Apolipoprotein A1-Unique Peptide as a Diagnostic Biomarker for Acute Ischemic Stroke
Abstract
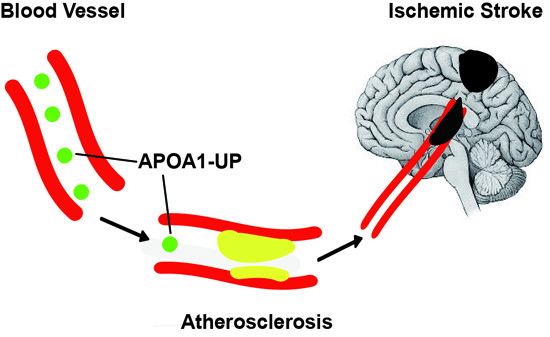
:1. Introduction
2. Results
2.1. Demographic Characteristics and Mass Spectrometry Measurements of the Study Population
2.2. Stratification of the Study Population Across Low, Medium, and High Levels of Serum APOA1-UP
2.3. Inverse Correlation between Serum APOA1-UP Level and the Presence of Ischemic Stroke
2.4. Evaluation of APOA1-UP as a Diagnostic Biomarker for Acute Ischemic Stroke
3. Discussion
3.1. Serum APOA1-UP and Initial CT Scans in the Diagnosis of Acute Ischemic Stroke
3.2. Serum Unique Peptides as Diagnostic Biomarkers for Acute Ischemic Stroke
3.3. Measurement of Serum Unique Peptides by MRM
3.4. Dyslipidemia and Acute Ischemic Stroke
4. Materials and Methods
4.1. Materials
4.2. Study Population
4.3. Laboratory Tests
4.4. Selection of Unique Peptides, Reference Peptides, and Q1/Q3 Transition
4.5. Statistical Analysis
5. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
Abbreviations
| APOA1-UP | apolipoprotein A1 unique peptide |
| AIS | acute ischemic stroke |
| HDL-C | high-density lipoprotein cholesterol |
| LDL-C | low-density lipoprotein cholesterol |
| TG | triglycerides |
| TC | total cholesterol |
| MRM | multiple reaction monitoring |
| LRP | labeled reference peptide |
| ROC | Receiver operator characteristic (ROC) curve |
| AUC | area under the curve |
| OR | odds ratio |
References
- Bhatia, M.; Howard, S.C.; Clark, T.G.; Neale, R.; Qizilbash, N.; Murphy, M.F.; Rothwell, P.M. Apolipoproteins as predictors of ischaemic stroke in patients with a previous transient ischaemic attack. Cerebrovasc. Dis. 2006, 21, 323–328. [Google Scholar] [CrossRef] [PubMed]
- Amarenco, P.; Labreuche, J.; Elbaz, A.; Touboul, P.J.; Driss, F.; Jaillard, A.; Bruckert, E. Blood lipids in brain infarction subtypes. Cerebrovasc. Dis. 2006, 22, 101–108. [Google Scholar] [CrossRef] [PubMed]
- Yuan, B.B.; Luo, G.G.; Gao, J.X.; Qiao, J.; Yang, J.B.; Huo, K.; Li, Y.B.; Liu, Y. Variance of serum lipid levels in stroke subtypes. Clin. Lab. 2015, 61, 1509–1514. [Google Scholar] [PubMed]
- Sheth, S.A.; Iavarone, A.T.; Liebeskind, D.S.; Won, S.J.; Swanson, R.A. Targeted lipid profiling discovers plasma biomarkers of acute brain injury. PLoS ONE 2015, 10, e0129735. [Google Scholar] [CrossRef] [PubMed]
- Pikula, A.; Beiser, A.S.; Wang, J.; Himali, J.J.; Kelly-Hayes, M.; Kase, C.S.; Yang, Q.; Seshadri, S.; Wolf, P.A. Lipid and lipoprotein measurements and the risk of ischemic vascular events: Framingham study. Neurology 2015, 84, 472–479. [Google Scholar] [CrossRef] [PubMed]
- Boekholdt, S.M.; Arsenault, B.J.; Hovingh, G.K.; Mora, S.; Pedersen, T.R.; Larosa, J.C.; Welch, K.M.; Amarenco, P.; Demicco, D.A.; Tonkin, A.M.; et al. Levels and changes of HDL cholesterol and apolipoprotein A-I in relation to risk of cardiovascular events among statin-treated patients: A meta-analysis. Circulation 2013, 128, 1504–1512. [Google Scholar] [CrossRef] [PubMed]
- Walldius, G.; Jungner, I. Apolipoprotein A-I versus HDL cholesterol in the prediction of risk for myocardial infarction and stroke. Curr. Opin. Cardiol. 2007, 22, 359–367. [Google Scholar] [CrossRef] [PubMed]
- Garfagnini, A.; Devoto, G.; Rosselli, P.; Boggiano, P.; Venturini, M. Relationship between HDL-cholesterol and apolipoprotein A1 and the severity of coronary artery disease. Eur. Heart J. 1995, 16, 465–470. [Google Scholar] [PubMed]
- Zhang, H.; Liu, Q.; Zimmerman, L.J.; Ham, A.J.; Slebos, R.J.; Rahman, J.; Kikuchi, T.; Massion, P.P.; Carbone, D.P.; Billheimer, D.; et al. Methods for peptide and protein quantitation by liquid chromatography-multiple reaction monitoring mass spectrometry. Mol. Cell. Proteom. MCP 2011, 10. [Google Scholar] [CrossRef] [PubMed]
- Lange, V.; Picotti, P.; Domon, B.; Aebersold, R. Selected reaction monitoring for quantitative proteomics: A tutorial. Mol. Syst. Biol. 2008, 4, 222. [Google Scholar] [CrossRef] [PubMed]
- Kaysen, G.A.; Hoye, E.; Jones, H., Jr.; van Tol, A.; Joles, J.A. Effect of oncotic pressure on apolipoprotein A-I metabolism in the rat. Am. J. Kidney Dis. Off. J. Natl. Kidney Found. 1995, 26, 178–186. [Google Scholar] [CrossRef]
- Lian, T.; Qu, D.; Zhao, X.; Yu, L.; Gao, B. Identification of site-specific stroke biomarker candidates by laser capture microdissection and labeled reference peptide. Int. J. Mol. Sci. 2015, 16, 13427–13441. [Google Scholar] [CrossRef] [PubMed]
- Unden, J.; Strandberg, K.; Malm, J.; Campbell, E.; Rosengren, L.; Stenflo, J.; Norrving, B.; Romner, B.; Lindgren, A.; Andsberg, G. Explorative investigation of biomarkers of brain damage and coagulation system activation in clinical stroke differentiation. J. Neurol. 2009, 256, 72–77. [Google Scholar] [CrossRef] [PubMed]
- Saenger, A.K.; Christenson, R.H. Stroke biomarkers: Progress and challenges for diagnosis, prognosis, differentiation, and treatment. Clin. Chem. 2010, 56, 21–33. [Google Scholar] [CrossRef] [PubMed]
- Jickling, G.C.; Sharp, F.R. Blood biomarkers of ischemic stroke. Neurother. J. Am. Soc. Exp. NeuroTherapeutics 2011, 8, 349–360. [Google Scholar] [CrossRef] [PubMed]
- Jauch, E.C.; Lindsell, C.; Broderick, J.; Fagan, S.C.; Tilley, B.C.; Levine, S.R. Association of serial biochemical markers with acute ischemic stroke: The national institute of neurological disorders and stroke recombinant tissue plasminogen activator stroke study. Stroke; J. Cereb. Circ. 2006, 37, 2508–2513. [Google Scholar] [CrossRef] [PubMed]
- Datta, A.; Chen, C.P.; Sze, S.K. Discovery of prognostic biomarker candidates of lacunar infarction by quantitative proteomics of microvesicles enriched plasma. PLoS ONE 2014, 9, e94663. [Google Scholar] [CrossRef] [PubMed]
- Lin, D.; Alborn, W.E.; Slebos, R.J.; Liebler, D.C. Comparison of protein immunoprecipitation-multiple reaction monitoring with elisa for assay of biomarker candidates in plasma. J. Proteome Res. 2013, 12, 5996–6003. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Qian, W.J.; Mottaz, H.M.; Clauss, T.R.; Anderson, D.J.; Moore, R.J.; Camp, D.G., 2nd; Khan, A.H.; Sforza, D.M.; Pallavicini, M.; et al. Development and evaluation of a micro- and nanoscale proteomic sample preparation method. J. Proteome Res. 2005, 4, 2397–2403. [Google Scholar] [CrossRef] [PubMed]
- Krueger, M.; Bechmann, I.; Immig, K.; Reichenbach, A.; Hartig, W.; Michalski, D. Blood-brain barrier breakdown involves four distinct stages of vascular damage in various models of experimental focal cerebral ischemia. J. Cereb. Blood Flow Metab. 2014, 35. [Google Scholar] [CrossRef] [PubMed]
- Fassbender, K.; Schmidt, R.; Schreiner, A.; Fatar, M.; Muhlhauser, F.; Daffertshofer, M.; Hennerici, M. Leakage of brain-originated proteins in peripheral blood: Temporal profile and diagnostic value in early ischemic stroke. J. Neurol. Sci. 1997, 148, 101–105. [Google Scholar] [CrossRef]
- Cuadrado-Godia, E.; Jimenez-Conde, J.; Ois, A.; Rodriguez-Campello, A.; Garcia-Ramallo, E.; Roquer, J. Sex differences in the prognostic value of the lipid profile after the first ischemic stroke. J. Neurol. 2009, 256, 989–995. [Google Scholar] [CrossRef] [PubMed]
- MacLean, B.; Tomazela, D.M.; Shulman, N.; Chambers, M.; Finney, G.L.; Frewen, B.; Kern, R.; Tabb, D.L.; Liebler, D.C.; MacCoss, M.J. Skyline: An open source document editor for creating and analyzing targeted proteomics experiments. Bioinformatics 2010, 26, 966–968. [Google Scholar] [CrossRef] [PubMed]

| Variables | Total (n = 131) | Control (n = 37) | Ischemic Stroke (n = 94) | p a |
|---|---|---|---|---|
| Age, Mean (SD) | 57.65 (15.14) | 47.08 (16.09) | 61.81 (12.58) | <0.0001 |
| Gender, % | ||||
| Male | 60.31 | 62.16 | 59.57 | 0.8445 |
| Female | 39.70 | 37.84 | 40.43 | |
| Diabetes Mellitus, % | 12.21 | 5.41 | 14.89 | 0.2338 |
| Hypertension, % | 30.53 | 5.41 | 40.43 | <0.0001 |
| Previous Ischemic heart disease, % | 12.21 | 2.70 | 15.96 | 0.0398 |
| LDL-C in mmol/L, Median (IQR) | 2.75 (0.82) | 2.73 (1.04) | 2.77 (0.75) | 0.9124 |
| TG in mmol/L, Median (IQR) | 1.52 (0.55) | 1.45 (0.38) | 1.59 (0.63) | 0.0619 |
| HDL-C in mmol/L, Median (IQR) | 1.06 (0.55) | 1.39 (0.59) | 1.03 (0.54) | <0.0001 |
| Total-C in mmol/L, Median (IQR) | 4.54 (1.03) | 4.43 (1.34) | 4.56 (0.94) | 0.0580 |
| APOA1-UP/LRP, Median (IQR) | 1.49 (0.77) | 2.14 (0.40) | 1.32 (0.44) | <0.0001 |
| Demographic Characteristics | No. of Patients | Serum APOA1-UP/LRP Ratio | p a for Trend | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Low | Medium | High | |||||||
| I.S. | Ctrl. | I.S. | Ctrl. | I.S. | Ctrl. | I.S. | Ctrl. | ||
| Overall | 94 | 37 | 14 | 0 | 76 | 10 | 4 | 27 | <0.0001 |
| Gender | |||||||||
| Male | 56 | 23 | 12 | 0 | 42 | 8 | 2 | 15 | <0.0001 |
| Female | 38 | 14 | 2 | 0 | 34 | 2 | 2 | 12 | <0.0001 |
| Age | |||||||||
| 0–29 | 1 | 4 | 0 | 0 | 1 | 0 | 0 | 4 | 0.0253 |
| 30–59 | 44 | 26 | 5 | 0 | 38 | 6 | 1 | 20 | <0.0001 |
| 60–89 | 49 | 7 | 9 | 0 | 37 | 4 | 3 | 3 | 0.0216 |
| Diabetes Mellitus | |||||||||
| Yes | 14 | 2 | 2 | 0 | 12 | 4 | 0 | 0 | 0.4489 |
| No | 80 | 35 | 12 | 0 | 12 | 8 | 4 | 27 | <0.0001 |
| Hypertension | |||||||||
| Yes | 38 | 2 | 4 | 0 | 33 | 1 | 1 | 1 | 0.1297 |
| No | 56 | 35 | 10 | 0 | 43 | 9 | 3 | 26 | <0.0001 |
| Previous IHD | |||||||||
| Yes | 15 | 1 | 3 | 0 | 11 | 1 | 1 | 0 | 0.7418 |
| No | 79 | 36 | 11 | 0 | 65 | 9 | 3 | 27 | <0.0001 |
| Variables | OR | 95% CI | p |
|---|---|---|---|
| Age | 1.03 | 0.97, 1.10 | 0.3376 |
| Diabetes Mellitus | 0.46 | 0.03, 11.74 | 0.5824 |
| Hypertension | 9.39 | 1.12, 150.04 | 0.0382 |
| Previous IHD | 3.40 | 0.19, 119.24 | 0.4184 |
| Variables | APOA1-UP/LRP | HDL-C | CT a |
|---|---|---|---|
| AUC | 0.9750 | 0.7488 | – |
| Sensitivity | 0.9063 | 0.4583 | 0.9149 (86/94) |
| Specificity | 0.9714 | 0.9714 | 0.8222 (37/45) |
| Diagnostic Index | 1.8777 | 1.4298 | 1.7371 |
| Cut-off Value | 1.8031 | 0.9400 | – |
| OR | 188.13 | 31.68 | – |
| 95% CI | 38.04, 930.43 | 4.17, 240.70 | – |
| p b | <0.0001 | <0.0001 | <0.0001 |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhao, X.; Yu, Y.; Xu, W.; Dong, L.; Wang, Y.; Gao, B.; Li, G.; Zhang, W. Apolipoprotein A1-Unique Peptide as a Diagnostic Biomarker for Acute Ischemic Stroke. Int. J. Mol. Sci. 2016, 17, 458. https://doi.org/10.3390/ijms17040458
Zhao X, Yu Y, Xu W, Dong L, Wang Y, Gao B, Li G, Zhang W. Apolipoprotein A1-Unique Peptide as a Diagnostic Biomarker for Acute Ischemic Stroke. International Journal of Molecular Sciences. 2016; 17(4):458. https://doi.org/10.3390/ijms17040458
Chicago/Turabian StyleZhao, Xu, Yue Yu, Wenlong Xu, Lei Dong, Yuan Wang, Bing Gao, Guangyu Li, and Wentao Zhang. 2016. "Apolipoprotein A1-Unique Peptide as a Diagnostic Biomarker for Acute Ischemic Stroke" International Journal of Molecular Sciences 17, no. 4: 458. https://doi.org/10.3390/ijms17040458
APA StyleZhao, X., Yu, Y., Xu, W., Dong, L., Wang, Y., Gao, B., Li, G., & Zhang, W. (2016). Apolipoprotein A1-Unique Peptide as a Diagnostic Biomarker for Acute Ischemic Stroke. International Journal of Molecular Sciences, 17(4), 458. https://doi.org/10.3390/ijms17040458