Polymeric Nanoparticle-Based Photodynamic Therapy for Chronic Periodontitis in Vivo
Abstract
:1. Introduction
2. Results
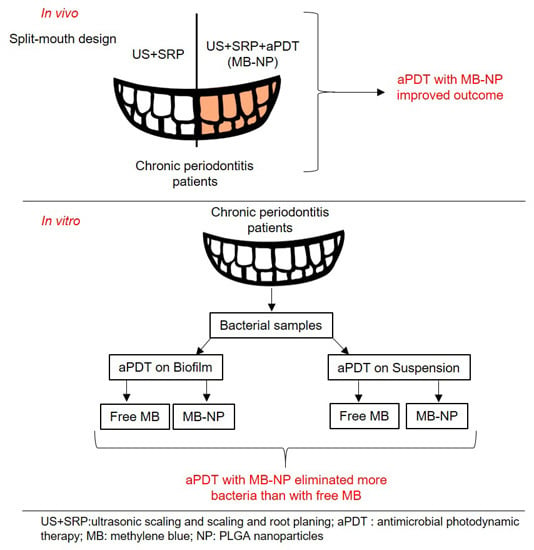
2.1. In Vitro Studies
2.2. In Vivo Study
3. Discussion
4. Materials and Methods
4.1. Subjects and Samples
4.2. In Vitro Study—Sample Collection
4.3. Preparation of Enriched Agar Plates
4.4. Development of Plaque-Derived Biofilms
4.5. Photodynamic Treatment in Vitro
4.5.1. Planktonic Bacteria
4.5.2. Biofilms
4.6. Preparation and Characterization of PLGA Nanocarriers
4.7. In Vivo Study
4.8. Data Collection—Measurement Reproducibility
4.9. Clinical Parameters
4.10. Statistical Analysis
Acknowledgments
Author Contributions
Conflicts of Interest
Abbreviations
| aPDT | antimicrobial photodynamic therapy |
| BOP | bleeding on probing |
| CFU | colony-forming unity |
| CAL | clinical attachment level |
| GBI | gingival bleeding index |
| MB | methylene blue |
| MB-NP | methylene blue-loaded PLGA nanoparticles |
| NP | nanoparticle |
| PLGA | poly(Lactic-co-Glycolic Acid) |
| PPD | probing pocket depth |
| ROS | reactive oxygen species |
| SRP | scaling and root planing |
| VPI | visible plaque index |
References
- Zuzanna, O.; Łabuzemail, P.; Macykemail, W.; Chomyszyn-Gajewskaemail, M. Antimicrobial photodynamic therapy—A discovery originating from the pre-antibiotic era in a novel periodontal therapy. Photodiagn. Photodyn. Ther. 2015, 12, 612–618. [Google Scholar] [CrossRef]
- Lin, J.; Bi, L.; Yu, X.; Kawai, T.; Taubman, M.A.; Shen, B.; Han, X. Porphyromonas gingivalis exacerbates ligature-induced, RANKL-dependent alveolar bone resorption via differential regulation of Toll-like receptor 2 (TLR2) and TLR4. Infect. Immun. 2014, 82, 4127–4134. [Google Scholar] [CrossRef] [PubMed]
- Hajishengallis, G. Periodontitis: From microbial immune subversion to systemic inflammation. Nat. Rev. Immunol. 2015, 15, 30–44. [Google Scholar] [CrossRef] [PubMed]
- Guthmiller, J.M.; Novak, K.F. Periodontal Diseases (chapter 8). In Polymicrobial Diseases; Brogden, K.A., Guthmiller, J.M., Eds.; ASM Press: Washington, DC, USA, 2002. [Google Scholar]
- Cieplik, F.; Tabenski, L.; Buchalla, W.; Maisch, T. Antimicrobial photodynamic therapy for inactivation of biofilms formed by oral key pathogens. Front. Microbiol. 2014, 5, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, S.; Hiorth, M. Advanced drug delivery systems for local treatment of the oral cavity. Ther. Deliv. 2015, 6, 595–608. [Google Scholar] [CrossRef] [PubMed]
- Allison, R.R.; Moghissi, K. Photodynamic therapy (PDT): PDT mechanisms. Clin. Endosc. 2013, 46, 24–29. [Google Scholar] [CrossRef] [PubMed]
- Dai, T.; Huang, Y.-Y.; Hamblin, M.R. Photodynamic therapy for localized infections—State of the art. Photodiagn. Photodyn. Ther. 2009, 6, 170–188. [Google Scholar] [CrossRef] [PubMed]
- Haag, P.A.; Steiger-Ronay, V.; Schmidlin, P.R. The in vitro Antimicrobial Efficacy of PDT against Periodontopathogenic Bacteria. Int. J. Mol. Sci. 2015, 16, 27327–27338. [Google Scholar] [CrossRef] [PubMed]
- Sreedhar, A.; Sarkar, I.; Rajan, P.; Pai, J.; Malagi, S.; Kamath, V.; Barmappa, R. Comparative evaluation of the efficacy of curcumin gel with and without photo activation as an adjunct to scaling and root planing in the treatment of chronic periodontitis: A split mouth clinical and microbiological study. J. Nat. Sci. Biol. Med. 2015, 6, S102–S109. [Google Scholar] [CrossRef] [PubMed]
- Moreira, A.L.; Novaes, A.B., Jr.; Grisi, M.F.; Taba, M., Jr.; Souza, S.L.; Palioto, D.B.; de Oliveira, P.G.; Casati, M.Z.; Casarin, R.C.; Messora, M.R. Antimicrobial photodynamic therapy as an adjunct to non-surgical treatment of aggressive periodontitis: A split-mouth randomized controlled trial. J. Periodontol. 2015, 86, 376–386. [Google Scholar] [CrossRef] [PubMed]
- Alwaeli, H.A.; Al-Khateeb, S.N.; Al-Sadi, A. Long-term clinical effect of adjunctive antimicrobial photodynamic therapy in periodontal treatment: A randomized clinical trial. Lasers Med. Sci. 2015, 30, 801–807. [Google Scholar] [CrossRef] [PubMed]
- Campanile Müller, V.S.; Giannopoulou, C.; Campanile, G.; Cancela, J.A.; Mombelli, A. Single or repeated antimicrobial photodynamic therapy as adjunct to ultrasonic debridement in residual periodontal pockets: Clinical, microbiological, and local biological effects. Lasers Med. Sci. 2015, 30, 27–34. [Google Scholar] [CrossRef]
- Azarpazhooh, A.; Shah, P.S.; Tenenbaum, H.C.; Goldberg, M.B. The effect of photodynamic therapy for periodontitis: A systematic review and meta-analysis. J. Periodontol. 2010, 81, 4–14. [Google Scholar] [CrossRef] [PubMed]
- Radcliffe, C.E.; Potouridou, L.; Qureshi, R.; Habahbeh, N.; Qualtrough, A.; Worthington, H.; Drucker, D.B. Antimicrobial activity of varying concentrations of sodium hypochlorite on the endodontic microorganisms Actinomyces israelii, A. naeslundii, Candida albicans and Enterococcus faecalis. Int. Endod. J. 2004, 37, 438–446. [Google Scholar] [CrossRef] [PubMed]
- Araújo, N.C.; Araújo, N.C.; Fontana, C.R.; Bagnato, V.S.; Gerbi, M.E. Photodynamic effects of curcumin against cariogenic pathogens. Photomed. Laser Surg. 2012, 30, 393–399. [Google Scholar] [CrossRef]
- Harris, F.; Chatfield, L.K.; Phoenix, D.A. Phenothiazinium based photosensitisers-photodynamic agents with a multiplicity of cellular targets and clinical applications. Curr. Drug Targets 2005, 6, 615–627. [Google Scholar] [CrossRef] [PubMed]
- Fontana, C.R.; Abernethy, A.D.; Som, S.; Ruggiero, K.; Doucette, S.; Marcantonio, R.C.; Boussios, C.I.; Kent, R.; Goodson, J.M.; Tanner, A.C.R.; et al. The antibacterial effect of photodynamic therapy in dental plaque-derived biofilms. J. Periodontal Res. 2009, 44, 751–759. [Google Scholar] [CrossRef] [PubMed]
- Ogura, M.; Abernethy, A.D.; Blissett, R.; Ruggiero, K.; Som, S.; Goodson, J.; Kent, R.; Doukas, A.; Soukos, N.S. Photomechanical wave-assisted molecular delivery in oral biofilms. World J. Microbiol. Biotechnol. 2007, 23, 1637–1646. [Google Scholar] [CrossRef]
- Müller, P.; Guggenheim, B.; Schmidlin, P.R. Efficacy of gasiform ozone and photodynamic therapy on a multispecies oral biofilm in vitro. Eur. J. Oral Sci. 2007, 115, 77–80. [Google Scholar] [CrossRef]
- Taraszkiewicz, A.; Fila, G.; Grinholc, M.; Nakonieczna, J. Innovative strategies to overcome biofilm resistance. BioMed Res. Int. 2013, 2013, 13. [Google Scholar] [CrossRef] [PubMed]
- Shiha, M.-H.; Huang, F.-C. Repetitive methylene blue-mediated photoantimicrobial chemotherapy changes the susceptibility and expression of the outer membrane proteins of Pseudomonas aeruginosa. Photodiagn. Photodyn. Ther. 2013, 10, 664–671. [Google Scholar] [CrossRef] [PubMed]
- Danhier, F.; Ansorena, E.; Silva, J.M.; Coco, R.; Le Breton, A.; Préat, V. PLGA-based nanoparticles: An overview of biomedical applications. J. Control. Release 2012, 161, 505–522. [Google Scholar] [CrossRef] [PubMed]
- Calixto, G.M.F.; Bernegossi, J.; de Freitas, L.M.; Fontana, C.R.; Chorilli, M. Nanotechnology-based drug delivery systems for photodynamic therapy of cancer: A review. Molecules 2016, 21, 342. [Google Scholar] [CrossRef] [PubMed]
- Nakanishia, W.; Minami, K.; Shrestha, L.K.; Ji, Q.; Hill, J.P.; Ariga, K. Bioactive nanocarbon assemblies: Nanoarchitectonics and applications. Nano Today 2014, 9, 378–394. [Google Scholar] [CrossRef]
- Ariga, K.; Kawakami, K.; Ebara, M.; Kotsuchibashi, Y.; Jia, Q.; Hillab, J.P. Bioinspired nanoarchitectonics as emerging drug delivery systems. New J. Chem. 2014, 38, 5149–5163. [Google Scholar] [CrossRef]
- Abdollahi, S.; Lotfipour, F. PLGA- and PLA-based polymeric nanoparticles for antimicrobial drug delivery. Biomed. Int. 2012, 3, 11–16. [Google Scholar]
- Santos, F.K.; Oyafuso, M.; Kiill, C.; Gremiao, M.P.; Chorilli, M. Nanotechnology-based drug delivery systems for treatment of hyperproliferative skin diseases—A review. Curr. Nanosci. 2013, 9, 159–167. [Google Scholar] [CrossRef]
- Souza, A.L.R.; Kiill, C.P.; Santos, F.K.; Luz, G.M.; Rocha e Silva, H.; Chorilli, M.; Gremiao, M.P.D. Nanotechnology-based drug delivery systems for dermatomycosis treatment. Curr. Nanosci. 2012, 8, 512–519. [Google Scholar] [CrossRef]
- Santos, B.F.; Gremião, M.P.D.; Chorilli, M. Nanotechnology-based drug delivery systems for the treatment of Alzheimer’s disease. Int. J. Nanomed. 2015, 10, 4981–5003. [Google Scholar] [CrossRef] [PubMed]
- Sato, M.R.; da Silva, P.B.; de Souza, R.A.; dos Santos, K.C.; Chorilli, M. Recent Advances in Nanoparticle Carriers for Coordination Complexes. Curr. Top. Med. Chem. 2015, 15, 287–297. [Google Scholar] [CrossRef] [PubMed]
- Koo, Y.E.L.; Fan, W.; Hah, H.; Xu, H.; Orringer, D.; Ross, B.; Rehemtulla, A.; Philbert, M.A.; Kopelman, R. Photonic explorers based on multifunctional nanoplatforms for biosensing and photodynamic therapy. Appl. Opt. 2007, 46, 1924–1930. [Google Scholar] [CrossRef] [PubMed]
- Pagonis, T.C.; Chen, J.; Fontana, C.R.; Devalapally, H.; Ruggiero, K.; Song, X.; Foschi, F.; Dunham, J.; Skobe, Z.; Yamazaki, H.; et al. Nanoparticle-based endodontic antimicrobial photodynamic therapy. J. Endod. 2010, 36, 322–328. [Google Scholar] [CrossRef] [PubMed]
- Deng, X.; Liang, Y.; Peng, X.; Su, T.; Luo, S.; Cao, J.; Gua, Z.; He, B. A facile strategy to generate polymeric nanoparticles for synergistic chemo-photodynamic therapy. Chem. Commun. 2015, 51, 4271–4274. [Google Scholar] [CrossRef] [PubMed]
- Lucky, S.S.; Soo, K.C.; Zhang, Y. Nanoparticles in photodynamic therapy. Chem. Rev. 2015, 115, 1990–2042. [Google Scholar] [CrossRef] [PubMed]
- Kumari, A.; Yadav, S.K.; Yadav, S.C. Biodegradable polymeric nanoparticles based drug delivery systems. Colloid Surf. B 2010, 75, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Sah, E.; Sahis, H. Recent trends in preparation of poly(lactide-co-glycolide) nanoparticles by mixing polymeric organic solution with antisolvent. J. Nanomater. 2015, 2015, 22. [Google Scholar] [CrossRef]
- Souza, E.; Medeiros, A.C.; Gurgel, B.C.; Sarmento, C. Antimicrobial photodynamic therapy in the treatment of aggressive periodontitis: A systematic review and meta-analysis. Lasers Med. Sci. 2016, 31, 187–196. [Google Scholar] [CrossRef] [PubMed]
- Sgolastra, F.; Petrucci, A.; Severino, M.; Graziani, F.; Gatto, R.; Monaco, A. Adjunctive photodynamic therapy to non-surgical treatment of chronic periodontitis: A systematic review and meta-analysis. J. Clin. Periodontol. 2013, 40, 514–526. [Google Scholar] [CrossRef] [PubMed]
- Del Pozo, J.L.; Patel, R. The challenge of treating biofilm-associated bacterial infections. Clin. Pharmacol. Ther. 2007, 82, 204–209. [Google Scholar] [CrossRef] [PubMed]
- Whiteley, M.; Bangera, M.G.; Bumgarner, R.E.; Parsek, M.R.; Teitzel, G.M.; Lory, S.; Greenberg, E.P. Gene expression in Pseudomonas aeruginosa biofilms. Nature 2001, 413, 860–864. [Google Scholar] [CrossRef] [PubMed]
- Brown, M.R.; Allison, D.G.; Gilbert, P. Resistance of bacterial biofilms to antibiotics: A growth-rate related effect? J. Antimicrob. Chemother. 1988, 22, 777–780. [Google Scholar] [CrossRef] [PubMed]
- Gilbert, P.; Das, J.; Foley, I. Biofilm susceptibility to antimicrobials. Adv. Dent. Res. 1997, 11, 160–167. [Google Scholar] [CrossRef] [PubMed]
- Tegos, G.P.; Masago, K.; Aziz, F.; Higginbotham, A.; Stermitz, F.R.; Hamblin, M.R. Inhibitors of bacterial multidrug efflux pumps potentiate antimicrobial photoinactivation. Antimicrob. Agents Chemother. 2008, 52, 3202–3209. [Google Scholar] [CrossRef] [PubMed]
- Nougayrède, J.P.; Kurokawa, K.; Tashiro, K.; Tobe, T.; Nakayama, K.; Kuhara, S.; Oswald, E.; Watanabe, H.; Hayashi, T. Extensive genomic diversity and selective conservation of virulence-determinants in enterohemorrhagic Escherichia coli strains of O157 and non-O157 serotypes. Genome Biol. 2007, 8, R138. [Google Scholar] [CrossRef]
- Klepac-Ceraj, V.; Patel, N.; Song, X.; Holewa, C.; Patel, C.; Kent, R.; Amiji, M.M.; Soukos, N.S. Photodynamic effects of methylene blue-loaded polymeric nanoparticles on dental plaque bacteria. Lasers Surg. Med. 2011, 43, 600–606. [Google Scholar] [CrossRef] [PubMed]
- McCarthy, J.R.; Perez, J.M.; Bruckner, C.; Weissleder, R. Polymeric nanoparticle preparation that eradicates tumors. Nano Lett. 2005, 5, 2552–2556. [Google Scholar] [CrossRef] [PubMed]
- Baeloa, A.; Levato, R.; Julián, E.; Crespo, A.; Astola, J.; Gavaldà, J.; Engel, E.; Mateos-Timoneda, M.A.; Torrents, E. Disassembling bacterial extracellular matrix with DNase-coated nanoparticles to enhance antibiotic delivery in biofilm infections. J. Control. Release 2015, 209, 150–158. [Google Scholar] [CrossRef] [PubMed]
- Forier, K.; Raemdonck, K.; de Smedt, S.C.; Demeester, J.; Coenye, T.; Braeckmans, K. Lipid and polymer nanoparticles for drug delivery to bacterial biofilms. J. Control. Release 2014, 190, 607–623. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Boda, S.K.; Broda, J.; Schiefer, F.; Weber-Heynemann, J.; Hoss, M.; Simon, U.; Basu, B.; Jahnen-Dechent, W. Cytotoxicity of ultrasmall gold nanoparticles on planktonic and biofilm encapsulated gram-positive Staphylococci. Small 2015, 11, 3183–3193. [Google Scholar] [CrossRef] [PubMed]
- Iannitelli, A.; Grande, R.; di Stefano, A.; di Giulio, M.; Sozio, P.; Bessa, L.J.; Laserra, S.; Paolini, C.; Protasi, F.; Cellini, L. Potential antibacterial activity of carvacrol-loaded poly (dl-lactide-co-glycolide)(PLGA) nanoparticles against microbial biofilm. Int. J. Mol. Sci. 2011, 12, 5039–5051. [Google Scholar] [CrossRef] [PubMed]
- Chronopoulou, L.; Massimi, M.; Giardi, M.F.; Cametti, C.; Devirgiliis, L.C.; Dentini, M.; Palocci, C. Chitosan-coated PLGA nanoparticles: A sustained drug release strategy for cell cultures. Colloids Surf. B Biointerfaces 2013, 103, 310–317. [Google Scholar] [CrossRef] [PubMed]
- Bechet, D.; Couleaud, P.; Frochot, C.; Viriot, M.L.; Guillemin, F.; Barberi-Heyob, M. Nanoparticles as vehicles for delivery of photodynamic therapy agents. Trends Biotechnol. 2008, 26, 612–621. [Google Scholar] [CrossRef] [PubMed]
- Keawchaoon, L.; Yoksan, R. Preparation, characterization and in vitro release study of carvacrol-loaded chitosan nanoparticles. Colloids Surf. B Biointerfaces 2011, 84, 163–171. [Google Scholar] [CrossRef] [PubMed]
- Ungaro, F.; d'Angelo, I.; Coletta, C.; d'Emmanuele di Villa Bianca, R.; Sorrentino, R.; Perfetto, B.; Tufano, M.A.; Miro, A.; La Rotonda, M.I.; Quaglia, F. Dry powders based on PLGA nanoparticles for pulmonary delivery of antibiotics: Modulation of encapsulation efficiency, release rate and lung deposition pattern by hydrophilic polymers. J. Control. Release 2012, 157, 149–159. [Google Scholar] [CrossRef] [PubMed]
- Petersilka, G.J.; Ehmke, B.; Flemmig, T.F. Antimicrobial effects of mechanical debridement. Periodontol. 2000 2002, 28, 56–71. [Google Scholar] [CrossRef] [PubMed]
- Zijnge, V.; Meijer, H.F.; Lie, M.A.; Tromp, J.A.; Degener, J.E.; Harmsen, H.J.; Abbas, F. The recolonization hypothesis in a full-mouth or multiple-session treatment protocol: A blinded, randomized clinical trial. J. Clin. Periodontol. 2010, 37, 518–525. [Google Scholar] [CrossRef] [PubMed]
- Teles, F.R.; Teles, R.P.; Uzel, N.G.; Song, X.Q.; Torresyap, G.; Socransky, S.S.; Haffajee, A.D. Early microbial succession in redeveloping dental biofilms in periodontal health and disease. J. Periodontal Res. 2012, 47, 95–104. [Google Scholar] [CrossRef] [PubMed]
- Sanz-Sánchez, I.; Ortiz-Vigón, A.; Herrera, D.; Sanz, M. Microbiological effects and recolonization patterns after adjunctive subgingival debridement with Er:YAG laser. Clin. Oral Investig. 2015. [Google Scholar] [CrossRef] [PubMed]
- Novaes, A.B., Jr.; Schwartz-Filho, H.O.; de Oliveira, R.R.; Feres, M.; Sato, S.; Figueiredo, L.C. Antimicrobial photodynamic therapy in the non-surgical treatment of aggressive periodontitis: Microbiological profile. Lasers Med. Sci. 2012, 27, 389–395. [Google Scholar] [CrossRef] [PubMed]
- Petelin, M.; Perkič, K.; Seme, K.; Gašpirc, B. Effect of repeated adjunctive antimicrobial photodynamic therapy on subgingival periodontal pathogens in the treatment of chronic periodontitis. Lasers Med. Sci. 2015, 30, 1647–1656. [Google Scholar] [CrossRef] [PubMed]
- Teles, R.; Benecha, H.K.; Preisser, J.S.; Moss, K.; Starr, J.R.; Corby, P.; Genco, R.; Garcia, N.; Giannobile, W.V.; Jared, H.; et al. Modelling changes in clinical attachment loss to classify periodontal disease progression. J. Clin. Periodontol. 2016. [Google Scholar] [CrossRef] [PubMed]
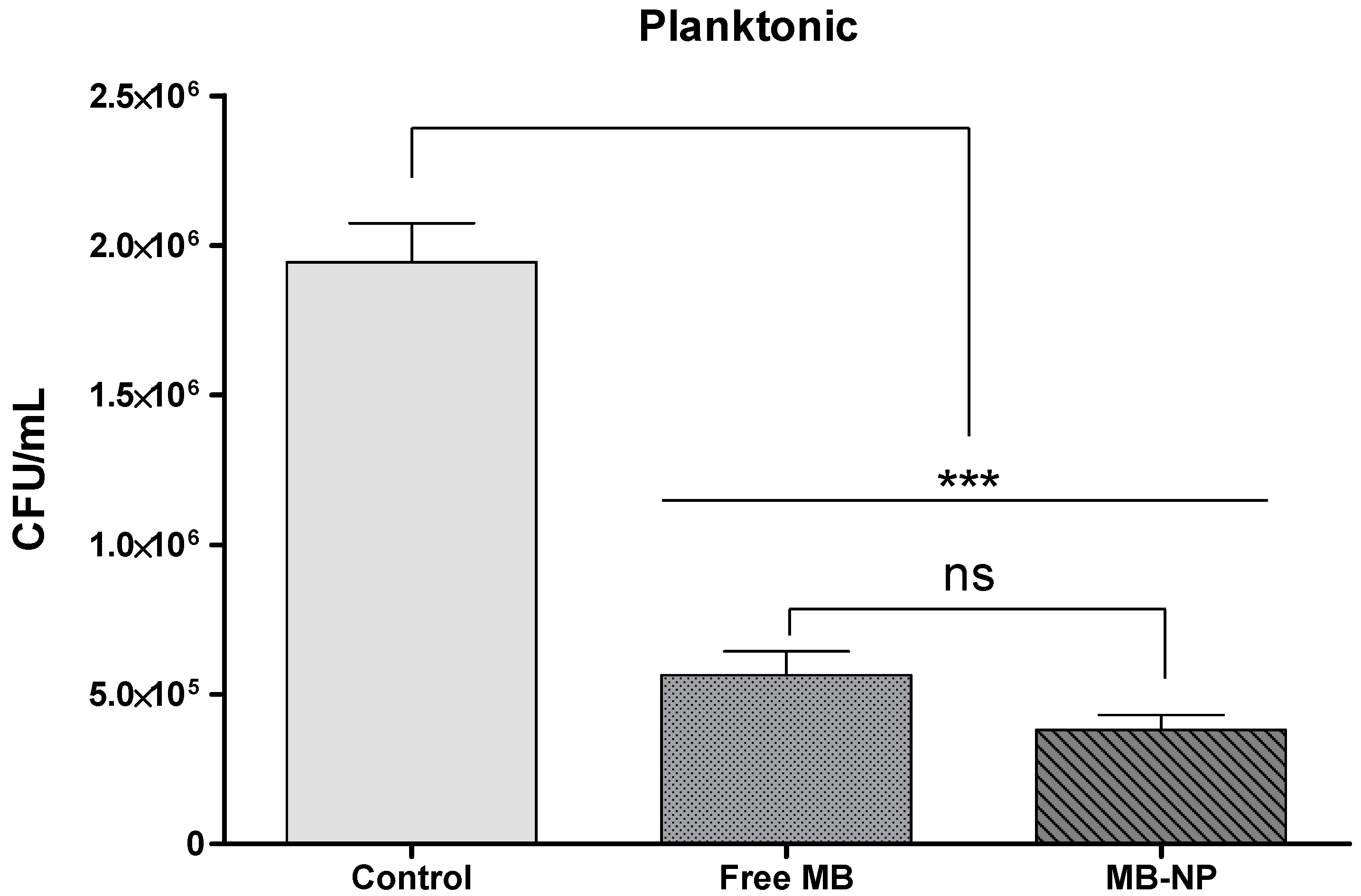





| Planktonic | Free MB | MB-NP |
|---|---|---|
| log10 steps reduction | 0.58 | 0.71 |
| % reduction | 70.97 | 80.40 |
| CFU/mL average | 564,167 | 380,833 |
| log10 average | 5.70 | 5.58 |
| SD | 0.21 | 0.20 |
| Biofilm | Free MB | MB-NP |
| log10 steps reduction | 0.35 | 0.69 |
| % reduction | 55.04 | 79.37 |
| CFU/mL average | 1,002,500 | 972,500 |
| log10 average | 6.00 | 5.99 |
| SD | 0.17 | 0.22 |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
De Freitas, L.M.; Calixto, G.M.F.; Chorilli, M.; Giusti, J.S.M.; Bagnato, V.S.; Soukos, N.S.; Amiji, M.M.; Fontana, C.R. Polymeric Nanoparticle-Based Photodynamic Therapy for Chronic Periodontitis in Vivo. Int. J. Mol. Sci. 2016, 17, 769. https://doi.org/10.3390/ijms17050769
De Freitas LM, Calixto GMF, Chorilli M, Giusti JSM, Bagnato VS, Soukos NS, Amiji MM, Fontana CR. Polymeric Nanoparticle-Based Photodynamic Therapy for Chronic Periodontitis in Vivo. International Journal of Molecular Sciences. 2016; 17(5):769. https://doi.org/10.3390/ijms17050769
Chicago/Turabian StyleDe Freitas, Laura Marise, Giovana Maria Fioramonti Calixto, Marlus Chorilli, Juçaíra Stella M. Giusti, Vanderlei Salvador Bagnato, Nikolaos S. Soukos, Mansoor M. Amiji, and Carla Raquel Fontana. 2016. "Polymeric Nanoparticle-Based Photodynamic Therapy for Chronic Periodontitis in Vivo" International Journal of Molecular Sciences 17, no. 5: 769. https://doi.org/10.3390/ijms17050769
APA StyleDe Freitas, L. M., Calixto, G. M. F., Chorilli, M., Giusti, J. S. M., Bagnato, V. S., Soukos, N. S., Amiji, M. M., & Fontana, C. R. (2016). Polymeric Nanoparticle-Based Photodynamic Therapy for Chronic Periodontitis in Vivo. International Journal of Molecular Sciences, 17(5), 769. https://doi.org/10.3390/ijms17050769