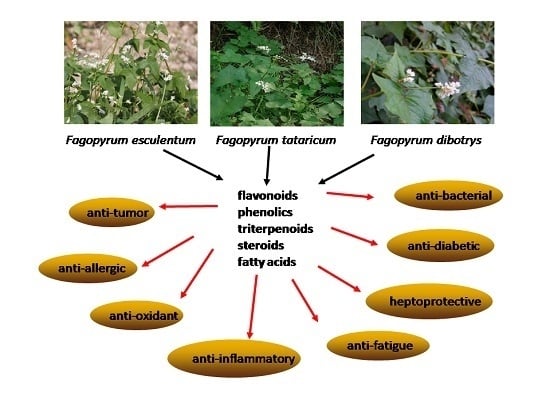
Phytochemical and Pharmacological Profiles of Three Fagopyrum Buckwheats
Abstract
:1. Introduction
2. Phytochemicals
2.1. Flavonoids
2.2. Phenolics
2.3. Fagopyritol
2.4. Triterpenoids
2.5. Steroids
2.6. Fatty Acid
2.7. Volatile Compounds
2.8. Other Compounds
3. Pharmacological Properties
3.1. Antitumor Activity
3.2. Anti-Oxidant Activity
3.3. Anti-Inflammatory Activity
3.4. Heptoprotective Activity
3.5. Anti-Diabetic Activity
3.6. Antibacterial Activity
3.7. Anti-Allergic Activity
3.8. Anti-Fatigue Activity
3.9. Other Activities
4. Conclusions
Acknowledgments
Conflicts of Interest
References
- Li, A.R. “Fagopyrum” Flora of China; Science Press and the Missouri Botanical Garden Press: Beijing, China, 2003. [Google Scholar]
- Inglett, G.E.; Chen, D.; Berhow, M.; Lee, S. Antioxidant activity of commercial buckwheat flours and their free and bound phenolic compositions. Food Chem. 2011, 125, 923–929. [Google Scholar] [CrossRef]
- Kim, S.J.; Zaidul, I.S.M.; Suzuki, T.; Mukasa, Y.; Hashimoto, N.; Takigawa, S.; Takahiro, N.; Chie, M.-E.; Hiroaki, Y. Comparison of phenolic compositions between common and tartary buckwheat (Fagopyrum) sprouts. Food Chem. 2008, 110, 814–820. [Google Scholar] [CrossRef] [PubMed]
- Wijngaard, H.H.; Arendt, E.K. Buckwheat. Cereal Chem. 2006, 83, 391–401. [Google Scholar] [CrossRef]
- Javornik, B.; Eggum, B.O.; Kreft, I. Studies on protein fractions and protein quality of buckwheat. Genetika 1981, 13, 115–121. [Google Scholar]
- Jiang, P.; Burczynski, F.; Campbell, C.; Pierce, G.; Austria, J.A.; Briggs, C.J. Rutin and flavonoid contents in three buckwheat species Fagopyrum esculentum, F. Tataricum, and F. Homotropicum and their protective effects against lipid peroxidation. Food Res. Int. 2007, 40, 356–364. [Google Scholar] [CrossRef]
- Nina, F.; Janko, R.; Jože, K.I.; Wang, Z.; Zhang, Z.; Ivan, K. Tartary buckwheat (Fagopyrum tataricum gaertn.) as a source of dietary rutin and quercitrin. J. Agric.Food Chem. 2003, 51, 6452–6455. [Google Scholar]
- Chai, Y.; Feng, B.L.; Hu, Y.G. Analysis on the variation of rutin content in different buckwheat genotypes. In Proceedings of the Ninth International Symposium on Buckwheat, Prague, Czech Republic, 18–22 August 2004; pp. 688–691.
- Zheng, C.J.; Hu, C.L.; Ma, X.Q.; Peng, C.; Zhang, H.; Qin, L.P. Cytotoxic phenylpropanoid glycosides from Fagopyrum tataricum (L.) gaertn. Food Chem. 2012, 132, 433–438. [Google Scholar] [CrossRef] [PubMed]
- Guo, X.; Zhu, K.; Zhang, H.; Yao, H. Anti-tumor activity of a novel protein obtained from tartary buckwheat. Int. Mol. Sci. 2010, 11, 5201–5211. [Google Scholar] [CrossRef] [PubMed]
- Sun, B.H.; Wu, Y.Q.; Gao, H.Y.; Huang, J.; Wu, L.J. Chemical constituents of Fagopyrum tataricum (L.) gaertn. J. Shenyang Pharm. Univ. 2008, 25, 541–543. [Google Scholar]
- Kim, C.D.; Lee, W.K.; No, K.O.; Park, S.K.; Lee, M.H.; Lim, S.R.; Roh, S.S. Anti-allergic action of buckwheat (Fagopyrum esculentum moench) grain extract. Int. Immunopharmacol. 2003, 3, 129–136. [Google Scholar] [CrossRef]
- Association, B.H.M. British Herbal Pharmacopoeia; Bournemouth: London, UK, 1990; Volume 1. [Google Scholar]
- Guo, X.; Zhu, K.; Zhang, H.; Yao, H. Purification and characterization of the antitumor protein from chinese tartary buckwheat (Fagopyrum tataricum gaertn.) water-soluble extracts. J. Agric.Food Chem. 2007, 55, 6958–6961. [Google Scholar] [CrossRef] [PubMed]
- Yao, Y.; Shan, F.; Bian, J.; Chen, F.; Wang, M.; Ren, G. d-Chiro-inositol-enriched tartary buckwheat bran extract lowers the blood glucose level in KK-Ay mice. J. Agric. Food Chem. 2008, 56, 10027–10031. [Google Scholar] [CrossRef] [PubMed]
- Tomotake, H.; Yamamoto, N.; Yanaka, N.; Ohinata, H.; Yamazaki, R.; Kayashita, J.; Kato, N. High protein buckwheat flour suppresses hypercholesterolemia in rats and gallstone formation in mice by hypercholesterolemic diet and body fat in rats because of its low protein digestibility. Nutrition 2006, 22, 166–173. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.L.; Chen, Y.S.; Yang, J.H.; Chiang, B.H. Antioxidant activity of tartary (Fagopyrum tataricum L.Gaertn.) and common (Fagopyrum esculentum moench) buckwheat sprouts. J. Agric. Food Chem. 2008, 56, 173–178. [Google Scholar] [CrossRef] [PubMed]
- Ren, W.; Qiao, Z.; Wang, H.; Zhu, L.; Zhang, L.; Lu, Y.; Cui, Y.; Zhang, Z.; Wang, Z. Tartary buckwheat flavonoid actvates caspase 3 and induces HL-60 cell apoptosis. Methods Find. Exp. Clin. Pharmacol. 2001, 23, 427–432. [Google Scholar] [CrossRef] [PubMed]
- Guo, X.N.; Yao, H.Y. Isolation, purification and structure analysis of antitumor protein from tartary buckwheat. Food Sci. 2007, 28, 462–465. [Google Scholar]
- Guo, Z.J.; Wang, J.X.; Su, Y.F.; Wei, Y.X.; Zhu, Y.H. Shaanxi qiyao; Shaanxi Science and Technology Press: Shaanxi, China, 2003; Volume 49. [Google Scholar]
- Grieve, M. A Modern Herbal; Dover Publications: New York, NY, USA, 1971. [Google Scholar]
- Maas, E.V. The Handbook of Plant Science in Agriculture: Salt Tolerance of Plants; CRC Press: Boca Raton, FL, USA, 1984. [Google Scholar]
- Manandhar, N.P.; Manandhar, S. Plants and People of Nepal; Timber Press: London, UK, 2002. [Google Scholar]
- Facciola, S. A Source Book of Edible Plants; Kampong Public: Vista, CA, USA, 1990; Volume 677. [Google Scholar]
- Chan, P.K. Inhibition of tumor growth in vitro by the extract of Fagopyrum cymosum (fago-c). Life Sci. 2003, 72, 1851–1858. [Google Scholar] [CrossRef]
- Gao, Z.; Meng, F. Effect of Fagopyrum cymosum rootin on clonal formation of four human tumor cells. China J. Chin. Mater. Med. 1993, 18, 498–500. [Google Scholar]
- Editorial Board of Zhong Hua Ben Cao of State Administration of Traditional Chinese Medicine. Zhong Hua Ben Cao; Shanghai Science and Technology Press: Shanghai, China, 1999; Volume 2. [Google Scholar]
- Sun, T.; Ho, C.T. Antioxidant activities of buckwheat extracts. Food Chem. 2005, 90, 743–749. [Google Scholar] [CrossRef]
- Choi, I.; Seog, H.; Park, Y.; Kim, Y.; Choi, H. Suppressive effects of germinated buckwheat on development of fatty liver in mice fed with high-fat diet. Phytomedicine 2007, 14, 563–567. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Qi, H.X.; Gu, B.B.; Huang, W.; Zhao, H.G. The therapeutic effects of tartarian buckwheat protein extracts on 2 type diabetic rats. Zhejiang J. Prev. Med. 2009, 21, 4–6. [Google Scholar]
- Hafeez, B.B.; Adhami, V.M.; Asim, M.; Siddiqui, I.A.; Bhat, K.M.; Zhong, W.; Saleem, M.; Din, M.; Setaluri, V.; Mukhtar, H. Targeted knockdown of Notch1 inhibits invasion of human prostate cancer cells concomitant with inhibition of matrix metalloproteinase-9 and urokinase plasminogen activator. Clin. Cancer Res. 2009, 15, 452–459. [Google Scholar] [CrossRef] [PubMed]
- Cook, N.C.; Samman, S. Flavonoids-chemistry, metabolism, cardio protective effects, and dietary sources. J. Nutr. Biochem. 1996, 7, 66–76. [Google Scholar] [CrossRef]
- Wang, K.; Zhang, Y.; Yang, C. Antioxidant phenolic constituents from Fagopyrum dibotrys. J. Ethnopharmacol. 2005, 99, 259–264. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, M. Catechins as antioxidants from buckwheat (Fagopyrum esculentum moench) groats. J. Agric. Food Chem. 1998, 46, 839–845. [Google Scholar] [CrossRef]
- Takuo, O.; Kazuko, M.; Tsutomu, H. Relationship of the structures of tannins to the binding activities with hemoglobin and methylene blue. Chem. Pharm. Bull. 1985, 33, 1424–1433. [Google Scholar]
- Nobuko, K.; Masao, H.; Tsuneo, N.; Makoto, N.; Takashi, Y.; Takuo, O. Inhibitory effect of tannins on reverse transcriptase from RNA tumor virus. J. Nat. Prod. 1985, 48, 614–621. [Google Scholar]
- Barbehenna, R.V.; Constabel, C.P. Tannins in plant-herbivore interactions. Phytochemistry 2011, 72, 1551–1565. [Google Scholar] [CrossRef] [PubMed]
- Bernadetta, K.; Zuzana, M. Prophylactic components of buckwheat. Food Res. Int. 2005, 38, 561–568. [Google Scholar]
- Dorota, D.-S.; Wieslaw, O. Effect of processing on the flavonoid content in buckwheat (Fagopyrum esculentum moench) grain. J. Agric. Food Chem. 1999, 47, 4383–4387. [Google Scholar]
- Watanabe, M.; Ohshita, Y.; Tsushida, T. Antioxidant compounds from buckwheat (Fagopyrum esculentum möench) hulls. J. Agric. Food Chem. 1997, 45, 1039–1044. [Google Scholar] [CrossRef]
- Saxena, V.K.; Samaiya, G.C. A new flavonoid from Fagopyrum tataricum. Fitoterapia 1987, 58, 283. [Google Scholar]
- Wang, L.B.; Shao, M.; Gao, H.Y.; Wu, B.; Wu, L.J. Study on bateriostasis of Fagopyrum cymosum meisn. Chin. J. Microecol. 2005, 17, 330–331. [Google Scholar]
- Lee, J.M.; Lee, K.H.; Yoon, Y.H.; Cho, E.J.; Lee, S. Identification of triterpenoids and flavonoids from the seeds of tartary buckwheat. Nat. Prod. Sci. 2013, 19, 137–144. [Google Scholar]
- Jiang, S.; Liu, Q.; Xie, Y.; Zeng, H.; Zhang, L.; Jiang, X.; Chen, X. Separation of five flavonoids from tartary buckwheat (Fagopyrum tataricum (L.) gaertn) grains via off-line two dimensional high-speed counter-current chromatography. Food Chem. 2015, 186, 153–159. [Google Scholar] [CrossRef] [PubMed]
- Ren, Q.; Wu, C.; Ren, Y.; Zhang, J. Characterization and identification of the chemical constituents from tartary buckwheat (Fagopyrum tataricum gaertn) by high performance liquid chromatography/photodiode array detector/linear ion trap FTICR hybrid mass spectrometry. Food Chem. 2013, 136, 1377–1389. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.Z.; Zhou, J.Y.; Pan, H.L. Study on chemical constituents of Fagopyrum dibotrys (D. Don) Hara. Chin. J. Hosp. Pharm. 2008, 28, 21–26. [Google Scholar]
- Kalinova, J.; Vrchotova, N. Level of catechin, myricetin, quercetin and isoquercitrin in buckwheat (Fagopyrum esculentum moench), changes of their levels during vegetation and their effect on the growth of selected weeds. J. Agric. Food Chem. 2009, 57, 2719–2725. [Google Scholar] [CrossRef] [PubMed]
- Shao, M.; Yang, Y.H.; Gao, H.Y.; Wu, B.; Wang, L.B.; Wu, L.J. Studies on the chemical constituents of Fagopyrum dibotrys. J. Shenyang Pharm. Univ. 2005, 22, 100–102. [Google Scholar]
- Bao, T.N.; Peng, S.L.; Zhou, Z.Z.; Li, B.G. Chemical constituents of Fagopytum tataricum (Linn) gaertn. Nat. Prod. Res. Dev. 2003, 15, 24–26. [Google Scholar]
- Bai, Z.Z.; Zhang, X.H.; Xuan, L.J.; Mo, F.K. A phenolic glycoside from Fagopyrum dibotrys (D. Don) Hara. Chin. Chem. Lett. 2007, 18, 1087–1088. [Google Scholar] [CrossRef]
- Tian, L.; Xu, L.Z. Studies on the chemical constituents of the aerial parts of Fagopyrum dibotrys. China J. Chin. Mater. Med. 1997, 22, 743–745. [Google Scholar]
- Xu, B.; Xiao, G.; Ding, X. Determination of phenolic acids and proanthocyanidin in buckwheat (Fagopytum tataricum (Linn) Gaench). Food Ferment. Ind. 2002, 28, 32–37. [Google Scholar]
- Obendorf, R.L.; Steadman, K.J.; Fuller, D.J.; Horbowicz, M.; Lewisc, B.A. Molecular structure of fagopyritol A1(O-α-d-galactopyranosyl-(1→3)-d-chiro-inositol) by NMR. Carbohydr. Res. 2000, 328, 623–627. [Google Scholar] [CrossRef]
- Steadmana, K.J.; Fullerb, D.J.; Obendorfa, R.L. Purification and molecular structure of two digalactosyl d-chiro-inositols and two trigalactosyl d-chiro-inositols from buckwheat seeds. Carbohydr. Res. 2001, 331, 19–25. [Google Scholar] [CrossRef]
- Obendorf, R.L. Oligosaccharides and galactosyl cyclitols in seed desiccation tolerance. Seed Sci. Res. 1997, 7, 63–74. [Google Scholar] [CrossRef]
- Zheng, F.; Song, Q.B.; Qiang, G.R.; Sun, P.L.; Zhu, X.Y.; Chen, D.X. GC/MS analysis of fatty acid in Fagopyrum esculentum seed oil. Food Sci. 2004, 25, 267–269. [Google Scholar]
- Cho, J.Y.; Moon, J.H.; Kim, H.K.; Ma, S.J.; Kim, S.J.; Jang, M.Y.; Kswazoe, K.; Takaishi, Y.; Park, K.H. Isolation and structural elucidation of antimicrobial compounds from buckwheat hull. J. Microbiol. Biotechnol. 2006, 16, 538–542. [Google Scholar]
- Wang, G.; Zhou, L.; Liang, R.; Geng, H. Effect of different extraction conditions on rutin hydrolysis of Fagopyrum tataricum seeds. Acta Bot. Boreali-Occident. Sin. 2005, 25, 1035–1038. [Google Scholar]
- Reena, R.; Lin, Y.T.; Shetty, K. Phenolics, their antioxidant and antimicrobial activity in dark germinated fenugreek sprouts in response to peptide and phytochemical elicitors. Asia Pac. J. Clin. Nutr. 2004, 13, 295–307. [Google Scholar]
- Balasundram, N.; Sundram, K.; Samman, S. Phenolic compounds in plants and agri-industrial by-products: Antioxidant activity, occurrence, and potential uses. Food Chem. 2006, 99, 191–203. [Google Scholar] [CrossRef]
- Horbowicz, M.; Brenac, P.; Obendorf, R.L. Fagopyritol B1, O-α-d-gal-(1→2)-d-chiro-inositol, a galactosyl cyclitol in maturing buckwheat seeds associated with desiccation tolerance. Planta 1998, 205, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Larner, J.; Huang, L.C.; Schwartz, C.F.W.; Oswald, A.S.; Shen, T.-Y.; Kinter, M.; Tang, G.; Zeller, K. Rat liver insulin mediator which stimulates pyruvate dehydrogenase phosphatase contains galactosamine and d-chiroinositol. Biochem. Biophys. Res. Commun. 1988, 151, 1416–1426. [Google Scholar] [CrossRef]
- Liu, Y.; Fang, Q.; Zhang, X.; Feng, X.; Zhang, L.; He, X. Study on the effective compounds of Fagopyrum dibotrys. Acta Pharm. Sin. 1983, 18, 545–547. [Google Scholar]
- Damjan, J.; Dragana, K.; Samo, K.; Helena, P. Identification of buckwheat (Fagopyrum esculentum moench) aroma compounds with GC–MS. Food Chem. 2009, 112, 120–124. [Google Scholar]
- Zhou, Q.; Wintersteen, C.L.; Cadwallader, K.R. Identification and quantification of aroma-active components that contribute to the distinct malty flavor of buckwheat honey. J. Agric. Food Chem. 2002, 50, 2016–2021. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Gu, Z.; Liang, Z. Antitemor effect of the extract of Fagopyrum cymosum. Acta Acad. Med. Suzhou 2001, 1, 23–25. [Google Scholar]
- Chen, J.; Gu, Z.; Liang, Z.; Zhou, W.e.; Guo, C. Study on anti-tumor efect of Fagopyrum cymosum Fr4. Chin. Wild Plant Resour. 2002, 21, 48–50. [Google Scholar]
- Chen, J.; Gu, Z.; Liang, Z.; Zhou, W.; Guo, C. Toxic effects of cyclophosphamide tumor suppression and antagonism of Fagopyrum cymosum Fr4. Chin. Tradit. Herbal Drugs 2003, 34, 938–940. [Google Scholar]
- Chen, X.F.; Gu, Z.L.; Yang, H.H.; Liang, Z.Q. Effect of MMP-9, TIMP-1 protein expression in Lewis lung cancer cell of Fagopyrum cymosum fh in mice. Suzhou Univ. J. Med. Sci. 2005, 25, 383–386. [Google Scholar]
- Kayashita, J.; Shimaoka, I.; Nakajoh, M.; Kishida, N.; Kato, N. Consumption of a buckwheat protein extract retards 7,12-dimethylbenz[α] anthracene-induced mammary carcinogenesis in rats. Biosci. Biotechnol. Biochem. 1999, 63, 1837–1839. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Ishikawa, W.; Huang, X.; Tomotake, H.; Kayashita, J.; Watanabe, H.; Kato, N. A buckwheat protein product suppresses 1,2-dimethylhydrazine-induced colon carcinogenesis in rats by reducing cell proliferation. J. Nutr. 2001, 131, 1850–1853. [Google Scholar] [PubMed]
- Chen, X.F.; Gu, Z.L.; Yang, H.H.; Liang, Z.Q.; Zhu, M.; Chen, B.G. Fagopyrum cymosum Fr4 induce HL-60 apoptosis and influence telomerase activity. J. China Pharmacol. Bull. 2006, 22, 836–840. [Google Scholar]
- Shen, M.; Chapman, R.S.; He, X.; Liu, L.Z.; Lai, H.; Chen, W.; Lan, Q. Dietary factors, food contamination and lung cancer risk in Xuanwei, China. Lung Cancer 2008, 61, 275–282. [Google Scholar] [CrossRef] [PubMed]
- Liu, F.; Lv, Y.; Ye, K.; Zhao, J.; Ye, C. Effect of FR/MA on invasion of HepG2 human hepatocellular liver carcinoma cell line in vitro. J. Pract. Oncol. 2009, 24, 337–340. [Google Scholar]
- Zhao, T.; Li, S.; Cui, J.; Zhang, H. Extracts of Fagopyrum cymosum and Rosa roxburghii tratt inhibit proliferation and induce apoptosis of human gastric and pulmonary carcinoma cells. J. Pract. Oncol. 2009, 24, 542–547. [Google Scholar]
- Guo, X.N.; Yao, H.Y. Anti-proliferative effect of tartary buckwheat protein fraction TBWSP31 on breast cancer cells. Food Sci. 2010, 31, 317–320. [Google Scholar]
- Tian, X.; Li, C.; Li, Y.Y.; Wang, Z.H. Analysis of inhibitory activity and antineoplastic effect of wild type rBTI and its mutants. Prog. Biochem. Biophys. 2010, 37, 654–661. [Google Scholar] [CrossRef]
- Tian, X.; Li, Y.Y.; Wang, Z.H. Expression of buckwheat protease inhibitor (aBTI) and its antitumor efficacy against human hepatoma (HepG2) cells. Chin. J. Cell Biol. 2010, 32, 589–595. [Google Scholar]
- Zhang, H.; Li, S.Y.; Cui, J.J.; Zhao, T. Effects of extract from Rosa roxburghii tratt or extract of Fagopyrum cymosum meissn on cell proliferation and apoptosis in human esophageal carcinoma CaEs-17 cell line. J. Oncol. 2010, 16, 35–39. [Google Scholar]
- Guo, L.; Zhao, Z.; Han, S. Study on antioxidative and antitumor effect of extraction of buckwheat flower and leaf. China J. Exp. Tradit. Med. Formul. 2012, 18, 176–179. [Google Scholar]
- Sun, G.; Cui, T.; Jin, Q.; Li, X.; Li, S.; Cui, C. Cytotoxicity of different extract parts of buckwheat sprout. Food Sci. Technol. 2012, 10, 200–203. [Google Scholar]
- Guo, L.; Wu, A.; Zhao, Z.; Han, S. Effect of the extraction of buckwheat flower and leaf on immunity function of H22 tumor mice. Her. Med. 2013, 32, 1422–1424. [Google Scholar]
- Guo, L.; Zhao, Z.; Han, S. Study on the anticancer effect in vitro of extraction from buckwheat flower and leaf. LiShiZhen Med. Mater. Med. Res. 2013, 24, 1849–1851. [Google Scholar]
- Guo, L.; Zhao, Z.; Han, S. Studies on antitumor activities of extraction from buckwheat flower and leaf in vitro and its mechanism. Pharmacol. Clin. Chin. Mater. Med. 2013, 29, 50–52. [Google Scholar]
- Christel, Q.-D.; Gressier, B.; Vasseur, J.; Dine, T.; Brunet, C.; Luyckx, M.; Cazin, M.; Cazin, J.C.; Bailleul, F.; Trotin, F. Phenolic compounds and antioxidant activities of buckwheat (Fagopyrum esculentum moench) hulls and Flour. J. Ethnopharmacol. 2000, 72, 35–42. [Google Scholar]
- Cai, Y.Z.; Sun, M.; Xing, J.; Luo, Q.; Corke, H. Structure-radical scavenging activity relationships of phenolic compounds from traditional chinese medicinal plants. Life Sci. 2006, 78, 2872–2888. [Google Scholar] [CrossRef] [PubMed]
- Şensoy, Í.; Rosen, R.T.; Ho, C.-T.; Karwe, M.V. Effect of processing on buckwheat phenolics and antioxidant activity. Food Chem. 2006, 99, 388–393. [Google Scholar] [CrossRef]
- Cao, W.; Chen, W.J.; Suo, Z.R.; Yao, Y.P. Protective effects of ethanolic extracts of buckwheat groats on DNA damage caused by hydroxyl radicals. Food Res. Int. 2008, 41, 924–929. [Google Scholar] [CrossRef]
- Yang, J.; Guo, J.; Yuan, J. In vitro antioxidant properties of rutin. LWT—Food Sci. Technol. 2008, 41, 1060–1066. [Google Scholar] [CrossRef]
- Tang, C.H.; Peng, J.; Zhen, D.W.; Chen, Z. Physicochemical and antioxidant properties of buckwheat (Fagopyrum esculentum moench) protein hydrolysates. Food Chem. 2009, 115, 672–678. [Google Scholar] [CrossRef]
- Inglett, G.E.; Rose, D.J.; Chen, D.; Stevenson, D.G.; Biswas, A. Phenolic content and antioxidant activity of extracts from whole buckwheat (Fagopyrum esculentum möench) with or without microwave irradiation. Food Chem. 2010, 119, 1216–1219. [Google Scholar] [CrossRef]
- Guo, X.D.; Ma, Y.J.; Parry, J.; Gao, J.M.; Yu, L.L.; Wang, M. Phenolics content and antioxidant activity of tartary buckwheat from different locations. Molecules 2011, 16, 9850–9867. [Google Scholar] [CrossRef] [PubMed]
- Hęś, M.; Górecka, D.; Dziedzic, K. Antioxidant properties of extracts from buckwheat by-products. Acta Sci. Pol. Technol. Aliment. 2012, 11, 167–174. [Google Scholar]
- Ishii, S.; Katsumura, T.; Shiozuka, C.; Ooyauchi, K.; Kawasaki, K.; Takigawa, S.; Fukushima, T.; Tokuji, Y.; Kinoshita, M.; Ohnishi, M.; et al. Anti-inflammatory effect of buckwheat sprouts in lipopolysaccharide-activated human colon cancer cells and mice. Biosci. Biotechnol. Biochem. 2008, 72, 3148–3157. [Google Scholar] [CrossRef] [PubMed]
- Bao, P.; Zhang, X.; Zhou, X.; Ding, C.; Liang, X.; Li, S. Pharmacodynamic research of Fagopyrum dibotrys (D. Don) Hara extract. Mod. Chin. Med. 2009, 11, 36–41. [Google Scholar]
- Cheng, Y.; Pan, H. Study on the anti-inflammatory active site of rhizoma Fagopyrum dibotrys. Lishizhen Med. Mater. Med. Res. 2009, 20, 2219–2220. [Google Scholar]
- Liu, L.; Sun, Z.; Lu, Y.; Shao, M.; Yan, J.; Chen, G.; Shi, H. The pharmacodynamic study on the effects of antinociception and anti-inflammation of the extracts of Fagopyrum cymosum (Trev.) Meisn. Med. Inf. 2012, 25, 49–50. [Google Scholar]
- Jun Kayashita, M.S.; Iwao Shimaoka, M.S.; Misao Nakajyoh, M.S. Hypocholesterolemic effect of buckwheat protein extract in rats fed cholesterol enriched diets. Nutr. Res. 1995, 15, 691–698. [Google Scholar] [CrossRef]
- Kayashita, J.; Shimaoka, I.; Nakajoh, M.; Yamazaki, M.; Kato, N. Consumption of buckwheat protein lowers plasma cholesterol and raises fecal neutral sterols in cholesterol-fed rats because of its low digestibility. J. Nutr. 1997, 127, 1395–1400. [Google Scholar] [PubMed]
- Tomotake, H.; Shimaoka, I.; Kayashita, J.; Yokoyama, F.; Nakajoh, M.; Kato, N. A buckwheat protein product suppresses gallstone formation and plasma cholesterol more strongly than soy protein isolate in hamsters. J. Nutr. 2000, 130, 1670–1674. [Google Scholar] [PubMed]
- Shu, C.; Qiu, J.; Wang, G. Effect of the seed extract of Fagopyrum tartaricum on the liver acutely injured by chemicals in mice. Her. Med. 2005, 24, 880–882. [Google Scholar]
- Kuwabara, T.; Han, K.-H.; Hashimoto, N.; Yamauchi, H.; Shimada, K.I.; Sekikawa, M.; Fukushima, M. Tartary buckwheat sprout powder lowers plasma cholesterol level in rats. J. Nutr. Sci. Vitaminol. 2007, 53, 501–507. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.C.; Shen, S.R.; Lai, Y.J.; Wu, S.C. Rutin and quercetin, bioactive compounds from tartary buckwheat, prevent liver inflammatory injury. Food Funct. 2013, 4, 794–802. [Google Scholar] [CrossRef] [PubMed]
- Qin, W.; Lun, C. The clinical observation of buckwheat for the treatment of diabetes. Chin. J. Endocrinol. Metab. 1992, 8, 52–53. [Google Scholar]
- Gao, T. Study of complex Fagopyrum tataricum prescription and its apart group on non-insulin-dependent diabetes mellitus rats. Mod. J. Integr. Tradit. Chin. West. Med. 2002, 11, 2209–2211. [Google Scholar]
- Lu, M.; Zhang, H.; Zhang, Y.; Tong, W.; Zhao, Y.; Shan, S.; Liu, H. An epidemiological study on relationship between buckwheat in diet and the prevalence of diabetes mellitus as blood glucose concentration. Mod. Prev. Med. 2002, 29, 326–327. [Google Scholar]
- Cao, W.; Zhang, Y.; Su, Y. Study on extracting of the d-chiro-inositol in Fagopyrium tartarian and curative of the extraction on diabetes. Cereals Oils 2006, 1, 22–24. [Google Scholar]
- Sheng, Y.T.; Xu, F.; Ruan, Q.X. Effects of tartary wheet flavone on neurofunction in diabetic rats. Pract. Pharm. Clin. Remedies 2006, 9, 219–221. [Google Scholar]
- Han, G.; Yao, G.; Lin, Q.; Zhai, G.; Fan, Y. Effect of extracts of buckwheat seed on blood glucose in type 2 diabetes mellitus rat. Mod. Preve. Med. 2008, 35, 4677–4678. [Google Scholar]
- Liu, R.; Wang, Y.; Guo, H.; Jia, S.; Hu, Y. Study on the effect of buckwheat protein in lowering blood glucose of diabetic mice. J. Jilin Agric. Univ. 2009, 31, 102–104. [Google Scholar]
- Li, G.; Chu, J.; Han, S. Role and mechanism of rutin from buckwheat flowers and leaves on metabolism of glucose and lipids. West China J. Pharm. Sci. 2010, 25, 426–428. [Google Scholar]
- Li, G.; Chu, J.; Han, S. Protective effect of rutin from buckwheat flowers and leaves on hepatic injury at early stage in diabetic rats. Jiangsu Med. J. 2010, 36, 935–937. [Google Scholar]
- Tian, Y.; Zhang, J.; Wang, M. Treatment effects on 80 cases of pneumonia in the elderly of Fagopyrum dibotrys. Chin. J. Geriatr. Care 2007, 5, 26. [Google Scholar]
- Dong, L.; Wang, C.; Wu, C.; Jiang, Q.; Zhang, Z. Effect of jinqiaomai on expression of TLR2/4, MyD88 mRNA and IκB-α in lung tissue of rats with Klebsiellla pneumonia. China J. Chin. Med. 2011, 36, 200–204. [Google Scholar]
- Dong, L.; Wang, C.; Wu, C.; Jiang, Q.; Zhang, Z. Protection and mechanism of Fagopyrum cymosum on lung injury in rats with Klebsiellla pneumonia. J. Chin. Med. Mater. 2012, 35, 603–607. [Google Scholar]
- Chaouloff, F.; Elghozi, J.L.; Guezennec, Y.; Laude, D. Effect of conditioned running on plasma, liver and brain tryptopgan and on brain 5-hydroxytryptamine metabolism of the rat. Br. J. Pharmacol. 1985, 86, 33–41. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Lu, Y.; Guo, G.X.; Zhang, H. Studies on antifatigue of buckwheat protein. J. Food Sci. Biotechnol. 2005, 24, 78–82. [Google Scholar]
- Wang, J.Q.; Wang, Z.X.; Huang, J.; Sun, B.H.; Gao, H.Y.; Wu, L.J. Chemical constituents from the seeds of Fagopyrum tataricum (L.) gaertn. J. Shenyang Pharm Univ. 2009, 26, 270–273. [Google Scholar]
- Hai, Q.; Liu, O. The antidiabetics effect of rats by pumpkin and buckwheat mixed feeding. J. Med. Forum 2011, 32, 84–85. [Google Scholar]
- Lai, Y.; Xiao, H.; Huan, Z. The study on the sleep effect and the spontaneous motion of Esculentum Polyaccharide in mice. J. Gannan Med. Univ. 2009, 29, 5–6. [Google Scholar]
- Urszula, G.D.; Dziki, D.; Baraniak, B.; Lin, R. The effect of simulated digestion in vitro on bioactivity of wheat bread with tartary buckwheat flavones addition. LWT-Food Sci. Technol. 2009, 42, 137–143. [Google Scholar]
- Yokozawa, T.; Kim, H.; Nonaka, G.; Kosuna, K. Buckwheat extract inhibits progression of renal failure. J. Agric. Food Chem. 2002, 50, 3341–3345. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.; Yi, L.; Ding, T.; Wang, J.; Chen, W. Wei Mai Ning capsule combined with radiotherapy for elderly patients with advanced non-small cell lung cancer. Pract. J. Cancer 2013, 28, 668–670. [Google Scholar]








| No. | Compounds | Source | Reference |
|---|---|---|---|
| Flavonoids | |||
| 1 | orientin | Fe, Ft | [3] |
| 2 | isoorientin | Fe, Ft | [3] |
| 3 | vitexin | Fe, Ft | [3] |
| 4 | isovitexin | Fe, Ft | [3] |
| 5 | quercetin | Fd, Fe, Ft | [33,40,42,43,44] |
| 6 | 3-methylquercetin | Fd | [33] |
| 7 | 3,5-dimethylquercetin | Fd | [33] |
| 8 | rutin | Fd, Fe, Ft | [33,40] |
| 9 | quercetin-3-O-(2′′-O-p-hydroxy-coumaroyl)-glucoside | Fd | [33] |
| 10 | 5,7,3′,4′-tetramethylquercetine-3-O-rutinoside | Ft | [41] |
| 11 | quercetin-3-O-rutinoside-7-O-galactoside | Ft | [41] |
| 12 | quercitrin (quercetin-3-O-rhamnoside) | Fd, Ft | [33,45] |
| 13 | quercetin-3-O-rutinoside-3′-O-β-glucopyranoside | Fd, Ft | [46] |
| 14 | hyperin/isoquercitrin (quercetin-3-O-glucoside) | Fe | [40,45,47] |
| 15 | quercetin-3-O-β-d-galactoside | Ft, Fe | [40,45] |
| 16 | quercetin-3-O-[β-d-xyloxyl-(1→2)-α-l-rhamnoside] | Ft | [45] |
| 17 | myricetin | Fe | [47] |
| 18 | kaempferol | Fd, Ft | [43,44,48] |
| 19 | kaempferol-3-O-glucoside | Ft | [45] |
| 20 | kaempferol-3-O-galactoside | Ft | [45] |
| 21 | kaempferol-3-O-rutinoside | Ft | [42,44,49] |
| 22 | kaempferol-3-O-sophoroside | Fe | [40] |
| 23 | kaempferol-3-O-glucoside-7-O-glucoside | Fe | [40] |
| 24 | luteolin | Fd | [48] |
| 25 | 3-methylgossypetin-8-O-β-d-glucopyranoside | Fd | [33] |
| 26 | 3′,4′-methylenedioxy-7-hydroxy-6-isopentenyl flavone | Fd | [41] |
| 27 | (−)-epicatechin | Fe, Fd | [34] |
| 28 | (−)-epicatechin-3-O-p-hydroxybenzoate | Fe | [34] |
| 29 | (−)-epicatechin-3-O-(3,4-di-O-methyl)-gallate | Fe | [34] |
| 30 | (+)-catechin | Fd | [33] |
| 31 | (+)-catechin-7-O-glucoside | Fe | [34] |
| 32 | aromadendrin-3-O-d-galactoside | Fe | [40] |
| 33 | taxifolin-3-O-d-xyloside | Fe | [40] |
| 34 | hesperidin | Fd | [46] |
| 35 | rhamnetin | Fd | [46] |
| Phenolics | |||
| 36 | tatariside A | Ft | [9] |
| 37 | tatariside B | Ft | [9] |
| 38 | tatariside C | Ft | [9] |
| 39 | tatariside D | Ft | [9] |
| 40 | tatariside E | Ft | [9] |
| 41 | tatariside F | Ft | [9] |
| 42 | tatariside G | Ft | [9] |
| 43 | diboside A | Ft, Fd | [33] |
| 44 | lapathoside A | Fd | [33] |
| 45 | 1,3,6-tri-p-coumaroyl-6′-feruloyl sucrose | Ft | [45] |
| 46 | 3,6-di-p-coumaroyl-1,6′-di-feruloyl sucrose | Ft | [45] |
| 47 | 1,3,6′-tri-feruloyl-6-p-coumaroyl sucrose | Ft | [45] |
| 48 | taroside (1,3,6,6′-tetra-feruloyl sucrose) | Ft | [45] |
| 49 | 1,3-dimethoxy-2-O-b-xylo-pyranosyl-5-O-β-glucopyranosyl-benzene | Fd | [50] |
| 50 | benzoic acid | Fd | [46] |
| 51 | gallic acid | Fd | [33] |
| 52 | p-hydroxybenzoic acid | Fd | [51] |
| 53 | syringic acid | Ft | [52] |
| 54 | vanillic acid | Ft | [52] |
| 55 | protocatechuic acid | Fd, Fe, Ft | [40,48] |
| 56 | protocatechuic acid methyl ester | Fd | [48] |
| 57 | 6-O-galloyl-d-glucose | Fd | [33] |
| 58 | 3,4-dihydroxybenzaldehyde | Fd, Fe | [40,48] |
| 59 | caffeic acid | Ft | [52] |
| 60 | ferulic acid | Ft | [52] |
| 61 | chlorogenic acid | Fe, Ft | [3] |
| 62 | p-coumaric acid | Ft | [52] |
| 63 | resveratrol | Fe | [40] |
| 64 | trans-p-hydroxy cinnamic methyl ester | Fd | [48] |
| Tannins | |||
| 65 | procyanidin B-1 | Fd | [33] |
| 66 | procyanidin B-2 | Fd | [33] |
| 67 | 3,3-di-O-galloyl-procyanidinB-2 | Fd | [33] |
| 68 | 3-O-galloyl-procyanidinB-2 | Fd | [33] |
| Cyclitol | |||
| 69 | fagopyritol A1 | Fe | [53] |
| 70 | fagopyritol A2 | Fe | [54] |
| 71 | fagopyritol A3 | Fe | [54] |
| 72 | fagopyritol B1 | Fe | [53] |
| 73 | fagopyritol B2 | Fe | [55] |
| 74 | fagopyritol B3 | Fe | [55] |
| Triterpenoids | |||
| 75 | ursolic acid | Ft | [42,43] |
| 76 | olean-12-en-3-ol | Fe | [56] |
| 77 | urs-12-en-3-ol | Fe | [56] |
| 78 | glutinone | Fd | [48] |
| 79 | glutinol | Fd | [48] |
| Steroids | |||
| 80 | β-sitosterol | Ft | [43,49] |
| 81 | β-sitosterol-palmitate | Ft | [49] |
| 82 | peroxidize-ergosterol | Ft | [49] |
| 83 | daucosterol | Ft | [43,49] |
| 84 | 6-hydroxystigmasta-4,22-dien-3-one | Fe | [56] |
| 85 | 23S-methylcholesterol | Fe | [56] |
| 86 | stigmast-5-en-3-ol | Fe | [56] |
| 87 | stigmast-5,24-dien-3-ol | Fe | [56] |
| 88 | trans-stigmast-5,22-dien-3-ol | Fe | [56] |
| 89 | stigmsat-4-en -3,6-dione | Ft | [49] |
| Fatty Acids | |||
| 90 | 6,7-dihydroxy-3,7-dimethyl-octa-2(Z),4(E)-dienoic acid | Fe | [57] |
| 91 | 6,7-dihydroxy-3,7-dimethyl-octa-2(E),4(E)-dienoic acid | Fe | [57] |
| 92 | 4,7-dihydroxy-3,7-dimethyl-octa-2(E),5(E)-dienoic acid | Fe | [57] |
| Others | |||
| 93 | uracil | Ft | [49] |
| 94 | (3-methoxyphenyl)-2-piperidinemethanol | Fd | [48] |
| 95 | N-trans-feruloyltyramine | Ft | [45] |
| 96 | succinic acid | Fd | [51] |
| 97 | 3, 4-dihydroxy benzamine | Fd | [48] |
| 98 | emodin | Fd | [49] |
| 99 | emodin-8-O-β-d-glucopyranoside | Fd | [33] |
| 100 | 5, 5′-di-α-furaldehyde dimethyl ester | Ft | [51] |
| 101 | 7-hydroxycoumarin | Ft | [11] |
| 102 | n-butyl-β-d-fructopyranoside | Fd | [48] |
| 103 | γ-tocopherol | Fe | [56] |
| 104 | squalene | Fe | [56] |
| 105 | sucrose | Ft | [58] |
| 106 | fructose | Ft | [58] |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jing, R.; Li, H.-Q.; Hu, C.-L.; Jiang, Y.-P.; Qin, L.-P.; Zheng, C.-J. Phytochemical and Pharmacological Profiles of Three Fagopyrum Buckwheats. Int. J. Mol. Sci. 2016, 17, 589. https://doi.org/10.3390/ijms17040589
Jing R, Li H-Q, Hu C-L, Jiang Y-P, Qin L-P, Zheng C-J. Phytochemical and Pharmacological Profiles of Three Fagopyrum Buckwheats. International Journal of Molecular Sciences. 2016; 17(4):589. https://doi.org/10.3390/ijms17040589
Chicago/Turabian StyleJing, Rui, Hua-Qiang Li, Chang-Ling Hu, Yi-Ping Jiang, Lu-Ping Qin, and Cheng-Jian Zheng. 2016. "Phytochemical and Pharmacological Profiles of Three Fagopyrum Buckwheats" International Journal of Molecular Sciences 17, no. 4: 589. https://doi.org/10.3390/ijms17040589
APA StyleJing, R., Li, H.-Q., Hu, C.-L., Jiang, Y.-P., Qin, L.-P., & Zheng, C.-J. (2016). Phytochemical and Pharmacological Profiles of Three Fagopyrum Buckwheats. International Journal of Molecular Sciences, 17(4), 589. https://doi.org/10.3390/ijms17040589