Immunogenicity and Cross-Protective Efficacy Induced by Outer Membrane Proteins from Salmonella Typhimurium Mutants with Truncated LPS in Mice
Abstract
:1. Introduction
2. Results
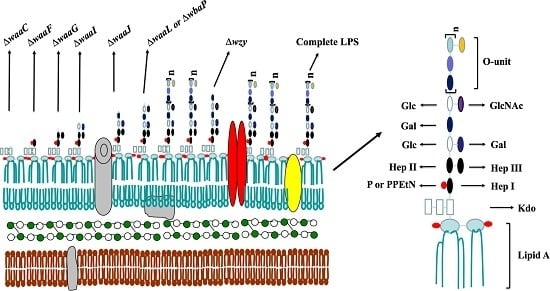
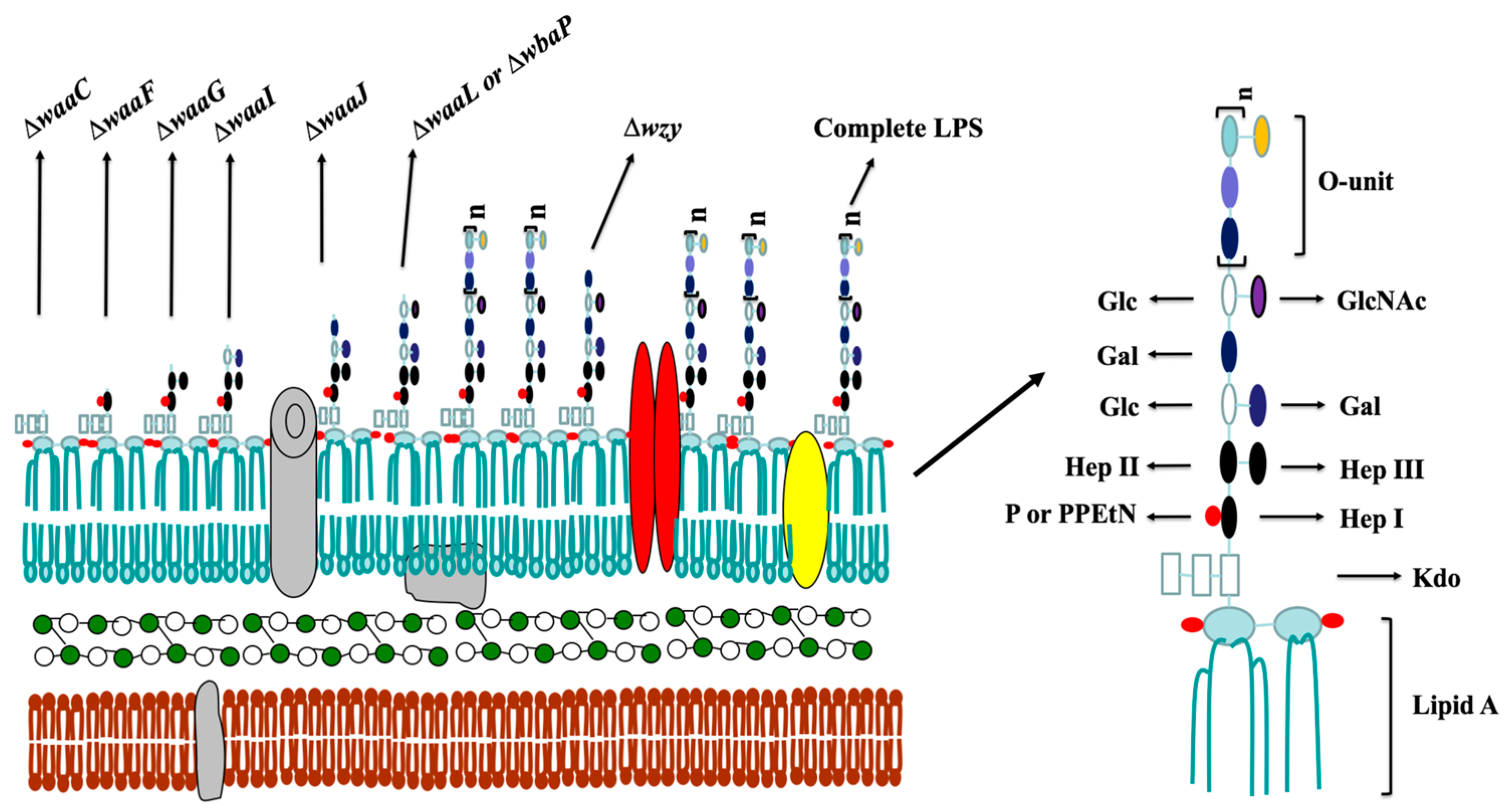
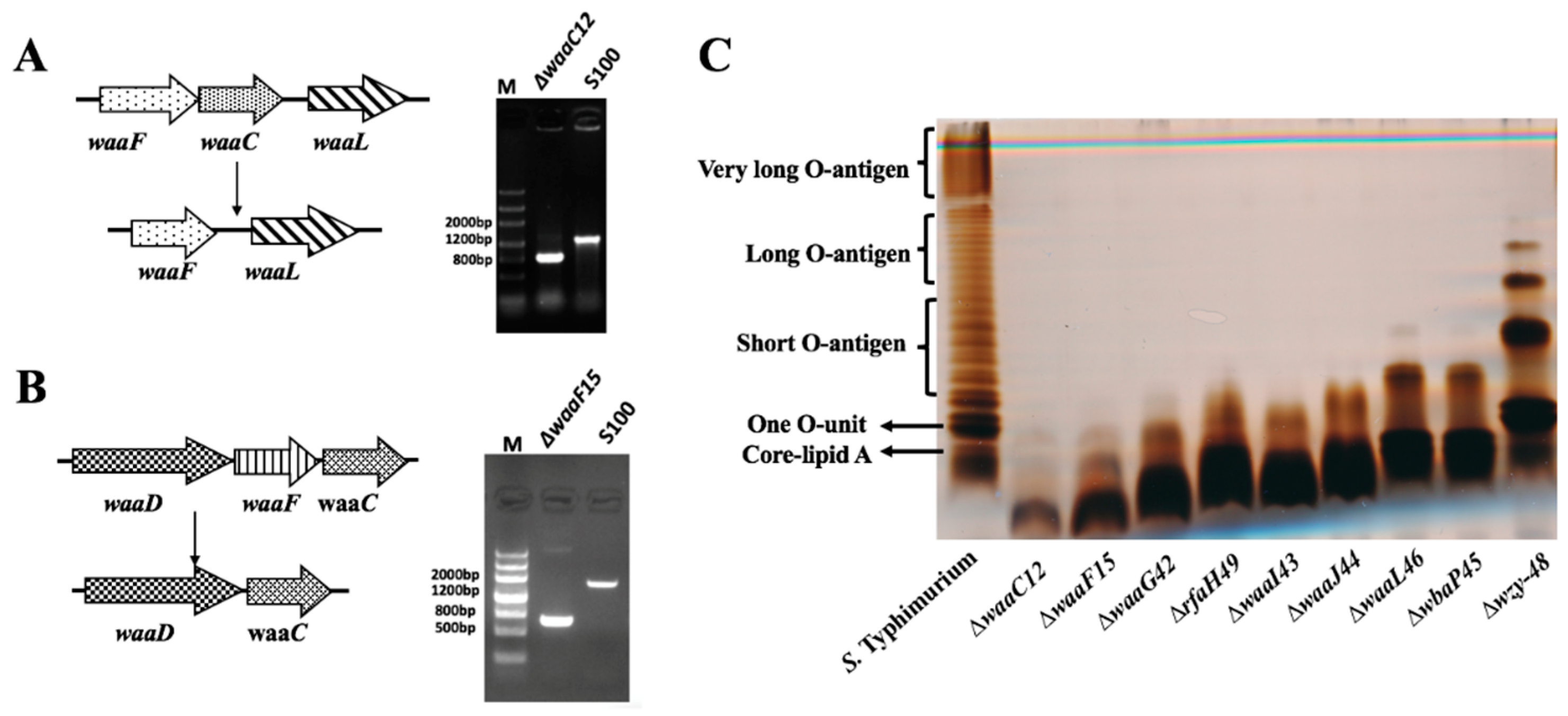
2.1. Mutant Construction and Phenotype Evaluation
2.2. Preparation of OMPs
2.3. Evaluation of the Immunogenicity of OMPs from Wild-Type and Truncated LPS Salmonella
2.4. Evaluation of Protection against Challenge by the S. Typhimurium Wild-Type Strain
2.5. Evaluation of Cross-Reactivity with OMPs from Heterologous Serotype Salmonella
2.6. Evaluation of Cross-Protection against Heterologous Serotype Salmonella
3. Discussion
4. Materials and Methods
4.1. Bacterial Strains, Plasmids, Media and Growth Conditions
4.2. Construction of Plasmids and Mutations
4.3. The Lipopolysaccharide (LPS) Profile of Salmonella Mutant Strains
4.4. Outer Membrane Proteins (OMPs) Purification
4.5. Animal Experiments
4.6. Analysis of Antibody Response in Mice
4.7. Statistical Analysis
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Zhang, S.; Walters, N.; Cao, L.; Robison, A.; Yang, X.H. Recombinant salmonella vaccination technology and its application to human bacterial pathogens. Curr. Pharm. Biotechnol. 2013, 14, 209–219. [Google Scholar] [CrossRef] [PubMed]
- Rabsch, W.; Tschape, H.; Baumler, A.J. Non-typhoidal salmonellosis: Emerging problems. Microbes Infect. 2001, 3, 237–247. [Google Scholar] [CrossRef]
- Hohmann, E.L. Nontyphoidal salmonellosis. Clin. Infect. Dis. 2001, 32, 263–269. [Google Scholar] [PubMed]
- Majowicz, S.E.; Musto, J.; Scallan, E.; Angulo, F.J.; Kirk, M.; O’Brien, S.J.; Jones, T.F.; Fazil, A.; Hoekstra, R.M. The global burden of nontyphoidal salmonella gastroenteritis. Clin. Infect. Dis. 2010, 50, 882–889. [Google Scholar] [CrossRef] [PubMed]
- Feasey, N.A.; Dougan, G.; Kingsley, R.A.; Heyderman, R.S.; Gordon, M.A. Invasive non-typhoidal salmonella disease: An emerging and neglected tropical disease in Africa. Lancet 2012, 379, 2489–2499. [Google Scholar] [CrossRef]
- Morpeth, S.C.; Ramadhani, H.O.; Crump, J.A. Invasive non-typhi Salmonella disease in Africa. Clin. Infect. Dis. 2009, 49, 606–611. [Google Scholar] [CrossRef] [PubMed]
- Reddy, E.A.; Shaw, A.V.; Crump, J.A. Community-acquired bloodstream infections in Africa: A systematic review and meta-analysis. Lancet Infect. Dis. 2010, 10, 417–432. [Google Scholar] [CrossRef]
- Mastroeni, P.; Chabalgoity, J.A.; Dunstan, S.J.; Maskell, D.J.; Dougan, G. Salmonella: Immune responses and vaccines. Vet. J. 2001, 161, 132–164. [Google Scholar] [CrossRef] [PubMed]
- Fraser, A.; Paul, M.; Goldberg, E.; Acosta, C.J.; Leibovici, L. Typhoid fever vaccines: Systematic review and meta-analysis of randomised controlled trials. Vaccine 2007, 25, 7848–7857. [Google Scholar] [CrossRef] [PubMed]
- Ortiz, D.; Siegal, E.M.; Kramer, C.; Khandheria, B.K.; Brauer, E. Nontyphoidal cardiac salmonellosis: Two case reports and a review of the literature. Tex. Heart Inst. J. 2014, 41, 401–406. [Google Scholar] [CrossRef] [PubMed]
- Singh, B. Salmonella vaccines for animals and birds and their future perspective. Open Vac. J. 2009, 2, 100–112. [Google Scholar] [CrossRef]
- De Marchis, G.M.; Braunschweig, M.; Greuter, S. Nontyphoidal salmonellosis and mycotic aneurysm: A case report. Mt. Sinai J. Med. 2005, 72, 351–353. [Google Scholar] [PubMed]
- Muhlradt, P.F.; Menzel, J. Outer membrane of salmonella—Sites of export of newly synthesized lipopolysaccharide on bacterial surface. Eur. J. Biochem. 1973, 35, 471–481. [Google Scholar] [CrossRef] [PubMed]
- Osborn, M.J.; Gander, J.E.; Parisi, E.; Carson, J. Mechanism of assembly of the outer membrane of Salmonella typhimurium. Isolation and characterization of cytoplasmic and outer membrane. J. Biol. Chem. 1972, 247, 3962–3972. [Google Scholar] [PubMed]
- Nikaido, H. Outer membrane of Salmonella typhimurium transmembrane diffusion of some hydrophobic substances. Biochim. Biophys. Acta 1976, 433, 118–132. [Google Scholar] [CrossRef]
- Kuusi, N.; Nurminen, M.; Saxen, H.; Valtonen, M.; Makela, P.H. Immunization with major outer-membrane proteins in experimental salmonellosis of mice. Infect. Immun. 1979, 25, 857–862. [Google Scholar] [PubMed]
- Schlecht, S.; Bhatnagar, N. Proteins from salmonella R-Mutants mediating protection against Salmonella typhimurium infection in mice. 2. Protection tests performed with proteins free from lipopolysaccharide. Zentralbl Bakteriol. Mikrobiol. Hyg. A 1985, 259, 367–377. [Google Scholar] [CrossRef]
- Udhayakumar, V.; Muthukkaruppan, V.R. Protective immunity induced by outer-membrane proteins of Salmonella typhimurium in mice. Infect. Immun. 1987, 55, 816–821. [Google Scholar] [PubMed]
- Isibasi, A.; Ortiz, V.; Vargas, M.; Paniagua, J.; Gonzalez, C.; Moreno, J.; Kumate, J. Protection against Salmonella typhi infection in mice after immunization with outer-membrane proteins isolated from Salmonella typhi 9,12,D,Vi. Infect. Immun. 1988, 56, 2953–2959. [Google Scholar] [PubMed]
- Tabaraie, B.; Sharma, B.K.; Sharma, P.R.; Sehgal, R.; Ganguly, N.K. Evaluation of salmonella porins as a broad-spectrum vaccine candidate. Microbiol. Immunol. 1994, 38, 553–559. [Google Scholar] [CrossRef] [PubMed]
- Wilson, G.; Fox, C.F. Biogenesis of microbial transport systems—Evidence for coupled incorporation of newly synthesized lipids and proteins into membrane. J. Mol. Biol. 1971, 55, 49–60. [Google Scholar] [CrossRef]
- Machtiger, N.A.; Fox, C.F. Biochemistry of bacterial membranes. Annu. Rev. Biochem. 1973, 42, 575–600. [Google Scholar] [CrossRef] [PubMed]
- Raetz, C.R.; Whitfield, C. Lipopolysaccharide endotoxins. Annu. Rev. Biochem. 2002, 71, 635–700. [Google Scholar] [CrossRef] [PubMed]
- Whitfield, C.; Kaniuk, N.; Frirdich, E. Molecular insights into the assembly and diversity of the outer core oligosaccharide in lipopolysaccharides from Escherichia coli and Salmonella. J. Endotoxin Res. 2003, 9, 244–249. [Google Scholar] [CrossRef] [PubMed]
- Frirdich, E.; Whitfield, C. Review: Lipopolysaccharide inner core oligosaccharide structure and outer membrane stability in human pathogens belonging to the Enterobacteriaceae. J. Endotoxin Res. 2005, 11, 133–144. [Google Scholar] [CrossRef] [PubMed]
- Kong, Q.; Yang, J.; Liu, Q.; Alamuri, P.; Roland, K.L.; Curtiss, R. Effect of deletion of genes involved in lipopolysaccharide core and O-antigen synthesis on virulence and immunogenicity of Salmonella enterica serovar Typhimurium. Infect. Immun. 2011, 79, 4227–4239. [Google Scholar] [CrossRef] [PubMed]
- Ernst, R.K.; Guina, T.; Miller, S.I. Salmonella typhimurium outer membrane remodeling: Role in resistance to host innate immunity. Microbes Infect. 2001, 3, 1327–1334. [Google Scholar] [CrossRef]
- Helander, I.M.; Latva-Kala, K.; Lounatmaa, K. Permeabilizing action of polyethyleneimine on Salmonella typhimurium involves disruption of the outer membrane and interactions with lipopolysaccharide. Microbiology 1998, 144, 385–390. [Google Scholar] [CrossRef] [PubMed]
- Van Alphen, W.; Lugtenberg, B.; Berendsen, W. Heptose-deficient mutants of Escherichia coli K12 deficient in up to three major outer membrane proteins. Mol. Gen. Genet. MGG 1976, 147, 263–269. [Google Scholar] [CrossRef] [PubMed]
- Ried, G.; Hindennach, I.; Henning, U. Role of lipopolysaccharide in assembly of Escherichia coli outer membrane proteins OmpA, OmpC, and OmpF. J. Bacteriol. 1990, 172, 6048–6053. [Google Scholar] [PubMed]
- Rivera, M.; Bertasso, A.; McCaffrey, C.; Georgopapadakou, N.H. Porins and lipopolysaccharide of Escherichia coli ATCC 25922 and isogenic rough mutants. FEMS Microbiol. Lett. 1993, 108, 183–187. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.P.; Williams, Y.U.; Miller, S.; Nikaido, H. The c-terminal domain of Salmonella enterica serovar Typhimurium OmpA is an immunodominant antigen in mice but appears to be only partially exposed on the bacterial cell surface. Infect. Immun. 2003, 71, 3937–3946. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.P.; Singh, S.R.; Williams, Y.U.; Jones, L.; Abdullah, T. Antigenic determinants of the OmpC porin from Salmonella typhimurium. Infect. Immun. 1995, 63, 4600–4605. [Google Scholar] [PubMed]
- Gaffga, N.H.; Barton Behravesh, C.; Ettestad, P.J.; Smelser, C.B.; Rhorer, A.R.; Cronquist, A.B.; Comstock, N.A.; Bidol, S.A.; Patel, N.J.; Gerner-Smidt, P.; et al. Outbreak of salmonellosis linked to live poultry from a mail-order hatchery. N. Engl. J. Med. 2012, 366, 2065–2073. [Google Scholar] [CrossRef] [PubMed]
- Havelaar, A.H.; Ivarsson, S.; Lofdahl, M.; Nauta, M.J. Estimating the true incidence of campylobacteriosis and salmonellosis in the European Union, 2009. Epidemiol. Infect. 2013, 141, 293–302. [Google Scholar] [CrossRef] [PubMed]
- Hoelzer, K.; Switt, A.I.M.; Wiedmann, M. Animal contact as a source of human non-typhoidal salmonellosis. Vet. Res. 2011, 42. [Google Scholar] [CrossRef] [PubMed]
- Carlone, G.M.; Thomas, M.L.; Rumschlag, H.S.; Sottnek, F.O. Rapid microprocedure for isolating detergent-insoluble outer-membrane proteins from Haemophilus species. J. Clin. Microbiol. 1986, 24, 330–332. [Google Scholar] [PubMed]
- Ruiz, A.; Oliveira, S.; Torremorell, M.; Pijoan, C. Outer membrane proteins and DNA profiles in strains of Haemophilus parasuis recovered from systemic and respiratory sites. J. Clin. Microbiol. 2001, 39, 1757–1762. [Google Scholar] [CrossRef] [PubMed]
- Fedorka-Cray, P.J.; Huether, M.J.; Stine, D.L.; Anderson, G.A. Efficacy of a cell extract from Actinobacillus (Haemophilus) pleuropneumoniae serotype 1 against disease in swine. Infect. Immun. 1990, 58, 358–365. [Google Scholar] [PubMed]
- Łaniewski, P.; Mitra, A.; Karaca, K.; Khan, A.; Prasad, R.; Curtiss, R.; Roland, K.L. Evaluation of protective efficacy of live attenuated Salmonella enterica serovar Gallinarum vaccine strains against fowl typhoid in chickens. Clin. Vaccine Immunol. 2014, 21, 1267–1276. [Google Scholar] [CrossRef] [PubMed]
- Schmidtke, J.R.; Dixon, F.J. Immune response to a hapten coupled to a nonimmunogenic carrier. Influence of lipopolysaccharide. J. Exp. Med. 1972, 136, 392–397. [Google Scholar] [CrossRef] [PubMed]
- Uchiyama, T.; Jacobs, D.M. Modulation of immune response by bacterial lipopolysaccharide (LPS): Cellular basis of stimulatory and inhibitory effects of LPS on the in vitro IgM antibody response to a T-dependent antigen. J. Immunol. 1978, 121, 2347–2351. [Google Scholar] [PubMed]
- Santos, R.L.; Zhang, S.; Tsolis, R.M.; Kingsley, R.A.; Adams, L.G.; Baumler, A.J. Animal models of Salmonella infections: Enteritis versus typhoid fever. Microbes Infect. 2001, 3, 1335–1344. [Google Scholar] [CrossRef]
- Humphrey, T.J.; Williams, A.; McAlpine, K.; Lever, M.S.; Guard-Petter, J.; Cox, J.M. Isolates of Salmonella enterica Enteritidis PT4 with enhanced heat and acid tolerance are more virulent in mice and more invasive in chickens. Epidemiol. Infect. 1996, 117, 79–88. [Google Scholar] [CrossRef] [PubMed]
- Carter, P.B.; Collins, F.M. The route of enteric infection in normal mice. J. Exp. Med. 1974, 139, 1189–1203. [Google Scholar] [CrossRef] [PubMed]
- Emoto, M.; Danbara, H.; Yoshikai, Y. Induction of γ/δ T cells in murine salmonellosis by an avirulent but not by a virulent strain of Salmonella choleraesuis. J. Exp. Med. 1992, 176, 363–372. [Google Scholar] [CrossRef] [PubMed]
- Ishigami, M.; Nishimura, H.; Naiki, Y.; Yoshioka, K.; Kawano, T.; Tanaka, Y.; Taniguchi, M.; Kakumu, S.; Yoshikai, Y. The roles of intrahepatic Valpha14+ NK1.1+ T cells for liver injury induced by Salmonella infection in mice. Hepatology 1999, 29, 1799–1808. [Google Scholar] [CrossRef] [PubMed]
- Greisman, S.; Johnston, C. Review: Evidence against the hypothesis that antibodies to the inner core of lipopolysaccharides in antisera raised by immunization with enterobacterial deep-rough mutants confer broad-spectrum protection during Gram-negative bacterial sepsis. J. Endotoxin Res. 1997, 4, 123–153. [Google Scholar] [CrossRef]
- Beltran, P.; Musser, J.M.; Helmuth, R.; Farmer, J.J.; Frerichs, W.M.; Wachsmuth, I.K.; Ferris, K.; McWhorter, A.C.; Wells, J.G.; Cravioto, A. Toward a population genetic analysis of Salmonella: Genetic diversity and relationships among strains of serotypes S. choleraesuis, S. derby, S. dublin, S. enteritidis, S. heidelberg, S. infantis, S. newport, and S. typhimurium. Proc. Natl. Acad. Sci. USA 1988, 85, 7753–7757. [Google Scholar] [CrossRef] [PubMed]
- Thomson, N.R.; Clayton, D.J.; Windhorst, D.; Vernikos, G.; Davidson, S.; Churcher, C.; Quail, M.A.; Stevens, M.; Jones, M.A.; Watson, M. Comparative genome analysis of Salmonella Enteritidis PT4 and Salmonella Gallinarum 287/91 provides insights into evolutionary and host adaptation pathways. Genome Res. 2008, 18, 1624–1637. [Google Scholar] [CrossRef] [PubMed]
- Masoud, H. LPS-based conjugate vaccines composed of saccharide antigens of smooth-type Salmonella enteritidis and rough-type S. gallinarum 9R bound to bovine serum albumin. Scand. J. Infect. Dis. 2007, 39, 315–322. [Google Scholar] [CrossRef] [PubMed]
- Johns, M.; Skehill, A.; McCabe, W.R. Immunization with rough mutants of Salmonella minnesota. IV. Protection by antisera to O and rough antigens against endotoxin. J. Infect. Dis. 1983, 147, 57–67. [Google Scholar] [CrossRef] [PubMed]
- Siber, G.R.; Kania, S.A.; Warren, H.S. Cross-reactivity of rabbit antibodies to lipopolysaccharides of Escherichia coli J5 and other gram-negative bacteria. J. Infect. Dis. 1985, 152, 954–964. [Google Scholar] [CrossRef] [PubMed]
- Gigliotti, F.; Shenep, J.L. Failure of monoclonal antibodies to core glycolipid to bind intact smooth strains of Escherichia coli. J. Infect. Dis. 1985, 151, 1005–1011. [Google Scholar] [CrossRef] [PubMed]
- Troncoso, G.; Sanchez, S.; Kolberg, J.; Rosenqvist, E.; Veiga, M.; Ferreiros, C.M.; Criado, M. Analysis of the expression of the putatively virulence-associated neisserial protein RmpM (class 4) in commensal Neisseria and Moraxella catarrhalis strains. FEMS Microbiol. Lett. 2001, 199, 171–176. [Google Scholar] [CrossRef] [PubMed]
- Nakayama, K.; Kelly, S.M.; Curtiss, R. Construction of an Asd+ expression-cloning vector: Stable maintenance and high level expression of cloned genes in a Salmonella vaccine strain. Nat. Biotechnol. 1988, 6, 693–697. [Google Scholar] [CrossRef]
- Roland, K.; Curtiss, R., III; Sizemore, D. Construction and evaluation of a Δcya Δcrp Salmonella typhimurium strain expressing avian pathogenic Escherichia coli O78 LPS as a vaccine to prevent airsacculitis in chickens. Avian Dis. 1999, 43, 429–441. [Google Scholar] [CrossRef] [PubMed]
- Shubeita, H.E.; Sambrook, J.F.; McCormick, A.M. Molecular cloning and analysis of functional cDNA and genomic clones encoding bovine cellular retinoic acid-binding protein. Proc. Natl. Acad. Sci. USA 1987, 84, 5645–5649. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.H.; Wang, S.F.; Xin, W.; Scarpellini, G.; Shi, Z.X.; Gunn, B.; Roland, K.L.; Curtiss, R. A sopB Deletion mutation enhances the immunogenicity and protective efficacy of a heterologous antigen delivered by live attenuated Salmonella enterica vaccines. Infect. Immun. 2008, 76, 5238–5246. [Google Scholar] [CrossRef] [PubMed]
- Hitchcock, P.J.; Brown, T.M. Morphological heterogeneity among Salmonella lipopolysaccharide chemotypes in silver-stained polyacrylamide gels. J. Bacteriol. 1983, 154, 269–277. [Google Scholar] [PubMed]
- Bjerre, A.; Brusletto, B.; Mollnes, T.E.; Fritzsonn, E.; Rosenqvist, E.; Wedege, E.; Namork, E.; Kierulf, P.; Brandtzaeg, P. Complement activation induced by purified Neisseria meningitidis lipopolysaccharide (LPS), outer membrane vesicles, whole bacteria, and an LPS-free mutant. J. Infect. Dis. 2002, 185, 220–228. [Google Scholar] [CrossRef] [PubMed]
- Cloeckaert, A.; Kerkhofs, P.; Limet, J.N. Antibody response to Brucella outer membrane proteins in bovine brucellosis: Immunoblot analysis and competitive enzyme-linked immunosorbent assay using monoclonal antibodies. J. Clin. Microbiol. 1992, 30, 3168–3174. [Google Scholar] [PubMed]
- Motulsky, H. Prism 5 statistics guide, 2007; GraphPad Software: La Jolla, CA, USA, 2007; pp. 1–26. [Google Scholar]





| Groups | No. of Surviving Mice/Total No. of Mice |
|---|---|
| S100 (wild-type) | 8/8 (100%) |
| S511 (∆waaC12) | 6/8 (75%) |
| S512 (∆waaF15) | 4/8 (50%) |
| S372 (∆waaG42) | 2/8 (25%) |
| S377 (∆rfaH49) | 2/8 (25%) |
| S373 (∆waaI43) | 4/8 (50%) |
| S374 (∆waaJ44) | 6/8 (75%) |
| S376 (∆waaL46) | 8/8 (100%) |
| S375 (∆wbaP45) | 6/8 (75%) |
| S378 (∆wzy-48) | 6/8 (75%) |
| Phosphate Buffered Saline (PBS) | 0/8 (0%) |
| Strains or Plasmids | Description | Source |
|---|---|---|
| Strains | ||
| S100 | S. Typhimurium, clinical isolate from duck | IPVM * |
| S511 | ∆waaC12 | This work |
| S512 | ∆waaF15 | This work |
| S372 | ∆waaG42 | [26] |
| S377 | ∆rfaH49 | [26] |
| S373 | ∆waaI43 | [26] |
| S374 | ∆waaJ44 | [26] |
| S376 | ∆waaL46 | [26] |
| S375 | ∆wbaP45 | [26] |
| S378 | ∆wzy-48 | [26] |
| S246 | S. Enteritidis, clinical isolate from chicken | IPVM |
| S340 | S. Choleraesuis, clinical isolate from pig | IPVM |
| E. coli | – | – |
| χ7232 | endA1 hsdR17 (rK-,mK+) supE44 thi-1 recA1 gyrArelA1Δ (lacZYA-argF) U169λpirdeoR (φ80dlac Δ (lacZ) M15) | [57] |
| χ7213 | thi-1 thr-1 leuB6 glnV44 tonA21 lacY1 recA1 RP4-2-Tc::μλpir ΔasdA4 Δzhf-2 ::Tn 10 | [57] |
| Plasmids | ||
| pYA4278 | Suicide plasmid (pRE112) | [26] |
| pQK256 | For deletion of rfaC | This work |
| pQK257 | For deletion of rfaF | This work |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, Q.; Liu, Q.; Zhao, X.; Liu, T.; Yi, J.; Liang, K.; Kong, Q. Immunogenicity and Cross-Protective Efficacy Induced by Outer Membrane Proteins from Salmonella Typhimurium Mutants with Truncated LPS in Mice. Int. J. Mol. Sci. 2016, 17, 416. https://doi.org/10.3390/ijms17030416
Liu Q, Liu Q, Zhao X, Liu T, Yi J, Liang K, Kong Q. Immunogenicity and Cross-Protective Efficacy Induced by Outer Membrane Proteins from Salmonella Typhimurium Mutants with Truncated LPS in Mice. International Journal of Molecular Sciences. 2016; 17(3):416. https://doi.org/10.3390/ijms17030416
Chicago/Turabian StyleLiu, Qiong, Qing Liu, Xinxin Zhao, Tian Liu, Jie Yi, Kang Liang, and Qingke Kong. 2016. "Immunogenicity and Cross-Protective Efficacy Induced by Outer Membrane Proteins from Salmonella Typhimurium Mutants with Truncated LPS in Mice" International Journal of Molecular Sciences 17, no. 3: 416. https://doi.org/10.3390/ijms17030416
APA StyleLiu, Q., Liu, Q., Zhao, X., Liu, T., Yi, J., Liang, K., & Kong, Q. (2016). Immunogenicity and Cross-Protective Efficacy Induced by Outer Membrane Proteins from Salmonella Typhimurium Mutants with Truncated LPS in Mice. International Journal of Molecular Sciences, 17(3), 416. https://doi.org/10.3390/ijms17030416