Phenolic Profile and Biological Activities of the Pepino (Solanum muricatum) Fruit and Its Wild Relative S. caripense
Abstract
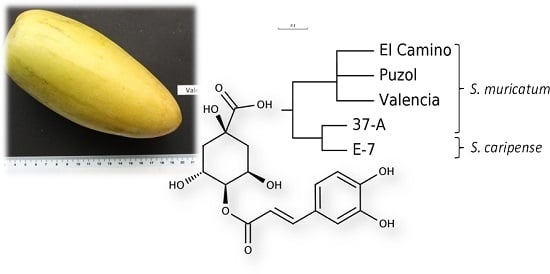
:1. Introduction
2. Results
2.1. Phenolic Composition
2.2. Antioxidant Activity
2.3. Biological Activity
2.4. Selection of Varieties for Phenolic Content and Biological Activities
3. Discussion
4. Materials and Methods
4.1. Plant Material
4.2. Sample Preparation
4.3. Phenolic Composition
4.4. Antioxidant Activity
4.5. Anti-Inflammatory Activity
4.6. Data Analysis
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
Abbreviations
| ORAC | Oxygen radical absorbance capacity |
| DPPH | 2,2-Diphenyl-1-picrylhydrazyl hydrate |
| TRC | Total reducing capacity |
| AUC | Area under the curve |
| MTT | 3-[4,5-Dimethylthiazol-2-yl]-2,5-diphenyl-tetrazolium bromide |
References
- Anderson, G.J.; Jansen, R.K.; Kim, Y. The origin and relationships of the pepino, Solanum muricatum (Solanaceae): DNA restriction fragment evidence. Econ. Bot. 1996, 50, 369–380. [Google Scholar] [CrossRef]
- Herraiz, F.J.; Vilanova, S.; Andújar, I.; Torrent, D.; Plazas, M.; Gramazio, P.; Prohens, J. Morphological and molecular characterization of local varieties, modern cultivars and wild relatives of an emerging vegetable crop, the pepino (Solanum muricatum), provides insight into its diversity, relationships and breeding history. Euphytica 2015, 206, 301–318. [Google Scholar] [CrossRef]
- Levy, D.; Kedar, N.; Levy, N. Pepino (Solanum muricatum Aiton): Breeding in Israel for better taste and aroma. Isr. J. Plant Sci. 2006, 54, 205–213. [Google Scholar] [CrossRef]
- Prohens, J.; Rodríguez-Burruezo, A.; Nuez, F. Utilization of genetic resources for the introduction and adaptation of exotic vegetable crops: The case of pepino (Solanum muricatum). Euphytica 2005, 146, 133–142. [Google Scholar] [CrossRef]
- Rodríguez-Burruezo, A.; Prohens, J.; Fita, A.M. Breeding strategies for improving the performance and fruit quality of the pepino (Solanum muricatum): A model for the enhancement of underutilized exotic fruits. Food Res. Int. 2011, 44, 1927–1935. [Google Scholar] [CrossRef]
- Prohens, J.; Ruiz, J.J.; Nuez, F. The pepino (Solanum muricatum, Solanaceae): A “new” crop with a history. Econ. Bot. 1996, 50, 355–368. [Google Scholar] [CrossRef]
- Muñoz, C.; Pertuzé, R.; Balzarini, M.; Bruno, C.; Salvatierra, A. Genetic variability in Chilean pepino (Solanum muricatum Aiton) fruit. Chil. J. Agric. Res. 2014, 74, 143–147. [Google Scholar] [CrossRef]
- Hsu, C.; Guo, Y.; Wang, Z.; Yin, M. Protective effects of an aqueous extract from pepino (Solanum muricatum Ait.) in diabetic mice. J. Sci. Food Agric. 2011, 91, 1517–1522. [Google Scholar] [CrossRef] [PubMed]
- Shathish, K.; Guruvayoorappan, C. Solanum muricatum Ait. Inhibits inflammation and cancer by modulating the immune system. J. Cancer Res. Ther. 2014, 10, 623–630. [Google Scholar] [PubMed]
- Sudha, G.; Sangeetha, P.M.; Indhu, S.R.; Vadivukkarasi, S. Antioxidant activity of ripe pepino fruit (Solanum muricatum Aiton). Int. J. Pharm. Pharm. Sci. 2011, 3, 257–261. [Google Scholar]
- Diamanti, J.; Battino, M.; Mezzetti, B. Breeding for fruit nutritional and nutraceutical quality. In Breeding for Fruit Quality; Jenks, M.A., Bebeli, P., Eds.; John Wiley & Sons: Hoboken, NJ, USA, 2011; pp. 61–79. [Google Scholar]
- Di Scala, K.; Vega-Gálvez, A.; Uribe, E.; Oyanadel, R.; Miranda, M.; Vergara, J.; Quispe, I.; Lemus-Mondaca, R. Changes of quality characteristics of pepino fruit (Solanum muricatum Ait) during convective drying. Int. J. Food Sci. Technol. 2011, 46, 746–753. [Google Scholar] [CrossRef]
- Acosta-Quezada, P.G.; Raigon, M.D.; Riofrio-Cuenca, T.; Garcia-Martinez, M.D.; Plazas, M.; Burneo, J.I.; Figueroa, J.G.; Vilanova, S.; Prohens, J. Diversity for chemical composition in a collection of different varietal types of tree tomato (Solanum betaceum Cav.), an Andean exotic fruit. Food Chem. 2015, 169, 327–335. [Google Scholar] [CrossRef] [PubMed]
- Kwon, Y.-I.; Apostolidis, E.; Shetty, K. In vitro studies of eggplant (Solanum melongena) phenolics as inhibitors of key enzymes relevant for type 2 diabetes and hypertension. Bioresour. Technol. 2008, 99, 2981–2988. [Google Scholar] [CrossRef] [PubMed]
- Plazas, M.; Prohens, J.; Cuñat, A.N.; Vilanova, S.; Gramazio, P.; Herraiz, F.J.; Prohens, J. Reducing capacity, chlorogenic acid content and biological activity in a collection of scarlet (Solanum aethiopicum) and gboma (S. macrocarpon) aeggplants. Int. J. Mol. Sci. 2014, 15, 17221–17241. [Google Scholar] [CrossRef] [PubMed]
- Slimestad, R.; Verheul, M. Review of flavonoids and other phenolics from fruits of different tomato (Lycopersicon esculentum Mill.) cultivars. J. Sci. Food Agric. 2009, 89, 1255–1270. [Google Scholar] [CrossRef]
- Wu, S.-B.; Meyer, R.S.; Whitaker, B.D.; Litt, A.; Kennelly, E.J. A new liquid chromatography–mass spectrometry-based strategy to integrate chemistry, morphology, and evolution of eggplant (Solanum) species. J. Chromatogr. A 2013, 1314, 154–172. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Burruezo, A.; Prohens, J.; Nuez, F. Genetic Analysis of Quantitative Traits in Pepino (Solanum muricatum) in Two Growing Seasons. J. Am. Soc. Hortic. Sci. 2002, 127, 271–278. [Google Scholar]
- Sudha, G.; Priya, M.S.; Shree, R.B.I.; Vadivukkarasi, S. Antioxidant activity of ripe and unripe pepino fruit (Solanum muricatum Aiton). J. Food Sci. 2012, 77, C1131–C1135. [Google Scholar] [CrossRef] [PubMed]
- Blanca, J.M.; Prohens, J.; Anderson, G.J.; Zuriaga, E.; Canizares, J.; Nuez, F. AFLP and DNA sequence variation in an Andean domesticate, pepino (Solanum muricatum, Solanaceae): Implications for evolution and domestication. Am. J. Bot. 2007, 94, 1219–1229. [Google Scholar] [CrossRef] [PubMed]
- Clifford, M.N.; Johnston, K.L.; Knight, S.; Kuhnert, N. Hierarchical scheme for LC-MSn identification of chlorogenic acids. J. Agric. Food Chem. 2003, 51, 2900–2911. [Google Scholar] [CrossRef] [PubMed]
- Clifford, M.N.; Knight, S.; Kuhnert, N. Discriminating between the six isomers of dicaffeoylquinic acid by LC-MSn. J. Agric. Food Chem. 2005, 53, 3821–3832. [Google Scholar] [CrossRef] [PubMed]
- Gomez-Romero, M.; Segura-Carretero, A.; Fernandez-Gutierrez, A. Metabolite profiling and quantification of phenolic compounds in methanol extracts of tomato fruit. Phytochemistry 2010, 71, 1848–1864. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-Rodríguez, E.; Ruiz, J.M.; Ferreres, F.; Moreno, D.A. Phenolic profiles of cherry tomatoes as influenced by hydric stress and rootstock technique. Food Chem. 2012, 134, 775–782. [Google Scholar] [CrossRef] [PubMed]
- Ferreres, F.; Taveira, M.; Gil-Izquierdo, A.; Oliveira, L.; Teixeira, T.; Valentao, P.; Simoes, N.; Andrade, P.B. High-performance liquid chromatography-diode array detection-electrospray ionization multi-stage mass spectrometric screening of an insect/plant system: The case of Spodoptera littoralis/Lycopersicon esculentum phenolics and alkaloids. Rapid Commun. Mass Spectrom. 2011, 25, 1972–1980. [Google Scholar] [CrossRef] [PubMed]
- Alarcón-Flores, M.I.; Romero-González, R.; Vidal, J.L.M.; Frenich, A.G. Multiclass determination of phytochemicals in vegetables and fruits by ultra high performance liquid chromatography coupled to tandem mass spectrometry. Food Chem. 2013, 141, 1120–1129. [Google Scholar] [CrossRef] [PubMed]
- Prohens, J.; Whitaker, B.D.; Plazas, M.; Vilanova, S.; Hurtado, M.; Blasco, M.; Gramazio, P.; Stommel, J.R. Genetic diversity in morphological characters and phenolic acids content resulting from an interspecific cross between eggplant, Solanum melongena, and its wild ancestor (S. incanum). Ann. Appl. Biol. 2013, 162, 242–257. [Google Scholar] [CrossRef]
- Helyes, L.; Lugasi, A.; Daood, H.G.; Pék, Z. The simultaneous effect of water supply and genotype on yield quantity, antioxidants content and composition of processing tomatoes. Not. Bot. Horti Agrobot. Cluj-Napoca 2014, 42, 143–149. [Google Scholar] [CrossRef]
- Hakkinen, S.H.; Torronen, A.R. Content of flavonols and selected phenolic acids in strawberries and Vaccinium species: Influence of cultivar, cultivation site and technique. Food Res. Int. 2000, 33, 517–524. [Google Scholar]
- Stalikas, C.D. Extraction, separation, and detection methods for phenolic acids and flavonoids. J. Sep. Sci. 2007, 30, 3268–3295. [Google Scholar] [CrossRef] [PubMed]
- Meyer, R.S.; Whitaker, B.D.; Little, D.P.; Wu, S.-B.; Kennelly, E.J.; Long, C.-L.; Litt, A. Parallel reductions in phenolic constituents resulting from the domestication of eggplant. Phytochemistry 2015, 115, 194–206. [Google Scholar] [CrossRef] [PubMed]
- Prohens, J.; Rodríguez-Burruezo, A.; Raigón, M.D.; Nuez, F. Total phenolic concentration and browning susceptibility in a collection of different varietal types and hybrids of eggplant: Implications for breeding for higher nutritional quality and reduced browning. J. Am. Soc. Hortic. Sci. 2007, 132, 638–646. [Google Scholar]
- Willits, M.G.; Kramer, C.M.; Prata, R.T. N.; De Luca, V.; Potter, B.G.; Steffens, J.C.; Graser, G. Utilization of the genetic resources of wild species to create a nontransgenic high flavonoid tomato. J. Agric. Food Chem. 2005, 53, 1231–1236. [Google Scholar] [CrossRef] [PubMed]
- Kaushik, P.; Andujar, I.; Vilanova, S.; Plazas, M.; Gramazio, P.; Herraiz, F.J.; Brar, N.S.; Prohens, J. Breeding vegetables with increased content in bioactive phenolic acids. Molecules 2015, 20, 18464–18481. [Google Scholar] [CrossRef] [PubMed]
- Jaiswal, R.; Patras, M.A.; Eravuchira, P.J.; Kuhnert, N. Profile and characterization of the chlorogenic acids in green Robusta coffee beans by LC-MSn: Identification of seven new classes of compounds. J. Agric. Food Chem. 2010, 58, 8722–8737. [Google Scholar] [CrossRef] [PubMed]
- Hwang, S.J.; Kim, Y.-W.; Park, Y.; Lee, H.-J.; Kim, K.-W. Anti-inflammatory effects of chlorogenic acid in lipopolysaccharide-stimulated RAW 264.7 cells. Inflamm. Res. 2014, 63, 81–90. [Google Scholar] [CrossRef] [PubMed]
- Girones-Vilaplana, A.; Moreno, D.A.; Garcia-Viguera, C. Phytochemistry and biological activity of Spanish Citrus fruits. Food Funct. 2014, 5, 764–772. [Google Scholar] [CrossRef] [PubMed]
- Speisky, H.; Lopez-Alarcon, C.; Gomez, M.; Fuentes, J.; Sandoval-Acuna, C. First web-based database on total phenolics and oxygen radical absorbance capacity (ORAC) of fruits produced and consumed within the south Andes region of South America. J. Agric. Food Chem. 2012, 60, 8851–8859. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Beecher, G.R.; Holden, J.M.; Haytowitz, D.B.; Gebhardt, S.E.; Prior, R.L. Lipophilic and hydrophilic antioxidant capacities of common foods in the United States. J. Agric. Food Chem. 2004, 52, 4026–4037. [Google Scholar] [CrossRef] [PubMed]
- Huang, D.; Ou, B.; Prior, R.L. The chemistry behind antioxidant capacity assays. J. Agric. Food Chem. 2005, 53, 1841–1856. [Google Scholar] [CrossRef] [PubMed]
- Stommel, J.R.; Whitaker, B.D. Phenolic acid content and composition of eggplant fruit in a germplasm core subset. J. Am. Soc. Hortic. Sci. 2003, 128, 704–710. [Google Scholar]
- Morales-Soto, A.; García-Salas, P.; Rodríguez-Pérez, C.; Jiménez-Sánchez, C.; de la Luz Cádiz-Gurrea, M.; Segura-Carretero, A.; Fernández-Gutiérrez, A. Antioxidant capacity of 44 cultivars of fruits and vegetables grown in Andalusia (Spain). Food Res. Int. 2014, 58, 35–46. [Google Scholar] [CrossRef]
- Chun, O.K.; Kim, D.-O.; Smith, N.; Schroeder, D.; Han, J.T.; Lee, C.Y. Daily consumption of phenolics and total antioxidant capacity from fruit and vegetables in the American diet. J. Sci. Food Agric. 2005, 85, 1715–1724. [Google Scholar] [CrossRef]
- Ferrari, M.; Fornasiero, M.C.; Isetta, A.M. MTT colorimetric assay for testing macrophage cytotoxic activity in vitro. J. Immunol. Methods 1990, 131, 165–172. [Google Scholar] [CrossRef]
- Cardenas, P.D.; Sonawane, P.D.; Heinig, U.; Bocobza, S.E.; Burdman, S.; Aharoni, A. The bitter side of the nightshades: Genomics drives discovery in Solanaceae steroidal alkaloid metabolism. Phytochemistry 2015, 113, 24–32. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-Mata, M.C.; Yokoyama, W.E.; Hong, Y.J.; Prohens, J. α-Solasonine and α-solamargine contents of gboma (Solanum macrocarpon L.) and scarlet (S. aethiopicum L.) eggplants. J. Agric. Food. Chem. 2010, 58, 5502–5508. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Mazza, G. Inhibitory effects of anthocyanins and other phenolic compounds on nitric oxide production in LPS/IFN-γ-activated RAW 264.7 macrophages. J. Agric. Food Chem. 2002, 50, 850–857. [Google Scholar] [CrossRef] [PubMed]
- Razzaghi-Asl, N.; Garrido, J.; Khazraei, H.; Borges, F.; Firuzi, O. Antioxidant properties of hydroxycinnamic acids: A review of structure- activity relationships. Curr. Med. Chem. 2013, 20, 4436–4450. [Google Scholar] [CrossRef] [PubMed]
- Thilakarathna, S.H.; Rupasinghe, H.P.V. Anti-atherosclerotic effects of fruit bioactive compounds: A review of current scientific evidence. Can. J. Plant Sci. 2012, 92, 407–419. [Google Scholar] [CrossRef]
- Heyes, J.A.; Blaikie, F.H.; Downs, C.G.; Sealey, D.F. Textural and physiological changes during pepino (Solanum muricatum Ait.) ripening. Sci. Hortic. (Amst.) 1994, 58, 1–15. [Google Scholar] [CrossRef]
- Prohens, J.; Leiva-Brondo, M.; Rodríguez-Burruezo, A.; Nuez, F. “Puzol”: A facultatively parthenocarpic hybrid of pepino (Solanum muricatum). HortScience 2002, 37, 418–419. [Google Scholar]
- Rodríguez-Burruezo, A.; Prohens, J.; Nuez, F. Valencia: A new pepino (Solanum muricatum) cultivar with improved fruit quality. HortScience 2004, 39, 1500–1502. [Google Scholar]
- Nuez, F.; Ruiz, J.J. El Pepino Dulce y su Cultivo; FAO: Rome, Italy, 1996. [Google Scholar]
- Mione, T.; Anderson, G.J. Pollen-ovule ratios and breeding system evolution in Solanum section Basarthrum (Solanaceae). Am. J. Bot. 1992, 279–287. [Google Scholar] [CrossRef]
- Mena, P.; Garcia-Viguera, C.; Navarro-Rico, J.; Moreno, D.A.; Bartual, J.; Saura, D.; Marti, N. Phytochemical characterisation for industrial use of pomegranate (Punica granatum L.) cultivars grown in Spain. J. Sci. Food Agric. 2011, 91, 1893–1906. [Google Scholar] [CrossRef] [PubMed]
- Ou, B.; Hampsch-Woodill, M.; Prior, R.L. Development and validation of an improved oxygen radical absorbance capacity assay using fluorescein as the fluorescent probe. J. Agric. Food Chem. 2001, 49, 4619–4626. [Google Scholar] [CrossRef] [PubMed]
- Grisham, M.B.; Johnson, G.G.; Lancaster, J.R.J. Quantitation of nitrate and nitrite in extracellular fluids. Methods Enzymol. 1996, 268, 237–246. [Google Scholar] [PubMed]
- Sokal, R.; Michener, C. A statistical method for evaluating systematic relationships. Univ. Kansas Sci. Bull. 1958, 38, 1409–1438. [Google Scholar]
- Rohlf, F.J. NTSYS-pc Version 2.02i Numerical Taxonomy and Multivariate Analysis System; Applied Biostatistics Inc., Exeter Software: Setauket, NY, USA, 1997. [Google Scholar]
- Page, R.D. TreeView: An application to display phylogenetic trees on personal computers. Comput. Appl. Biosci. 1996, 12, 357–358. [Google Scholar] [PubMed]
- Dunnett, C.W. A multiple comparison procedure for comparing several treatments with a control. J. Am. Stat. Assoc. 1955, 50, 1096–1121. [Google Scholar] [CrossRef]




| Peak | Compound | Rt | [M − H]− | MS2[M − H]−, m/z (%) | 37-A | El Camino | Puzol | Valencia | E-7 |
|---|---|---|---|---|---|---|---|---|---|
| 1 | Di-caffeoyl-quinic acid I | 15.2 | 515 | 353 (54), 191(100) | – | – | – | – | X |
| 2 | 3-Caffeoyl-quinic acid | 16.4 | 353 | 191 (100), 179 (42) | X | X | X | X | X |
| 3 | Caffeoyl-hexoside I | 17.4 | 341 | 179 (100), 135 (21) | – | X | X | X | X |
| 4 | Caffeoyl-hexoside II | 17.6 | 341 | 179 (64), 135 (9) | – | X | X | X | – |
| 5 | Di-caffeoyl-quinic acid II | 18.4 | 515 | 353 (78), 191 (88) | – | – | – | – | X |
| 6 | Caffeoyl-hexoside III | 18.4 | 341 | 179 (100), 135 (19) | – | X | X | X | – |
| 7 | Feruloyl-hexoside | 19.2 | 355 | 193 (100), 175 (53) | – | – | X | – | – |
| 8 | Caffeoyl-di-hexoside | 19.3 | 503 | 341 (36), 179 (100) | – | X | X | X | – |
| 9 | Caffeoyl-hexose IV | 19.8 | 341 | 179 (100), 135 (12) | X | X | X | X | X |
| 10 | 5-Caffeoyl-quinic acid | 20.6 | 353 | 191 (100) | X | X | X | X | X |
| 11 | 4-Caffeoyl-quinic acid | 20.7 | 353 | 173 (100) | X | X | X | X | X |
| 12 | p-Coumaroyl-di-hexoside | 22.0 | 487 | 325 (14), 163 (100) | – | X | X | X | X |
| 13 | Feruloyl-hexoside | 22.3 | 355 | 193 (100), 175 (56) | – | X | X | X | – |
| 14 | Caffeoyl-quinic acid isomer | 23.0 | 353 | 191 (100) | – | X | – | – | – |
| 15 | Feruloyl-dihexoside | 23.2 | 517 | 235 (50), 193 (100), 175 (76) | – | X | X | X | – |
| 16 | Sinapoyl-di-hexoside | 23.4 | 547 | 265 (82), 324 (43), 223 (28) | – | X | – | – | – |
| 17 | Feruloyl-hexoside | 24.4 | 355 | 193 (100), 175 (19) | – | – | – | X | – |
| 18 | Caffeoyl-hexoside derivative | 27.8 | 441 | 341 (100) | – | – | – | X | – |
| 19 | Di-caffeoyl-quinic acid | 30.6 | 515 | 353 (100), 191 (12) | – | – | X | – | – |
| 20 | Sinapoyl-quinic acid derivative | 31.1 | 577 | 415 (100), 353 (13), 191 (9) | – | X | – | – | – |
| 21 | Feruloyl-di-hexoside derivative | 31.7 | 517 | 323 (100), 193 (41), 179 (19) | – | – | X | – | – |
| 22 | Di-caffeoyl-quinic acid | 32.9 | 515 | 353 (100), 191 (2) | – | X | X | X | – |
| 23 | Caffeoyl-hexoside derivative | 34.6 | 423 | 179 (100), 135 (28) | – | – | – | X | – |
| 24 | Caffeoyl-sinapoyl-quinic acid | 35.7 | 559 | 397 (100), 223 (18), 173 (3) | X | X | X | X | X |
| Peak | Compound | S. muricatum | S. caripense | |||
|---|---|---|---|---|---|---|
| 37-A | El Camino | Puzol | Valencia | E-7 | ||
| 2 | 3-Caffeoyl-quinic acid | 0.90 ± 0.31 | <LOQ | <LOQ | <LOQ | <LOQ |
| 9 | Caffeoyl-hexose IV | 0.07 ± 0.01 | <LOQ | <LOQ | <LOQ | <LOQ |
| 10 | 5-Caffeoyl-quinic acid | <LOQ | 0.89 ± 0.46 | 1.44 ± 0.24 | 1.38 ± 0.35 | 0.19 ± 0.03 |
| 11 | 4-Caffeoyl-quinic acid | 0.03 ± 0.01 | <LOQ | <LOQ | <LOQ | <LOQ |
| 12 | p-Coumaroyl-di-hexose | n.d. | 0.06 ± 0.01 | 0.14 ± 0.04 | 0.41 ± 0.05 | 0.06 ± 0.01 |
| 13 | Feruloyl-hexose | n.d. | 0.14 ± 0.04 | 0.28 ± 0.08 | <LOQ | n.d. |
| 14 | 5-Caffeoyl-quinic acid isomer | n.d. | 0.03 ± 0.01 | n.d. | n.d. | n.d. |
| 15 | Feruloyl-dihexose | n.d. | 0.26 ± 0.04 | 0.16 ± 0.02 | 0.37 ± 0.07 | n.d. |
| 16 | Sinapoyl-di-hexose | n.d. | 0.05 ± 0.01 | n.d. | n.d. | n.d. |
| 18 | Caffeoyl-hexose derivative | n.d. | n.d. | n.d. | 0.06 ± 0.01 | n.d. |
| 19 | Di-caffeoyl-quinic acid | n.d. | <LOQ | 0.06 ± 0.01 | <LOQ | n.d. |
| 20 | Sinapoyl-quinic acid derivative | n.d. | 0.05 ± 0.02 | n.d. | n.d. | n.d. |
| 24 | Caffeoyl-sinapoyl-quinic acid | 0.10 ± 0.02 | 0.04 ± 0.01 | 0.09 ± 0.01 | 0.13 ± 0.02 | 1.13 ± 0.15 |
| – | Total hydroxycinnamic acids | 1.11 ± 0.08 | 1.51 ± 0.07 | 2.17 ± 0.07 | 2.35 ± 0.10 | 1.37 ± 0.06 |
| Accession | ORAC (µmol Trolox/g d.w.) | DPPH (µmol Trolox/g d.w.) | TRC (µmol caffeic acid/g d.w.) |
|---|---|---|---|
| S. muricatum | |||
| 37-A | 83.5 ± 7.0 | 26.1 ± 3.4 | 99.2 ± 11.7 |
| El Camino | 76.0 ± 2.6 | 22.2 ± 1.5 | 73.6 ± 1.7 |
| Puzol | 80.9 ± 8.2 | 34.5 ± 2.1 | 66.2 ± 4.8 |
| Valencia | 51.9 ± 11.9 | 29.2 ± 2.1 | 76.8 ± 6.7 |
| S. caripense | |||
| E-7 | 170.7 ± 22.9 | 36.3 ± 2.9 | 127.9 ± 4.8 |
| Accession | Hydroxycinnamic Acids | ORAC | DPPH | TRC | NO Inhibition |
|---|---|---|---|---|---|
| S. muricatum | |||||
| 37-A | 5 | 2 | 4 | 2 | 1 |
| El Camino | 3 | 4 | 5 | 4 | 5 |
| Puzol | 2 | 3 | 2 | 5 | 2 |
| Valencia | 1 | 5 | 3 | 3 | 4 |
| S. caripense | |||||
| E-7 | 4 | 1 | 1 | 1 | 3 |
| Accession | Origin | Main Use | Fruit Shape | Fruit Weight (g) | Soluble Solids Content (%) |
|---|---|---|---|---|---|
| S. muricatum | |||||
| 37-A | Ecuador | Fresh fruit | Conical | 72 ± 9 | 5.4 ± 0.5 |
| El Camino | New Zealand | Fresh fruit | Heart-shaped | 127 ± 12 | 6.7 ± 0.5 |
| Puzol | Spain | Salads | Ellipsoid | 213 ± 24 | 7.2 ± 0.4 |
| Valencia | Spain | Fresh fruit | Elongated | 192 ± 22 | 7.6 ± 0.6 |
| S. caripense | |||||
| E-7 | Ecuador | Occasionally picked for its sweet fruits | Round | 19 ± 2 | 10.1 ± 0.9 |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Herraiz, F.J.; Villaño, D.; Plazas, M.; Vilanova, S.; Ferreres, F.; Prohens, J.; Moreno, D.A. Phenolic Profile and Biological Activities of the Pepino (Solanum muricatum) Fruit and Its Wild Relative S. caripense. Int. J. Mol. Sci. 2016, 17, 394. https://doi.org/10.3390/ijms17030394
Herraiz FJ, Villaño D, Plazas M, Vilanova S, Ferreres F, Prohens J, Moreno DA. Phenolic Profile and Biological Activities of the Pepino (Solanum muricatum) Fruit and Its Wild Relative S. caripense. International Journal of Molecular Sciences. 2016; 17(3):394. https://doi.org/10.3390/ijms17030394
Chicago/Turabian StyleHerraiz, Francisco J., Débora Villaño, Mariola Plazas, Santiago Vilanova, Federico Ferreres, Jaime Prohens, and Diego A. Moreno. 2016. "Phenolic Profile and Biological Activities of the Pepino (Solanum muricatum) Fruit and Its Wild Relative S. caripense" International Journal of Molecular Sciences 17, no. 3: 394. https://doi.org/10.3390/ijms17030394
APA StyleHerraiz, F. J., Villaño, D., Plazas, M., Vilanova, S., Ferreres, F., Prohens, J., & Moreno, D. A. (2016). Phenolic Profile and Biological Activities of the Pepino (Solanum muricatum) Fruit and Its Wild Relative S. caripense. International Journal of Molecular Sciences, 17(3), 394. https://doi.org/10.3390/ijms17030394