Ionogels Based on Poly(methyl methacrylate) and Metal-Containing Ionic Liquids: Correlation between Structure and Mechanical and Electrical Properties
Abstract
:1. Introduction
2. Results
2.1. Ionic Liquids
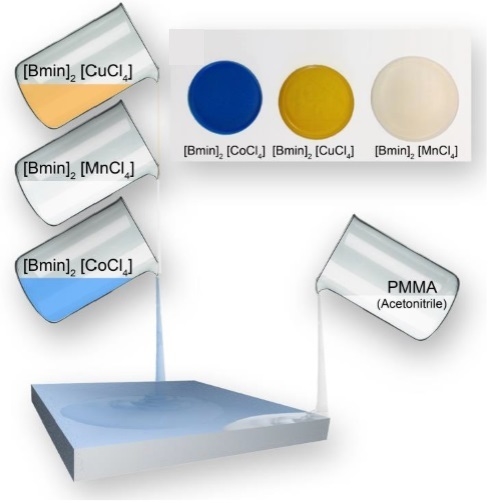
2.2. Ionogels (IGs)
3. Discussion
4. Materials and Methods
4.1. Materials
4.2. General Procedure for [Bmim]2[CuCl4], [Bmim]2[CoCl4] and [Bmim]2[MnCl4] Synthesis
4.3. IG Synthesis
4.4. Infrared Spectroscopy
4.5. UV/Vis Spectroscopy
4.6. Magnetic Properties
4.7. Thermal Analysis
4.8. Optical Microscopy
4.9. Dielectric Spectroscopy. IG Thickness
4.10. Mechanical Characterization
4.11. Dielectric and Electrical Characterization
5. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
Abbreviations
| MDPI | Multidisciplinary Digital Publishing Institute |
| DOAJ | Directory of open access journals |
| TLA | Three letter acronym |
| LD | linear dichroism |
References
- Brennecke, J.F.; Maginn, E.J. Ionic liquids: Innovative fluids for chemical processing. AIChE J. 2001, 47, 2384–2389. [Google Scholar] [CrossRef]
- Wei, D.; Ivaska, A. Applications of ionic liquids in electrochemical sensors. Anal. Chim. Acta 2008, 607, 126–135. [Google Scholar] [CrossRef] [PubMed]
- Pandey, S. Analytical applications of room-temperature ionic liquids: A review of recent efforts. Anal. Chim. Acta 2006, 556, 38–45. [Google Scholar] [CrossRef] [PubMed]
- Binnemans, K. Ionic liquid crystals. Chem. Rev. 2005, 105, 4148–4204. [Google Scholar] [CrossRef] [PubMed]
- Douce, L.; Suisse, J.-M.; Guillon, D.; Taubert, A. Imidazolium-based liquid crystals: A modular platform for versatile new materials with finely tuneable properties and behaviour. Liquid Cryst. 2011, 38, 1653–1661. [Google Scholar] [CrossRef]
- Ji, Q.; Honma, I.; Paek, S.M.; Akada, M.; Hill, J.P.; Vinu, A.; Ariga, K. Layer-by-layer films of graphene and ionic liquids for highly selective gas sensing. Angew. Chem. 2010, 122, 9931–9933. [Google Scholar] [CrossRef]
- Mallick, B.; Balke, B.; Felser, C.; Mudring, A.V. Dysprosium room-temperature ionic liquids with strong luminescence and response to magnetic fields. Angew. Chem. Int. Ed. 2008, 47, 7635–7638. [Google Scholar] [CrossRef] [PubMed]
- Mudring, A.-V.; Babai, A.; Arenz, S.; Giernoth, R.; Binnemans, K.; Driesen, K.; Nockemann, P. Strong luminescence of rare earth compounds in ionic liquids: Luminescent properties of lanthanide(III) iodides in the ionic liquid 1-dodecyl-3-methylimidazolium bis(trifluoromethanesulfonyl) imide. J. Alloys Compd. 2006, 418, 204–208. [Google Scholar] [CrossRef]
- Wasserscheid, P.; Keim, W. Ionic liquids-new “solutions” for transition metal catalysis. Angew. Chem. 2000, 39, 3772–3789. [Google Scholar] [CrossRef]
- Dupont, J.; de Souza, R.F.; Suarez, P.A. Ionic liquid (molten salt) phase organometallic catalysis. Chem. Rev. 2002, 102, 3667–3692. [Google Scholar] [CrossRef] [PubMed]
- Welton, T. Ionic liquids in catalysis. Coord. Chem. Rev. 2004, 248, 2459–2477. [Google Scholar] [CrossRef]
- Lin, I.J.; Vasam, C.S. Preparation and application of N-heterocyclic carbene complexes of Ag (I). Coord. Chem. Rev. 2007, 251, 642–670. [Google Scholar] [CrossRef]
- Binnemans, K. Lanthanides and actinides in ionic liquids. Chem. Rev. 2007, 107, 2592–2614. [Google Scholar] [CrossRef] [PubMed]
- Binnemans, K. Luminescence of metallomesogens in the liquid crystal state. J. Mater. Chem. 2009, 19, 448–453. [Google Scholar] [CrossRef] [Green Version]
- Bica, K.; Gaertner, P. Applications of chiral ionic liquids. Eur. J. Org. Chem. 2008, 2008, 3235–3250. [Google Scholar] [CrossRef]
- Olivier-Bourbigou, H.; Magna, L. Ionic liquids: Perspectives for organic and catalytic reactions. J. Mol. Catal. A Chem. 2002, 182, 419–437. [Google Scholar] [CrossRef]
- Olivier-Bourbigou, H.; Magna, L.; Morvan, D. Ionic liquids and catalysis: Recent progress from knowledge to applications. Appl. Catal. A Gen. 2010, 373, 1–56. [Google Scholar] [CrossRef]
- Petkovic, M.; Seddon, K.R.; Rebelo, L.P.N.; Pereira, C.S. Ionic liquids: A pathway to environmental acceptability. Chem. Soc. Rev. 2011, 40, 1383–1403. [Google Scholar] [CrossRef] [PubMed]
- Plechkova, N.V.; Seddon, K.R. Applications of ionic liquids in the chemical industry. Chem. Soc. Rev. 2008, 37, 123–150. [Google Scholar] [CrossRef] [PubMed]
- Wasserscheid, P.; Welton, T. Ionic Liquids in Synthesis; Wiley Online Library: Hoboken, NJ, USA, 2008; Volume 1. [Google Scholar]
- Endres, F. Ionic liquids: Solvents for the electrodeposition of metals and semiconductors. ChemPhysChem 2002, 3, 144–154. [Google Scholar] [CrossRef]
- Sun, X.-G.; Dai, S. Electrochemical investigations of ionic liquids with vinylene carbonate for applications in rechargeable lithium ion batteries. Electrochim. Acta 2010, 55, 4618–4626. [Google Scholar] [CrossRef]
- Chen, H.; Choi, J.-H.; Salas-de la Cruz, D.; Winey, K.; Elabd, Y.A. Polymerized ionic liquids: The effect of random copolymer composition on ion conduction. Macromolecules 2009, 42, 4809–4816. [Google Scholar] [CrossRef]
- Shimura, H.; Yoshio, M.; Hoshino, K.; Mukai, T.; Ohno, H.; Kato, T. Noncovalent approach to one-dimensional ion conductors: Enhancement of ionic conductivities in nanostructured columnar liquid crystals. J. Am. Chem. Soc. 2008, 130, 1759–1765. [Google Scholar] [CrossRef] [PubMed]
- Martinelli, A.; Iojoiu, C.; Sergent, N. A H2/O2 fuel cell for in situ μ-raman measurements. In-depth characterization of an ionic liquid filled nafion membrane. Fuel Cells 2012, 12, 169–178. [Google Scholar] [CrossRef]
- Khodagholy, D.; Curto, V.F.; Fraser, K.J.; Gurfinkel, M.; Byrne, R.; Diamond, D.; Malliaras, G.G.; Benito-Lopez, F.; Owens, R.M. Organic electrochemical transistor incorporating an ionogel as a solid state electrolyte for lactate sensing. J. Mater. Chem. 2012, 22, 4440–4443. [Google Scholar] [CrossRef] [Green Version]
- Anastasova-Ivanova, S.; Mattinen, U.; Radu, A.; Bobacka, J.; Lewenstam, A.; Migdalski, J.; Danielewski, M.; Diamond, D. Development of miniature all-solid-state potentiometric sensing system. Sens. Actuators B Chem. 2010, 146, 199–205. [Google Scholar] [CrossRef] [Green Version]
- Vasiloiu, M.; Rainer, D.; Gaertner, P.; Reichel, C.; Schröder, C.; Bica, K. Basic chiral ionic liquids: A novel strategy for acid-free organocatalysis. Catal. Today 2013, 200, 80–86. [Google Scholar] [CrossRef]
- Lunstroot, K.; Driesen, K.; Nockemann, P.; Görller-Walrand, C.; Binnemans, K.; Bellayer, S.; Le Bideau, J.; Vioux, A. Luminescent ionogels based on europium-doped ionic liquids confined within silica-derived networks. Chem. Mater. 2006, 18, 5711–5715. [Google Scholar] [CrossRef]
- Giernoth, R. Task-Specific Ionic Liquids. Angew. Chem. Int. Ed. 2010, 49, 2834–2839. [Google Scholar] [CrossRef] [PubMed]
- Nockemann, P.; Thijs, B.; Postelmans, N.; Van Hecke, K.; Van Meervelt, L.; Binnemans, K. Anionic rare-earth thiocyanate complexes as building blocks for low-melting metal-containing ionic liquids. J. Am. Chem. Soc. 2006, 128, 13658–13659. [Google Scholar] [CrossRef] [PubMed]
- Gray, D.H.; Gin, D.L. Polymerizable lyotropic liquid crystals containing transition-metal ions as building blocks for nanostructured polymers and composites. Chem. Mater. 1998, 10, 1827–1832. [Google Scholar] [CrossRef]
- Zakrzewska, M.E.; Bogel-Łukasik, E.; Bogel-Łukasik, R. Ionic liquid-mediated formation of 5-hydroxymethylfurfural—A promising biomass-derived building block. Chem. Rev. 2010, 111, 397–417. [Google Scholar] [CrossRef] [PubMed]
- Bica, K.; Gaertner, P. An iron-containing ionic liquid as recyclable catalyst for aryl Grignard cross-coupling of alkyl halides. Org. Lett. 2006, 8, 733–735. [Google Scholar] [CrossRef] [PubMed]
- Bica, K.; Gaertner, P. Metal-containing ionic liquids as efficient catalysts for hydroxymethylation in water. Eur. J. Org. Chem. 2008, 2008, 3453–3456. [Google Scholar] [CrossRef]
- Chen, X.; Peng, Y. Chloroferrate (III) ionic liquid: Efficient and recyclable catalyst for solvent-free synthesis of 3, 4-dihydropyrimidin-2 (1H)-ones. Catal. Lett. 2008, 122, 310–313. [Google Scholar] [CrossRef]
- Sakal, S.A.; Lu, Y.-Z.; Jiang, X.-C.; Shen, C.; Li, C.-X. A Promising ionic liquid [BMIM][FeCl4] for the extractive separation of aromatic and aliphatic hydrocarbons. J. Chem. Eng. Data 2014, 59, 533–539. [Google Scholar] [CrossRef]
- Xie, Z.-L.; Jeličić, A.; Wang, F.-P.; Rabu, P.; Friedrich, A.; Beuermann, S.; Taubert, A. Transparent, flexible, and paramagnetic ionogels based on PMMA and the iron-based ionic liquid 1-butyl-3-methylimidazolium tetrachloroferrate(III)[Bmim][FeCl4]. J. Mater. Chem. 2010, 20, 9543–9549. [Google Scholar] [CrossRef]
- Nockemann, P.; Glorieux, C.; Van Hecke, K.; van Meervelt, L.; Kirchner, B.; Binnemans, K. Task-specific ionic liquid for solubilizing metal oxides. J. Phys. Chem. B 2006, 110, 20978–20992. [Google Scholar] [CrossRef] [PubMed]
- Werner, S.; Haumann, M.; Wasserscheid, P. Ionic liquids in chemical engineering. Annu. Rev. Chem. Biomol. Eng. 2010, 1, 203–230. [Google Scholar] [CrossRef] [PubMed]
- Burguete, M.I.; Galindo, F.; García-Verdugo, E.; Karbass, N.; Luis, S.V. Polymer supported ionic liquid phases (SILPs) versus ionic liquids (ILs): How much do they look alike. Chem. Commun. 2007, 3086–3088. [Google Scholar] [CrossRef] [PubMed]
- Le Bideau, J.; Viau, L.; Vioux, A. Ionogels, ionic liquid based hybrid materials. Chem. Soc. Rev. 2011, 40, 907–925. [Google Scholar] [CrossRef] [PubMed]
- Hindson, K.J. Hybrid materials, the materials for today’s technology. Eur. J. Inorg. Chem. 2015, 2015, 1085. [Google Scholar] [CrossRef]
- Taubert, A. Electrospinning of ionogels: Current status and future perspectives. Eur. J. Inorg.Chem. 2015, 2015, 1148–1159. [Google Scholar] [CrossRef]
- Viau, L.; Tourné-Péteilh, C.; Devoisselle, J.-M.; Vioux, A. Ionogels as drug delivery system: One-step sol–gel synthesis using imidazolium ibuprofenate ionic liquid. Chem. Commun. 2010, 46, 228–230. [Google Scholar] [CrossRef] [PubMed]
- Horowitz, A.I.; Panzer, M.J. High-performance, mechanically compliant silica-based ionogels for electrical energy storage applications. J. Mater. Chem. 2012, 22, 16534–16539. [Google Scholar]
- Lee, J.; Panzer, M.J.; He, Y.; Lodge, T.P.; Frisbie, C.D. Ion gel gated polymer thin-film transistors. J. Am. Chem. Soc. 2007, 129, 4532–4533. [Google Scholar] [CrossRef] [PubMed]
- Vioux, A.; Le Bideau, J.; Mutin, P.H.; Leclercq, D. Hybrid organic-inorganic materials based on organophosphorus derivatives. In New Aspects in Phosphorus Chemistry IV; Springer: Berlin, Germany, 2004; pp. 145–174. [Google Scholar]
- Vioux, A.; Viau, L.; Volland, S.; Le Bideau, J. Use of ionic liquids in sol-gel; ionogels and applications. Comptes Rendus Chim. 2010, 13, 242–255. [Google Scholar] [CrossRef]
- Lunstroot, K.; Driesen, K.; Nockemann, P.; van Hecke, K.; Van Meervelt, L.; Görller-Walrand, C.; Binnemans, K.; Bellayer, S.; Viau, L.; Le Bideau, J. Lanthanide-doped luminescent ionogels. Dalton Trans. 2009, 14, 298–306. [Google Scholar] [CrossRef] [PubMed]
- Lunstroot, K.; Driesen, K.; Nockemann, P.; Viau, L.; Mutin, P.H.; Vioux, A.; Binnemans, K. Ionic liquid as plasticizer for europium(III)-doped luminescent poly (methyl methacrylate) films. Phys. Chem. Chem. Phys. 2010, 12, 1879–1885. [Google Scholar] [CrossRef] [PubMed]
- Feng, Y.; Li, H.; Gan, Q.; Wang, Y.; Liu, B.; Zhang, H. A transparent and luminescent ionogel based on organosilica and ionic liquid coordinating to Eu3+ ions. J. Mater. Chem. 2010, 20, 972–975. [Google Scholar] [CrossRef]
- Feng, J.; Zhang, H. Hybrid materials based on lanthanide organic complexes: A review. Chem. Soc. Rev. 2013, 42, 387–410. [Google Scholar] [CrossRef] [PubMed]
- Saricilar, S.; Antiohos, D.; Shu, K.; Whitten, P.G.; Wagner, K.; Wang, C.; Wallace, G.G. High strain stretchable solid electrolytes. Electrochem. Commun. 2013, 32, 47–50. [Google Scholar] [CrossRef]
- Guyomard-Lack, A.; Abusleme, J.; Soudan, P.; Lestriez, B.; Guyomard, D.; Bideau, J.L. Hybrid silica–Polymer ionogel solid electrolyte with tunable properties. Adv. Energy Mater. 2014, 4. [Google Scholar] [CrossRef]
- Ribot, J.C.; Guerrero-Sanchez, C.; Hoogenboom, R.; Schubert, U.S. Thermoreversible ionogels with tunable properties via aqueous gelation of an amphiphilic quaternary ammonium oligoether-based ionic liquid. J. Mater. Chem. 2010, 20, 8279–8284. [Google Scholar] [CrossRef]
- Göbel, R.; Friedrich, A.; Taubert, A. Tuning the phase behavior of ionic liquids in organically functionalized silica ionogels. Dalton Trans. 2010, 39, 603–611. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Zhou, X.; Chen, Q.; Chu, B.; Zhang, Q. Recent development of high energy density polymers for dielectric capacitors. Dielectr. Electr. Insul. IEEE Trans. 2010, 17, 1036–1042. [Google Scholar] [CrossRef]
- Zhou, Z.; Mackey, M.; Carr, J.; Zhu, L.; Flandin, L.; Baer, E. Multilayered polycarbonate/poly (vinylidene fluoride-co-hexafluoropropylene) for high energy density capacitors with enhanced lifetime. J. Polym. Sci. Part B Polym. Phys. 2012, 50, 993–1003. [Google Scholar] [CrossRef]
- Zheng, J.; Jow, T. High energy and high power density electrochemical capacitors. J. Power Sources 1996, 62, 155–159. [Google Scholar] [CrossRef]
- Rogers, J.A.; Someya, T.; Huang, Y. Materials and mechanics for stretchable electronics. Science 2010, 327, 1603–1607. [Google Scholar] [CrossRef] [PubMed]
- Ueno, K.; Watanabe, M. From colloidal stability in ionic liquids to advanced soft materials using unique media. Langmuir 2011, 27, 9105–9115. [Google Scholar] [CrossRef] [PubMed]
- Neve, F.; Impéror-Clerc, M. An Ia3¯d thermotropic cubic phase from N-alkylpyridinium tetrahalocuprates. Liquid Cryst. 2004, 31, 907–912. [Google Scholar] [CrossRef]
- Neve, F.; Crispini, A.; Armentano, S.; Francescangeli, O. Synthesis, structure, and thermotropic mesomorphism of layered N-alkylpyridinium tetrahalopalladate(II) salts. Chem. Mater. 1998, 10, 1904–1913. [Google Scholar] [CrossRef]
- Neve, F.; Francescangeli, O.; Crispini, A. Crystal architecture and mesophase structure of long-chain N-alkylpyridinium tetrachlorometallates. Inorg. Chim. Acta 2002, 338, 51–58. [Google Scholar] [CrossRef]
- Amirnasr, M.; Mahmoudkhani, A.H.; Gorji, A.; Dehghanpour, S.; Bijanzadeh, H.R. Cobalt(II), nickel(II), and zinc(II) complexes with bidentate N,N′-bis(β-phenylcinnamaldehyde)-1,2-diiminoethane Schiff base: Synthesis and structures. Polyhedron 2002, 21, 2733–2742. [Google Scholar] [CrossRef]
- Zhong, C.; Sasaki, T.; Jimbo-Kobayashi, A.; Fujiwara, E.; Kobayashi, A.; Tada, M.; Iwasawa, Y. Syntheses, structures, and properties of a series of metal ion-containing dialkylimidazolium ionic liquids. Bull. Chem. Soc. Jpn. 2007, 80, 2365–2374. [Google Scholar] [CrossRef]
- Wilkins, J.L.R.G. Modern Coordination Chemistry; Interscience: New York, NY, USA, 1960; p. 406. [Google Scholar]
- Cotton, F.A.; Goodgame, D.; Goodgame, M. The electronic structures of tetrahedral cobalt(II) complexes. J. Am. Chem. Soc. 1961, 83, 4690–4699. [Google Scholar] [CrossRef]
- Winter, A.; Zabel, A.; Strauch, P. Tetrachloridocuprates(II)—Synthesis and electron paramagnetic resonance (EPR) spectroscopy. Int. J. Mol. Sci. 2012, 13, 1612–1619. [Google Scholar] [CrossRef] [PubMed]
- Tsunekawa, M.; Nishio, S.; Sato, H. Multiphoton ionization-mass spectrometric study on laser ablation of polymethylmethacrylate and polystyrene at 308 nm. Jpn. J. Appl. Phys. 1995, 34, 218. [Google Scholar] [CrossRef]
- Nakajima, H.; Ohno, H. Preparation of thermally stable polymer electrolytes from imidazolium-type ionic liquid derivatives. Polymer 2005, 46, 11499–11504. [Google Scholar] [CrossRef]
- Gallo, V.; Mastrorilli, P.; Nobile, C.F.; Romanazzi, G.; Suranna, G.P. How does the presence of impurities change the performance of catalytic systems in ionic liquids? A case study: The Michael addition of acetylacetone to methyl vinyl ketone. J. Chem. Soc. Dalton Trans. 2002, 4339–4342. [Google Scholar] [CrossRef]
- Bhargava, R.; Gallagher, D.; Hong, X.; Nurmikko, A. Optical properties of manganese-doped nanocrystals of ZnS. Phys. Rev. Lett. 1994, 72, 416–419. [Google Scholar] [CrossRef] [PubMed]
- Hitchcock, P.B.; Seddon, K.R.; Welton, T. Hydrogen-bond acceptor abilities of tetrachlorometalate(II) complexes in ionic liquids. J. Chem. Soc. Dalton Trans. 1993, 2639–2643. [Google Scholar] [CrossRef]
- Hashim, A.A. Polymer Dipoles Relaxation and Potential Energy (New Simulation Model); INTECH Open Access Publisher: Vienna, Austria, 2010. [Google Scholar]
- Tsangaris, G.; Psarras, G.; Kouloumbi, N. Electric modulus and interfacial polarization in composite polymeric systems. J. Mater. Sci. 1998, 33, 2027–2037. [Google Scholar] [CrossRef]
- Marsh, K.; Boxall, J.; Lichtenthaler, R. Room temperature ionic liquids and their mixtures—A review. Fluid Phase Equilib. 2004, 219, 93–98. [Google Scholar] [CrossRef]
- Ueki, T.; Watanabe, M. Upper critical solution temperature behavior of poly (N-isopropylacrylamide) in an ionic liquid and preparation of thermo-sensitive nonvolatile gels. Chem. Lett. 2006, 35, 964–965. [Google Scholar] [CrossRef]
- Hayashi, S.; Hamaguchi, H.-O. Discovery of a magnetic ionic liquid [Bmim]FeCl4. Chem. Lett. 2004, 33, 1590–1591. [Google Scholar] [CrossRef]
- Winter, A.; Thiel, K.; Zabel, A.; Klamroth, T.; Pöppl, A.; Kelling, A.; Schilde, U.; Taubert, A.; Strauch, P. Tetrahalidocuprates(II)—Structure and EPR spectroscopy. Part 2: Tetrachloridocuprates(II). New J. Chem. 2014, 38, 1019–1030. [Google Scholar] [CrossRef]
- Farra, R.; Thiel, K.; Winter, A.; Klamroth, T.; Pöppl, A.; Kelling, A.; Schilde, U.; Taubert, A.; Strauch, P. Tetrahalidocuprates(II)—Structure and EPR spectroscopy. Part 1: Tetrabromidocuprates(II). New J. Chem. 2011, 35, 2793–2803. [Google Scholar] [CrossRef]
- Lodge, T.P. A unique platform for materials design. Science 2008, 321, 50–51. [Google Scholar] [CrossRef] [PubMed]
- Weber, R.L.; Ye, Y.; Schmitt, A.L.; Banik, S.M.; Elabd, Y.A.; Mahanthappa, M.K. Effect of nanoscale morphology on the conductivity of polymerized ionic liquid block copolymers. Macromolecules 2011, 44, 5727–5735. [Google Scholar] [CrossRef]
- Choi, J.-H.; Ye, Y.; Elabd, Y.A.; Winey, K.I. Network structure and strong microphase separation for high ion conductivity in polymerized ionic liquid block copolymers. Macromolecules 2013, 46, 5290–5300. [Google Scholar] [CrossRef]
- Neve, F.; Francescangeli, O.; Crispini, A.; Charmant, J. A2 [MX4] copper(II) pyridinium salts. From ionic liquids to layered solids to liquid crystals. Chem. Mater. 2001, 13, 2032–2041. [Google Scholar] [CrossRef]









| Parameter | [Bmim]2[CuCl4] | [Bmim]2[CoCl4] | [Bmim]2[MnCl4] |
|---|---|---|---|
| Chemical Formula | C16H30Cl4CuN4 | C16H30Cl4CoN4 | C16H30Cl4MnN4 |
| Formula weight | 483.78 | 479.17 | 475.18 |
| Temperature/K | 150(2) | 150(2) | 150(2) |
| Crystal system | monoclinic | monoclinic | monoclinic |
| Space group | Cc | Cc | Cc |
| Unit cell dimension a/Å | 14.1014(5) | 14.3976(7) | 14.4272(7) |
| Unit cell dimension b/Å | 9.7074(4) | 9.7151(6) | 9.7344(3) |
| Unit cell dimension c/Å | 17.1303(6) | 16.8773(9) | 16.9521(7) |
| β/° | 107.431(3) | 107.699(4) | 107.563(3) |
| Volume/Å3 | 2237.25(15) | 2249.0(2) | 2269.78(16) |
| Z | 4 | 4 | 4 |
| Calculated density (ρcalc)/mg·m−³ | 1.436 | 1.415 | 1.391 |
| μ/mm−1 | 1.461 | 1.246 | 1.060 |
| F(000) | 1004 | 996 | 988 |
| Crystal size | 1.0 × 1.2 × 1.4 | 0.05 × 0.2 × 0.4 | 0.2 × 0.3 × 0.4 |
| Crystal color | orange | blue | colourless |
| Crystal description | block | plate | block |
| Theta range for data collection | 2.49 to 24.99 | 2.53 to 25.00 | 2.52 to 25.00 |
| Miller Index ranges | −16 ≤ h ≤ 16 −11 ≤ k ≤ 11 −20 ≤ l ≤ 20 | −17 ≤ h ≤ 17 −11 ≤ k ≤ 11 −19 ≤ l ≤ 20 | −17 ≤ h ≤ 17 −11 ≤ k ≤ 11 −20 ≤ l ≤ 19 |
| Reflections collected | 14160 | 9990 | 14352 |
| Unique reflections | 3937 (Rint = 0.0961) | 3770 (Rint = 0.0279) | 3866 (Rint = 0.0167) |
| Data/restraints/parameters | 3937/2/227 | 3770/2/227 | 3866/2/227 |
| Final R indices (I > 2σ(I)) | R1 = 0.0316 wR2 = 0.0848 | R1 = 0.0210 wR2 = 0.0517 | R1 = 0.0155 wR2 = 0.0423 |
| R indices (all data) | R1 = 0.0316 wR2 = 0.0848 | R1 = 0.0231 wR2 = 0.0525 | R1 = 0.0157 wR2 = 0.0423 |
| Goodness-of-fit on F2 | 1.042 | 1.036 | 1.060 |
| Largest diff. peak and hole/Å3 | 0.314/−0.431 | 0.253/−0.287 | 0.299/−0.329 |
| CCDC | 1452214 | 1452215 | 1452218 |
| IL | Tg (°C) | Tm (°C) | µeff | (*) µeff [68] |
|---|---|---|---|---|
| [Bmim]2[CuCl4] | −48.6 ± 0.5 | 26.1 ± 0.5 | 1.78 ± 0.1 | 1.8–2.1 |
| [Bmim]2[CoCl4] | −42.5 ± 1.4 | 61.5 ± 1.4 | 4.47 ± 0.1 | 4.3–5.2 |
| [Bmim]2[MnCl4] | −49.2 ± 0.4 | 53.3 ± 0.4 | 5.42 ± 0.1 | 5.7–6.0 |
| IG | Tg, onset/°C | Tg, onset/°C |
|---|---|---|
| 0 (Pure PMMA film) | - | 108.0 ± 0.6 |
| CuIG10 | −48.3 ± 0.3 | 116.6 ± 0.7 |
| CuIG20 | −50.2 ± 0.8 | 114.3 ± 0.7 |
| CuIG30 | −55.2 ± 0.6 | 114.7 ± 0.7 |
| CuIG40 | −51.0 ± 0.7 | 115.9 ± 0.6 |
| CoIG10 | −47.4 ± 2.5 | 113.9 ± 2.4 |
| CoIG20 | −46.3 ± 1.6 | 114.4 ± 2.6 |
| CoIG30 | −44.9 ± 1.4 | 116.4 ± 2.3 |
| CoIG40 | −45.5 ± 1.3 | 114.8 ± 2.3 |
| MnIG10 | −55.3 ± 2.4 | 114.0 ± 1.1 |
| MnIG20 | −53.5 ± 0.4 | 114.5 ± 1.2 |
| MnIG30 | −53.8 ± 0.7 | 113.6 ± 1.0 |
| MnIG40 | −51.6 ± 0.6 | 114.7 ± 1.3 |
| ID | Young’s Modulus [GPa] | Yield Strain [%] |
|---|---|---|
| PMMA | 1.8011 ± 120.9 | 2.3 ± 0.52 |
| CuIG40 | 0.8328 ± 211.8 | 1.00 ± 0.55 |
| CoIG40 | 0.9344 ± 86.6 | 1.07 ± 0.19 |
| MnIG40 | 0.6956 ± 72.9 | 0.67 ± 0.24 |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zehbe, K.; Kollosche, M.; Lardong, S.; Kelling, A.; Schilde, U.; Taubert, A. Ionogels Based on Poly(methyl methacrylate) and Metal-Containing Ionic Liquids: Correlation between Structure and Mechanical and Electrical Properties. Int. J. Mol. Sci. 2016, 17, 391. https://doi.org/10.3390/ijms17030391
Zehbe K, Kollosche M, Lardong S, Kelling A, Schilde U, Taubert A. Ionogels Based on Poly(methyl methacrylate) and Metal-Containing Ionic Liquids: Correlation between Structure and Mechanical and Electrical Properties. International Journal of Molecular Sciences. 2016; 17(3):391. https://doi.org/10.3390/ijms17030391
Chicago/Turabian StyleZehbe, Kerstin, Matthias Kollosche, Sebastian Lardong, Alexandra Kelling, Uwe Schilde, and Andreas Taubert. 2016. "Ionogels Based on Poly(methyl methacrylate) and Metal-Containing Ionic Liquids: Correlation between Structure and Mechanical and Electrical Properties" International Journal of Molecular Sciences 17, no. 3: 391. https://doi.org/10.3390/ijms17030391
APA StyleZehbe, K., Kollosche, M., Lardong, S., Kelling, A., Schilde, U., & Taubert, A. (2016). Ionogels Based on Poly(methyl methacrylate) and Metal-Containing Ionic Liquids: Correlation between Structure and Mechanical and Electrical Properties. International Journal of Molecular Sciences, 17(3), 391. https://doi.org/10.3390/ijms17030391