against UVB radiation-induced DNA damage and apoptosis: Potential for prevention of skin carcinogenesis. Nanomed. Nanotechnol. Biol. Med. 2015, 11, 1265–1275. [Google Scholar] [CrossRef] [PubMed]
- Jeyaraj, M.; Rajesha, M.; Arunb, R.; MubarakAlic, D.; Sathishkumara, G.; Sivanandhana, G.; Kapil Deva, G.; Manickavasagama, M.; Premkumarb, K.; Thajuddinc, N.; et al. An investigation on the cytotoxicity and caspase-mediated apoptotic effect of biologically synthesized silver nanoparticles using Podophyllum hexandrum on human cervical carcinoma cells. Coll. Surf. B Biointerfaces 2013, 102, 708–717. [Google Scholar] [CrossRef] [PubMed]
- Lokina, S.; Stephen, A.; Kaviyarasan, V.; Arulvasu, C.; Narayanan, V. Cytotoxicity and antimicrobial activities of green synthesized silver nanoparticles. Eur. J. Med. Chem. 2014, 76, 256–263. [Google Scholar] [CrossRef] [PubMed]
- Husseiny, S.M.; Salah, T.A.; Anter, H.A. Biosynthesis of size controlled silver nanoparticles by Fusarium oxysporum, their antibacterial and antitumor activities. Beni-Suef Univ. J. Basic Appl. Sci. 2015, 4, 225–231. [Google Scholar] [CrossRef]
- Mahmoud, M.A.; Al-sohaibani, S.A.; Alothman, M.R.; Abd el aziz, A.R.M.; Eifan, S.A. Synthesis of extracellular silver nanoparticles using fusarium semitectum (ksu-4) isolated from Saudi Arabia. Dig. J. Nanomater. Biostruct. 2013, 8, 589–596. [Google Scholar]
- Roy, S.; Mukherjee, T.; Chakraborty, S.; Das, T.K. Biosynthesis, characterisation & antifungal activity of Silver nanoparticles synthesized by the fungus aspergillus Foetidus mtcc8876. Dig. J. Nanomater. Biostruct. 2015, 8, 197–205. [Google Scholar]
- Selvi, V.K.; Sivakumar, T. Isolation and characterization of silver nanoparticles from Fusarium oxysporum. Int. J. Curr. Microbiol. Appl. Sci. 2012, 1, 56–62. [Google Scholar]
- Elechiguerra, J.L.; Burt, J.L.; Morones, J.R.; Camacho-Bragado, A.; Gao, X.; Lara, H.H.; Yacaman, M.J. Interaction of silver nanoparticles with HIV-1. J. Nanobiotechnol. 2005, 3, 6. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wani, I.A.; Ganguly, A.; Ahmed, J.; Ahmad, T. Silver nanoparticles: Ultrasonic wave assisted synthesis, optical characterization and surface area studies. Mater. Lett. 2011, 65, 520–522. [Google Scholar] [CrossRef]
- Ahmad, A.; Mukherjee, P.; Senapati, S.; Mandal, D.; Khan, M.I.; Kumar, R.; Sastry, M. Extracellular biosynthesis of silver nanoparticles using the fungus Fusarium oxysporum. Coll. Surf. B Biointerfaces 2003, 8, 313–318. [Google Scholar] [CrossRef]
- Xu, J.K.; Zhang, F.F.; Sun, J.J.; Sheng, J.; Wang, F.; Sun, M. Bio and nanomaterials based on Fe3O4. Molecules 2014, 19, 21506–21528. [Google Scholar] [CrossRef] [PubMed]
- Syed, A.; Supriya, S.; Gopal, K.; Absar, A. Biological synthesis of silver nanoparticles using the fungus Humicola sp. and evaluation of their cytoxicity using normal and cancer cell lines. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2013, 114, 144–147. [Google Scholar] [CrossRef] [PubMed]
- Kathiravan, V.; Ravi, S.; Ashokkumar, S. Synthesis of silver nanoparticles from Melia dubia leaf extract and their in vitro anticancer activity. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2014, 130, 116–121. [Google Scholar] [CrossRef] [PubMed]
- Vasanth, K.; Ilango, K.; MohanKumar, R.; Agrawal, A.; Dubey, G.P. Anticancer activity of Moringa oleifera mediated silver nanoparticleson human cervical carcinoma cells by apoptosis induction. Coll. Surf. B Biointerfaces 2014, 117, 354–359. [Google Scholar] [CrossRef] [PubMed]
- Sambale, F.; Wagner, S.; Stahh, F.; Khaydarov, R.R.; Scheper, T.; Bahnemann, D. Investigations of the toxic effect of silver nanoparticles on mammalian cell lines. J. Nanomater. 2015, 2015, 6. [Google Scholar] [CrossRef]
- Jeong, Y.; Lim, D.W.; Choi1, J. Assessment of size-dependent antimicrobial and cytotoxic properties of silver nanoparticles. Adv. Mater. Sci. Eng. 2014. [Google Scholar] [CrossRef]
- Brewer, M.; Zhang, T.; Dong, W.; Rutherford, M.; Tian, Z.R. Future approaches of nanomedicine in clinical science. Med. Clin. N. Am. 2007, 91, 963–1016. [Google Scholar] [CrossRef] [PubMed]
- Mishra, A.; Mehdi, S.J.; Irshad, M.; Ali, A.; Sardar, M.; Moshahid, M.; Rizvi, A. Effect of Biologically Synthesized silver nanoparticles on human cancer cells. Sci. Adv. Mater. 2012, 4, 1200–1206. [Google Scholar] [CrossRef]
- Zhang, L.W.; Monteiro-Riviere, N.A. Use of confocal microscopy for nanoparticle drug delivery through skin. J. Biomed. Opt. 2013, 18, 061214. [Google Scholar] [CrossRef] [PubMed]
- Ngamwongsatit, P.; Banada, P.P.; Panbangred, W.; Bhunia, A.K. WST-1-based cell cytotoxicity assay as a substitute for MTT-based assay for rapid detection of toxigenic Bacillus species using CHO cell line. J. Microbiol. Methods 2008, 73, 211–215. [Google Scholar] [CrossRef] [PubMed]
- Goma, S.M.; Salah, T.A.; Abdelhamid, A.O. Synthesis, characterization, and pharmacological evaluation of some novel thiadiazoles and thiazoles incorporating pyrazole moiety as anticancer agents. Monatshefte für Chem. Chem. Mon. 2015, 146, 149–158. [Google Scholar] [CrossRef]





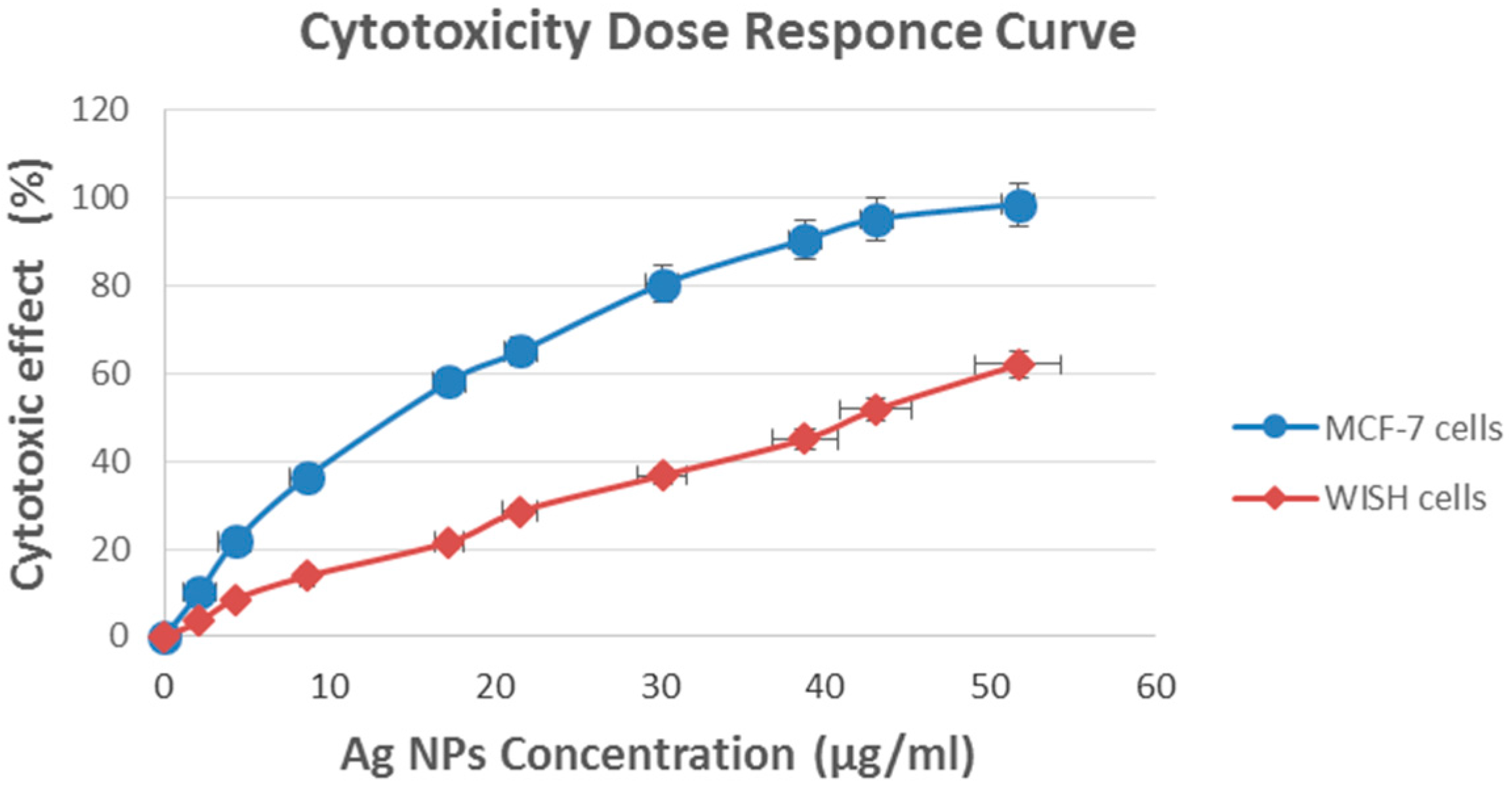


| Concentration (µg/mL) | Cytotoxic Effect (%) | |
|---|---|---|
| MCF-7 Cells | Normal WISH Cells | |
| 0 | 0 | 0 |
| 2.2 | 10.2 ± 2.7 | 3.5 ± 2.3 |
| 4.3 | 21.7 ± 2.4 | 8.6 ± 2.1 |
| 8.6 | 36.4 ± 2.1 | 13.8 ± 2.0 |
| 17.3 | 58.1 ± 1.8 | 21.6 ± 1.6 |
| 21.6 | 65.1 ± 1.5 | 28.6 ± 1.2 |
| 30.2 | 80.5 ± 1.0 | 36.8 ± 1.3 |
| 38.8 | 90.6 ± 0.9 | 45.1 ± 0.8 |
| 43.2 | 95.2 ± 0.6 | 51.8 ± 1.3 |
| 51.8 | 98.7 ± 0.7 | 62.1 ± 2.0 |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Salaheldin, T.A.; Husseiny, S.M.; Al-Enizi, A.M.; Elzatahry, A.; Cowley, A.H. Evaluation of the Cytotoxic Behavior of Fungal Extracellular Synthesized Ag Nanoparticles Using Confocal Laser Scanning Microscope. Int. J. Mol. Sci. 2016, 17, 329. https://doi.org/10.3390/ijms17030329
Salaheldin TA, Husseiny SM, Al-Enizi AM, Elzatahry A, Cowley AH. Evaluation of the Cytotoxic Behavior of Fungal Extracellular Synthesized Ag Nanoparticles Using Confocal Laser Scanning Microscope. International Journal of Molecular Sciences. 2016; 17(3):329. https://doi.org/10.3390/ijms17030329
Chicago/Turabian StyleSalaheldin, Taher A., Sherif M. Husseiny, Abdullah M. Al-Enizi, Ahmed Elzatahry, and Alan H. Cowley. 2016. "Evaluation of the Cytotoxic Behavior of Fungal Extracellular Synthesized Ag Nanoparticles Using Confocal Laser Scanning Microscope" International Journal of Molecular Sciences 17, no. 3: 329. https://doi.org/10.3390/ijms17030329
APA StyleSalaheldin, T. A., Husseiny, S. M., Al-Enizi, A. M., Elzatahry, A., & Cowley, A. H. (2016). Evaluation of the Cytotoxic Behavior of Fungal Extracellular Synthesized Ag Nanoparticles Using Confocal Laser Scanning Microscope. International Journal of Molecular Sciences, 17(3), 329. https://doi.org/10.3390/ijms17030329