The Development of Sugar-Based Anti-Melanogenic Agents
Abstract
:1. Introduction
2. Sugars as Anti-Melanogenic Agents
2.1. Natural Sugars
2.2. Sugar Derivatives
2.3. Membrane-Associated Transporter Protein (MATP): An Alternative Target for Anti-Melanogenic Sugars
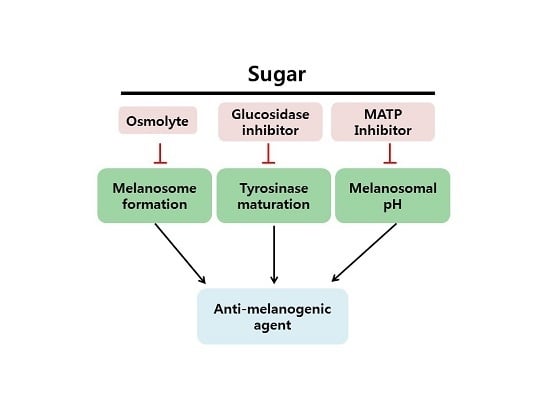
3. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Slominski, A.; Paus, R.; Schadendorf, D. Melanocytes as “sensory” and regulatory cells in the epidermis. J. Theor. Biol. 1993, 164, 103–120. [Google Scholar] [CrossRef] [PubMed]
- Slominski, A.T.; Zmijewski, M.A.; Zbytek, B.; Tobin, D.J.; Theoharides, T.C.; Rivier, J. Key role of crf in the skin stress response system. Endocr. Rev. 2013, 34, 827–884. [Google Scholar] [CrossRef] [PubMed]
- Kvam, E.; Tyrrell, R.M. The role of melanin in the induction of oxidative DNA base damage by ultraviolet a irradiation of DNA or melanoma cells. J. Investig. Dermatol. 1999, 113, 209–213. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Weng, Q.Y.; Fisher, D.E. UV signaling pathways within the skin. J. Investig. Dermatol. 2014, 134, 2080–2085. [Google Scholar] [CrossRef] [PubMed]
- Hearing, V.J. Unraveling the melanocyte. Am. J. Hum. Genet. 1993, 52, 1–7. [Google Scholar] [PubMed]
- Slominski, A.; Moellmann, G.; Kuklinska, E. l-tyrosine, l-dopa, and tyrosinase as positive regulators of the subcellular apparatus of melanogenesis in bomirski ab amelanotic melanoma cells. Pigment Cell Res. 1989, 2, 109–116. [Google Scholar] [CrossRef] [PubMed]
- Slominski, A.; Zmijewski, M.A.; Pawelek, J. l-Tyrosine and l-dihydroxyphenylalanine as hormone-like regulators of melanocyte functions. Pigment Cell Melanoma Res. 2012, 25, 14–27. [Google Scholar] [CrossRef] [PubMed]
- Slominski, A.T.; Zmijewski, M.A.; Skobowiat, C.; Zbytek, B.; Slominski, R.M.; Steketee, J.D. Sensing the environment: Regulation of local and global homeostasis by the skin’s neuroendocrine system. Adv. Anat. Embryol. Cell Biol. 2012, 212, 1–115. [Google Scholar]
- Slominski, A.; Wortsman, J. Neuroendocrinology of the skin. Endocr. Rev. 2000, 21, 457–487. [Google Scholar] [CrossRef] [PubMed]
- Slominski, A.; Wortsman, J.; Luger, T.; Paus, R.; Solomon, S. Corticotropin releasing hormone and proopiomelanocortin involvement in the cutaneous response to stress. Physiol. Rev. 2000, 80, 979–1020. [Google Scholar] [PubMed]
- Davis, E.C.; Callender, V.D. Postinflammatory hyperpigmentation: A review of the epidemiology, clinical features, and treatment options in skin of color. J. Clin. Aesth. Dermatol. 2010, 3, 20–31. [Google Scholar]
- Handel, A.C.; Lima, P.B.; Tonolli, V.M.; Miot, L.D.; Miot, H.A. Risk factors for facial melasma in women: A case-control study. Br. J. Dermatol. 2014, 171, 588–594. [Google Scholar] [CrossRef] [PubMed]
- Slominski, A.; Tobin, D.J.; Shibahara, S.; Wortsman, J. Melanin pigmentation in mammalian skin and its hormonal regulation. Physiol. Rev. 2004, 84, 1155–1228. [Google Scholar] [CrossRef] [PubMed]
- Yamaguchi, Y.; Hearing, V.J. Physiological factors that regulate skin pigmentation. Biofactors 2009, 35, 193–199. [Google Scholar] [CrossRef] [PubMed]
- Bellei, B.; Pitisci, A.; Catricala, C.; Larue, L.; Picardo, M. Wnt/β-catenin signaling is stimulated by α-melanocyte-stimulating hormone in melanoma and melanocyte cells: Implication in cell differentiation. Pigment Cell Melanoma Res. 2011, 24, 309–325. [Google Scholar] [CrossRef] [PubMed]
- Bae-Harboe, Y.S.; Park, H.Y. Tyrosinase: A central regulatory protein for cutaneous pigmentation. J. Investig. Dermatol. 2012, 132, 2678–2680. [Google Scholar] [CrossRef] [PubMed]
- Schiaffino, M.V. Signaling pathways in melanosome biogenesis and pathology. Int. J. Biochem. Cell Biol. 2010, 42, 1094–1104. [Google Scholar] [CrossRef] [PubMed]
- Brozyna, A.A.; Jozwicki, W.; Carlson, J.A.; Slominski, A.T. Melanogenesis affects overall and disease-free survival in patients with stage III and IV melanoma. Hum. Pathol. 2013, 44, 2071–2074. [Google Scholar] [CrossRef] [PubMed]
- Slominski, R.M.; Zmijewski, M.A.; Slominski, A.T. The role of melanin pigment in melanoma. Exp. Dermatol. 2015, 24, 258–259. [Google Scholar] [CrossRef] [PubMed]
- Slominski, A.T.; Carlson, J.A. Melanoma resistance: A bright future for academicians and a challenge for patient advocates. Mayo Clin. Proc. 2014, 89, 429–433. [Google Scholar] [CrossRef] [PubMed]
- Iannella, G.; Greco, A.; Didona, D.; Didona, B.; Granata, G.; Manno, A.; Pasquariello, B.; Magliulo, G. Vitiligo: Pathogenesis, clinical variants and treatment approaches. Autoimmun. Rrev. 2015, 15, 335–343. [Google Scholar] [CrossRef] [PubMed]
- Okura, M.; Yamashita, T.; Ishii-Osai, Y.; Yoshikawa, M.; Sumikawa, Y.; Wakamatsu, K.; Ito, S. Effects of rhododendrol and its metabolic products on melanocytic cell growth. J. Dermatol. Sci. 2015, 80, 142–149. [Google Scholar] [CrossRef] [PubMed]
- Spritz, R.A. Six decades of vitiligo genetics: Genome-wide studies provide insights into autoimmune pathogenesis. J. Investig. Dermatol. 2012, 132, 268–273. [Google Scholar] [CrossRef] [PubMed]
- Tanemura, A.; Yang, L.; Yang, F.; Nagata, Y.; Wataya-Kaneda, M.; Fukai, K.; Tsuruta, D.; Ohe, R.; Yamakawa, M.; Suzuki, T.; et al. An immune pathological and ultrastructural skin analysis for rhododenol-induced leukoderma patients. J. Dermatol. Sci. 2015, 77, 185–188. [Google Scholar] [CrossRef] [PubMed]
- Imokawa, G.; Ishida, K. Inhibitors of intracellular signaling pathways that lead to stimulated epidermal pigmentation: Perspective of anti-pigmenting agents. Int. J. Mol. Sci. 2014, 15, 8293–8315. [Google Scholar] [CrossRef] [PubMed]
- Slominski, A.; Wortsman, J.; Tobin, D.J. The cutaneous serotoninergic/melatoninergic system: Securing a place under the sun. FASEB J. 2005, 19, 176–194. [Google Scholar] [CrossRef] [PubMed]
- Fukamachi, S.; Shimada, A.; Shima, A. Mutations in the gene encoding B, a novel transporter protein, reduce melanin content in medaka. Nat. Genet. 2001, 28, 381–385. [Google Scholar] [CrossRef] [PubMed]
- Bin, B.H.; Bhin, J.; Yang, S.H.; Choi, D.H.; Park, K.; Shin, D.W.; Lee, A.Y.; Hwang, D.; Cho, E.G.; Lee, T.R. Hyperosmotic stress reduces melanin production by altering melanosome formation. PLoS ONE 2014, 9, e105965. [Google Scholar] [CrossRef] [PubMed]
- Meyer, H.; Vitavska, O.; Wieczorek, H. Identification of an animal sucrose transporter. J. Cell Sci. 2011, 124, 1984–1991. [Google Scholar] [CrossRef] [PubMed]
- Wang, N.; Hebert, D.N. Tyrosinase maturation through the mammalian secretory pathway: Bringing color to life. Pigment Cell Res. 2006, 19, 3–18. [Google Scholar] [CrossRef] [PubMed]
- Sturm, R.A. Molecular genetics of human pigmentation diversity. Hum. Mol. Genet. 2009, 18, R9–R17. [Google Scholar] [CrossRef] [PubMed]
- Svedine, S.; Wang, T.; Halaban, R.; Hebert, D.N. Carbohydrates act as sorting determinants in er-associated degradation of tyrosinase. J. Cell Sci. 2004, 117, 2937–2949. [Google Scholar] [CrossRef] [PubMed]
- Burg, M.B. Molecular basis for osmoregulation of organic osmolytes in renal medullary cells. J. Exp. Zool. 1994, 268, 171–175. [Google Scholar] [CrossRef] [PubMed]
- Warskulat, U.; Brookmann, S.; Felsner, I.; Brenden, H.; Grether-Beck, S.; Haussinger, D. Ultraviolet a induces transport of compatible organic osmolytes in human dermal fibroblasts. Exp. Dermatol. 2008, 17, 1031–1036. [Google Scholar] [CrossRef] [PubMed]
- Warskulat, U.; Reinen, A.; Grether-Beck, S.; Krutmann, J.; Haussinger, D. The osmolyte strategy of normal human keratinocytes in maintaining cell homeostasis. J. Investig. Dermatol. 2004, 123, 516–521. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Perez, A.; Burg, M.B. Role of organic osmolytes in adaptation of renal cells to high osmolality. J. Membr. Biol. 1991, 119, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Mavrogonatou, E.; Kletsas, D. Differential response of nucleus pulposus intervertebral disc cells to high salt, sorbitol, and urea. J. Cell. Physiol. 2012, 227, 1179–1187. [Google Scholar] [CrossRef] [PubMed]
- Bin, B.H.; Bhin, J.; Yang, S.H.; Shin, M.; Nam, Y.J.; Choi, D.H.; Shin, D.W.; Lee, A.Y.; Hwang, D.; Cho, E.G.; et al. Membrane-associated transporter protein (MATP) regulates melanosomal ph and influences tyrosinase activity. PLoS ONE 2015, 10, e0129273. [Google Scholar] [CrossRef] [PubMed]
- Bin, B.H.; Joo, Y.H.; Lee, A.Y.; Shin, S.S.; Cho, E.G.; Lee, T.R. Novel inhibitory effect of N-(2-hydroxycyclohexyl)valiolamine on melanin production in a human skin model. Int. J. Mol. Sci. 2014, 15, 12188–12195. [Google Scholar] [CrossRef] [PubMed]
- Bin, B.H.; Seo, J.; Yang, S.H.; Lee, E.; Choi, H.; Kim, K.H.; Cho, E.G.; Lee, T.R. Novel inhibitory effect of the antidiabetic drug voglibose on melanogenesis. Exp. Dermatol. 2013, 22, 541–546. [Google Scholar] [CrossRef] [PubMed]
- Yun, E.J.; Lee, S.; Kim, J.H.; Kim, B.B.; Kim, H.T.; Lee, S.H.; Pelton, J.G.; Kang, N.J.; Choi, I.G.; Kim, K.H. Enzymatic production of 3,6-anhydro-l-galactose from agarose and its purification and in vitro skin whitening and anti-inflammatory activities. Appl. Microbiol. Biotechnol. 2013, 97, 2961–2970. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, R.; Takisada, M.; Suzuki, T.; Kirimura, K.; Usami, S. Neoagarobiose as a novel moisturizer with whitening effect. Biosci. Biotechnol. Biochem. 1997, 61, 162–163. [Google Scholar] [CrossRef] [PubMed]
- Nakayasu, M.; Saeki, H.; Tohda, H.; Oikawa, A. Effects of sugars on melanogenesis in cultured melanoma cells. J.Cell. Physiol. 1977, 92, 49–55. [Google Scholar] [CrossRef] [PubMed]
- Imokawa, G.; Mishima, Y. Importance of glycoproteins in the initiation of melanogenesis: An electron microscopic study of b-16 melanoma cells after release from inhibition of glycosylation. J. Investig. Dermatol. 1986, 87, 319–325. [Google Scholar] [CrossRef] [PubMed]
- Dmitrieva, N.I.; Burg, M.B. Hypertonic stress response. Mutat. Res. 2005, 569, 65–74. [Google Scholar] [CrossRef] [PubMed]
- Sougrat, R.; Morand, M.; Gondran, C.; Barre, P.; Gobin, R.; Bonte, F.; Dumas, M.; Verbavatz, J.M. Functional expression of AQP3 in human skin epidermis and reconstructed epidermis. J. Investig. Dermatol. 2002, 118, 678–685. [Google Scholar] [CrossRef] [PubMed]
- Warner, R.R.; Myers, M.C.; Taylor, D.A. Electron probe analysis of human skin: Determination of the water concentration profile. J. Investig. Dermatol. 1988, 90, 218–224. [Google Scholar] [CrossRef] [PubMed]
- Bright, N.A.; Reaves, B.J.; Mullock, B.M.; Luzio, J.P. Dense core lysosomes can fuse with late endosomes and are re-formed from the resultant hybrid organelles. J. Cell Sci. 1997, 110, 2027–2040. [Google Scholar] [PubMed]
- Bright, N.A.; Lindsay, M.R.; Stewart, A.; Luzio, J.P. The relationship between lumenal and limiting membranes in swollen late endocytic compartments formed after wortmannin treatment or sucrose accumulation. Traffic 2001, 2, 631–642. [Google Scholar] [CrossRef] [PubMed]
- Wasmeier, C.; Hume, A.N.; Bolasco, G.; Seabra, M.C. Melanosomes at a glance. J. Cell Sci. 2008, 121, 3995–3999. [Google Scholar] [CrossRef] [PubMed]
- Branza-Nichita, N.; Negroiu, G.; Petrescu, A.J.; Garman, E.F.; Platt, F.M.; Wormald, M.R.; Dwek, R.A.; Petrescu, S.M. Mutations at critical n-glycosylation sites reduce tyrosinase activity by altering folding and quality control. J. Biol. Chem. 2000, 275, 8169–8175. [Google Scholar] [CrossRef] [PubMed]
- Choi, H.; Ahn, S.; Chang, H.; Cho, N.S.; Joo, K.; Lee, B.G.; Chang, I.; Hwang, J.S. Influence of N-glycan processing disruption on tyrosinase and melanin synthesis in HM3KO melanoma cells. Exp. Dermatol. 2007, 16, 110–117. [Google Scholar] [CrossRef] [PubMed]
- Petrescu, S.M.; Petrescu, A.J.; Titu, H.N.; Dwek, R.A.; Platt, F.M. Inhibition of N-glycan processing in B16 melanoma cells results in inactivation of tyrosinase but does not prevent its transport to the melanosome. J. Biol. Chem. 1997, 272, 15796–15803. [Google Scholar] [CrossRef] [PubMed]
- Ujvari, A.; Aron, R.; Eisenhaure, T.; Cheng, E.; Parag, H.A.; Smicun, Y.; Halaban, R.; Hebert, D.N. Translation rate of human tyrosinase determines its N-linked glycosylation level. J. Biol. Chem. 2001, 276, 5924–5931. [Google Scholar] [CrossRef] [PubMed]
- Briganti, S.; Camera, E.; Picardo, M. Chemical and instrumental approaches to treat hyperpigmentation. Pigment Cell Res. 2003, 16, 101–110. [Google Scholar] [CrossRef] [PubMed]
- Ando, H.; Kondoh, H.; Ichihashi, M.; Hearing, V.J. Approaches to identify inhibitors of melanin biosynthesis via the quality control of tyrosinase. J. Investig. Dermatol. 2007, 127, 751–761. [Google Scholar] [CrossRef] [PubMed]
- Imokawa, G.; Mishima, Y. Loss of melanogenic properties in tyrosinases induced by glucosylation inhibitors within malignant melanoma cells. Cancer Res. 1982, 42, 1994–2002. [Google Scholar] [PubMed]
- Horii, S.; Fukase, H.; Matsuo, T.; Kameda, Y.; Asano, N.; Matsui, K. Synthesis and α-d-glucosidase inhibitory activity of N-substituted valiolamine derivatives as potential oral antidiabetic agents. J. Med. Chem. 1986, 29, 1038–1046. [Google Scholar] [CrossRef] [PubMed]
- Bin, B.H.; Kim, S.T.; Bhin, J.; Byoun, K.; Lee, T.R.; Cho, E.G. Synergistic effect of maltose enhances the anti-melanogenic activity of acarbose. Unpublished work. 2016. [Google Scholar]
- Kamaraj, B.; Purohit, R. Mutational analysis of oculocutaneous albinism: A compact review. Biomed Res. Int. 2014, 2014, 905472. [Google Scholar] [CrossRef] [PubMed]
- Gronskov, K.; Ek, J.; Brondum-Nielsen, K. Oculocutaneous albinism. Orphanet J. Rare Dis. 2007, 2, 43–51. [Google Scholar] [CrossRef] [PubMed]
- Li, H.Y.; Duan, H.L.; Zheng, H. A new form of oculocutaneous albinism, OCA4. Yi Chuan 2006, 28, 1149–1152. [Google Scholar] [CrossRef] [PubMed]
- Newton, J.M.; Cohen-Barak, O.; Hagiwara, N.; Gardner, J.M.; Davisson, M.T.; King, R.A.; Brilliant, M.H. Mutations in the human orthologue of the mouse underwhite gene (UW) underlie a new form of oculocutaneous albinism, oca4. Am. J. Hum. Genet. 2001, 69, 981–988. [Google Scholar] [CrossRef] [PubMed]
- Bartolke, R.; Heinisch, J.J.; Wieczorek, H.; Vitavska, O. Proton-associated sucrose transport of mammalian solute carrier family 45: An analysis in saccharomyces cerevisiae. Biochem. J. 2014, 464, 193–201. [Google Scholar] [CrossRef] [PubMed]
- Setty, S.R.; Tenza, D.; Sviderskaya, E.V.; Bennett, D.C.; Raposo, G.; Marks, M.S. Cell-specific atp7a transport sustains copper-dependent tyrosinase activity in melanosomes. Nature 2008, 454, 1142–1146. [Google Scholar] [CrossRef] [PubMed]
- Martinez, J.H.; Solano, F.; Garcia-Borron, J.C.; Iborra, J.L.; Lozano, J.A. The involvement of histidine at the active site of harding-passey mouse melanoma tyrosinase. Biochem. Int. 1985, 11, 729–738. [Google Scholar] [PubMed]
- Ancans, J.; Tobin, D.J.; Hoogduijn, M.J.; Smit, N.P.; Wakamatsu, K.; Thody, A.J. Melanosomal ph controls rate of melanogenesis, eumelanin/phaeomelanin ratio and melanosome maturation in melanocytes and melanoma cells. Exp. Cell Res. 2001, 268, 26–35. [Google Scholar] [CrossRef] [PubMed]



© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bin, B.-H.; Kim, S.T.; Bhin, J.; Lee, T.R.; Cho, E.-G. The Development of Sugar-Based Anti-Melanogenic Agents. Int. J. Mol. Sci. 2016, 17, 583. https://doi.org/10.3390/ijms17040583
Bin B-H, Kim ST, Bhin J, Lee TR, Cho E-G. The Development of Sugar-Based Anti-Melanogenic Agents. International Journal of Molecular Sciences. 2016; 17(4):583. https://doi.org/10.3390/ijms17040583
Chicago/Turabian StyleBin, Bum-Ho, Sung Tae Kim, Jinhyuk Bhin, Tae Ryong Lee, and Eun-Gyung Cho. 2016. "The Development of Sugar-Based Anti-Melanogenic Agents" International Journal of Molecular Sciences 17, no. 4: 583. https://doi.org/10.3390/ijms17040583
APA StyleBin, B.-H., Kim, S. T., Bhin, J., Lee, T. R., & Cho, E.-G. (2016). The Development of Sugar-Based Anti-Melanogenic Agents. International Journal of Molecular Sciences, 17(4), 583. https://doi.org/10.3390/ijms17040583