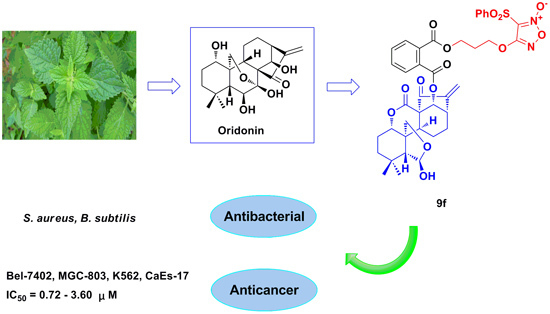
Synthesis, Biological Activity, and Apoptotic Properties of NO-Donor/Enmein-Type ent-Kauranoid Hybrids
Abstract
:1. Introduction
2. Results and Discussion
2.1. Chemistry
2.2. Antimicrobial Activity
2.3. Antiproliferative Activity
2.4. NO-Releasing Ability
2.5. Influence of 9f on the Bel-7402 Cell Cycle
2.6. Induction of Apoptosis by 9f
2.7. 9f Induced Mitochondrial Depolarization
3. Materials and Methods
3.1. Chemistry
3.1.1. General
3.1.2. General Procedure to Synthesize 9a–i
3.2. Biology
3.2.1. Antibacterial Assay
3.2.2. MTT Assay
3.2.3. Griess Assay
3.2.4. Cell Cycle Arrest
3.2.5. Cellular Apoptosis
3.2.6. Mitochondrial Membrane Potential
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
Abbreviations
| NO | Nitric Oxide |
| SAR | Structure Activity Relationship |
| DMAP | 4-Dimethylaminopyridine |
| EDCI | 1-Ethyl-3-(3-dimethylaminopropyl)carbodiimide Hydrochloride |
| rt | Room Temperature |
| THF | Tetrahydrofuran |
| SI | Selectivity Index |
| MTT | 3-(4,5-Dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide |
| TLC | Thin Layer Chromatograph |
| FBS | Fetal Bovine Serum |
| TMS | Tetramethylsilane |
| 1H NMR | Proton Nuclear Magnetic Resonance |
| TMS | Tetramethlysilane |
| ESI MS | Electrospray Ionization-Mass Spectrometry |
| HR-MS | High Resolution Mass Spectrum |
| DCM | Dichloromethane |
| MIC | Minimal Inhibitory Concentration |
| PI | Propidium Iodide |
| NT | Not Test |
References
- Pfeltz, R.F.; Wilkinson, B.J. The escalating challenge of vancomycin resistance in Staphylococcus aureus. Curr. Drug Targets Infect. Disord. 2004, 4, 273–294. [Google Scholar] [CrossRef] [PubMed]
- Roberts, M.C. Distribution of macrolide, lincosamide, streptogramin, ketolide and oxazolidinone (MLSKO) resistance genes in Gram-negative bacteria. Curr. Drug Targets Infect. Disord. 2004, 4, 207–215. [Google Scholar] [CrossRef] [PubMed]
- Hoadley, K.A.; Yau, C.; Wolf, D.M.; Cherniack, A.D.; Tamborero, D.; Ng, S.; Leiserson, M.D.; Niu, B.; McLellan, M.D.; Uzunangelov, V.; et al. Multiplatform analysis of 12 cancer types reveals molecular classification within and across tissues of origin. Cell 2014, 158, 929–944. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.B.; Di, Y.T.; Bao, Y.; Yuan, C.M.; Chen, G.; Li, D.H.; Bai, J.; He, H.P.; Hao, X.J.; Pei, Y.H.; et al. Peganumine A, a β-carboline dimer with a new octacyclic scaffold from Peganum harmala. Org. Lett. 2014, 16, 4028–4031. [Google Scholar] [CrossRef] [PubMed]
- Sai, C.M.; Li, D.H.; Xue, C.M.; Wang, K.B.; Hu, P.; Pei, Y.H.; Bai, J.; Jing, Y.K.; Li, Z.L.; Hua, H.M. Two pairs of enantiomeric alkaloid dimers from Macleaya cordata. Org. Lett. 2015, 17, 4102–4105. [Google Scholar] [CrossRef] [PubMed]
- Rostom, S.A.; Faidallah, H.M.; Radwan, M.F.; Badr, M.H. Bifunctional ethyl 2-amino-4-methylthiazole-5-carboxylate derivatives: Synthesis and in vitro biological evaluation as antimicrobial and anticancer agents. Eur. J. Med. Chem. 2014, 76, 170–181. [Google Scholar] [CrossRef] [PubMed]
- Climo, M.W.; Yokoe, D.S.; Warren, D.K.; Perl, T.M.; Bolon, M.; Herwaldt, L.A.; Weinstein, R.A.; Sepkowitz, K.A.; Jernigan, J.A.; Sanogo, K.; et al. Effect of daily chlorhexidine bathing on hospital-acquired infection. N. Engl. J. Med. 2013, 368, 533–542. [Google Scholar] [CrossRef] [PubMed]
- Vinšová, J.; Kozic, J.; Krátký, M.; Stolaříková, J.; Mandíková, J.; Trejtnar, F.; Buchta, V. Salicylanilide diethyl phosphates: Synthesis, antimicrobial activity and cytotoxicity. Bioorg. Med. Chem. 2014, 22, 728–737. [Google Scholar] [CrossRef] [PubMed]
- Huczyński, A.; Rutkowski, J.; Popiel, K.; Maj, E.; Wietrzyk, J.; Stefańska, J.; Majcher, U.; Bartl, F. Synthesis, antiproliferative and antibacterial evaluation of C-ring modified colchicine analogues. Eur. J. Med. Chem. 2015, 90, 296–301. [Google Scholar] [CrossRef] [PubMed]
- Emirdağ-Öztürk, S.; Karayıldırım, T.; Çapcı-Karagöz, A.; Alankuş-Çalışkan, Ö.; Özmen, A.; Poyrazoğlu-Çoban, E. Synthesis, antimicrobial and cytotoxic activities, and structure-activity relationships of gypsogenin derivatives against human cancer cells. Eur. J. Med. Chem. 2014, 82, 565–573. [Google Scholar] [CrossRef] [PubMed]
- Gu, W.; Hao, Y.; Zhang, G.; Wang, S.F.; Miao, T.T.; Zhang, K.P. Synthesis, in vitro antimicrobial and cytotoxic activities of new carbazole derivatives of ursolic acid. Bioorg. Med. Chem. Lett. 2015, 25, 554–557. [Google Scholar] [CrossRef] [PubMed]
- Harvey, A.L. Natural products in drug discovery. Drug Discov. Today 2008, 13, 894–901. [Google Scholar] [CrossRef] [PubMed]
- Newman, D.J.; Cragg, G.M. Natural products as sources of new drugs over the 30 years from 1981 to 2010. J. Nat. Prod. 2012, 75, 311–335. [Google Scholar] [CrossRef] [PubMed]
- Sun, H.D.; Huang, S.X.; Han, Q.B. Diterpenoids from Isodon species and their biological activities. Nat. Prod. Rep. 2006, 23, 673–698. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Li, D.H.; Wang, C.L.; Zhang, Y.H.; Xu, J.Y. Recent progress in the development of natural ent-kaurane diterpenoids with anti-tumor activity. Mini Rev. Med. Chem. 2011, 11, 910–919. [Google Scholar] [CrossRef] [PubMed]
- Cha, J.Y.; Yeoman, J.T.S.; Reisman, S.E. A concise total synthesis of (−)-maoecrystal Z. J. Am. Chem. Soc. 2011, 133, 14964–14967. [Google Scholar] [CrossRef] [PubMed]
- Gong, J.X.; Lin, G.A.; Sun, W.B.; Li, C.C.; Yang, Z. Total synthesis of (±) maoecrystal V. J. Am. Chem. Soc. 2010, 132, 16745–16746. [Google Scholar] [CrossRef] [PubMed]
- Pan, Z.; Zheng, C.; Wang, H.; Chen, Y.; Li, Y.; Cheng, B.; Zhai, H. Total synthesis of (±)-sculponeatin N. Org. Lett. 2014, 16, 216–219. [Google Scholar] [CrossRef] [PubMed]
- Carpenter, A.W.; Schoenfisch, M.H. Nitric oxide release: Part II. Therapeutic applications. Chem. Soc. Rev. 2012, 41, 3742–3752. [Google Scholar] [CrossRef] [PubMed]
- Bogdan, C. Nitric oxide and the immune response. Nat. Immunol. 2001, 2, 907–916. [Google Scholar] [CrossRef] [PubMed]
- Mocellin, S. Nitric oxide: Cancer target or anticancer agent? Curr. Cancer Drug Targets 2009, 9, 214–236. [Google Scholar] [CrossRef] [PubMed]
- Han, C.; Huang, Z.J.; Zheng, C.; Wan, L.D.; Zhang, L.W.; Peng, S.X.; Ding, K.; Ji, H.B.; Tian, J.D.; Zhang, Y.H. Novel hybrids of (phenylsulfonyl)furoxan and anilinopyrimidine as potent and selective epidermal growth factor receptor inhibitors for intervention of non-small-cell lung cancer. J. Med. Chem. 2013, 56, 4738–4748. [Google Scholar] [CrossRef] [PubMed]
- Ai, Y.; Kang, F.; Huang, Z.; Xue, X.; Lai, Y.; Peng, S.; Tian, J.; Zhang, Y. Synthesis of CDDO-amino acid-nitric oxide donor trihybrids as potential antitumor agents against both drug-sensitive and drug-resistant colon cancer. J. Med. Chem. 2015, 58, 2452–2464. [Google Scholar] [CrossRef] [PubMed]
- Fang, F.C. Antimicrobial reactive oxygen and nitrogen species: Concepts and controversies. Nat. Rev. Microbiol. 2004, 2, 820–832. [Google Scholar] [CrossRef] [PubMed]
- Privett, B.J.; Broadnax, A.D.; Bauman, S.J.; Riccio, D.A.; Schoenfisch, M.H. Examination of bacterial resistance to exogenous nitric oxide. Nitric Oxide 2012, 26, 169–173. [Google Scholar] [CrossRef] [PubMed]
- Backlund, C.J.; Worley, B.V.; Schoenfisch, M.H. Anti-biofilm action of nitric oxide-releasing alkyl-modified poly(amidoamine) dendrimers against Streptococcus mutans. Acta Biomater. 2016, 29, 198–205. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Li, D.; Xu, S.; Cai, H.; Yao, H.; Zhang, Y.; Jiang, J.; Xu, J. The conversion of oridonin to spirolactone-type or enmein-type diterpenoid: Synthesis and biological evaluation of ent-6,7-seco-oridonin derivatives as novel potential anticancer agents. Eur. J. Med. Chem. 2012, 52, 242–250. [Google Scholar] [CrossRef] [PubMed]
- Fu, J.; Liu, L.; Huang, Z.; Lai, Y.; Ji, H.; Peng, S.; Tian, J.; Zhang, Y. Hybrid molecule from O2-(2,4-dinitrophenyl)diazeniumdiolate and oleanolic acid: A glutathione S-transferase π-activated nitric oxide prodrug with selective anti-human hepatocellular carcinoma activity and improved stability. J. Med. Chem. 2013, 56, 4641–4655. [Google Scholar] [CrossRef] [PubMed]
- Fang, L.; Appenroth, D.; Decker, M.; Kiehntopf, M.; Lupp, A.; Peng, S.; Fleck, C.; Zhang, Y.; Lehmann, J. NO-donating tacrine hybrid compounds improve scopolamine-induced cognition impairment and show less hepatotoxicity. J. Med. Chem. 2008, 51, 7666–7669. [Google Scholar] [CrossRef]
- Zhang, X.M.; Guo, H.; Li, Z.S.; Song, F.H.; Wang, W.M.; Dai, H.Q.; Zhang, L.X.; Wang, J.G. Synthesis and evaluation of isatin-β-thiosemicarbazones as novel agents against antibiotic-resistant Gram-positive bacterial species. Eur. J. Med. Chem. 2015, 101, 419–430. [Google Scholar]
- Liao, J.; Yang, F.; Zhang, L.; Chai, X.; Zhao, Q.; Yu, S.; Zou, Y.; Meng, Q.; Wu, Q. Synthesis and biological evaluation of novel fluconazole analogues bearing 1,3,4-oxadiazole moiety as potent antifungal agents. Arch. Pharm. Res. 2015, 38, 470–479. [Google Scholar]
- Chen, T.C.; Wu, C.L.; Lee, C.C.; Chen, C.L.; Yu, D.S.; Huang, H.S. Structure-based hybridization, synthesis and biological evaluation of novel tetracyclic heterocyclic azathioxanthone analogues as potential antitumor agents. Eur. J. Med. Chem. 2015, 103, 615–627. [Google Scholar]
- Bi, Y.; Yang, X.; Zhang, T.; Liu, Z.; Zhang, X.; Lu, J.; Cheng, K.; Xu, J.; Wang, H.; Lv, G.; et al. Design, synthesis, nitric oxide release and antibacterial evaluation of novel nitrated ocotillol-type derivatives. Eur. J. Med. Chem. 2015, 101, 71–80. [Google Scholar]
- Shen, T.; Li, W.; Wang, Y.Y.; Zhong, Q.Q.; Wang, S.Q.; Wang, X.N.; Ren, D.M.; Lou, H.X. Antiproliferative activities of Garcinia bracteata extract and its active ingredient, isobractatin, against human tumor cell lines. Arch. Pharm. Res. 2014, 37, 412–420. [Google Scholar]
- Lepiarczyk, M.; Kałuża, Z.; Bielawska, A.; Czarnomysy, R.; Gornowicz, A.; Bielawski, K. Cytotoxic activity of octahydropyrazin[2,1-a:5,4-a′]diisoquinoline derivatives in human breast cancer cells. Arch. Pharm. Res. 2015, 38, 628–641. [Google Scholar]
- Yugandhar, D.; Nayak, V.L.; Archana, S.; Shekar, K.C.; Srivastava, A.K. Design, synthesis and anticancer properties of novel oxa/azaspiro[4,5]trienones as potent apoptosis inducers through mitochondrial disruption. Eur. J. Med. Chem. 2015, 101, 348–357. [Google Scholar]





| Compound | E. coli | S. aureus | B. subtilis | M. albicans |
|---|---|---|---|---|
| 7 | >100 | 32 | 32 | >100 |
| 8 | >100 | >100 | >100 | >100 |
| 9a | >100 | 16 | 64 | >100 |
| 9b | >100 | 4 | 2 | >100 |
| 9c | >100 | 8 | 4 | >100 |
| 9d | >100 | 4 | 2 | >100 |
| 9e | >100 | 16 | 16 | >100 |
| 9f | >100 | 32 | 64 | >100 |
| 9g | >100 | 16 | 16 | >100 |
| 9h | >100 | 16 | 16 | >100 |
| 9i | >100 | 16 | 2 | >100 |
| Chloromycetin | 4 | 4 | 8 | NT 1 |
| Fluconazole | NT | NT | NT | 4 |
| Compound | K562 | MGC-803 | CaEs-17 | Bel-7402 | L-02 | SI 2 |
|---|---|---|---|---|---|---|
| 7 | 4.76 ± 0.32 | 5.69 ± 0.39 | 11.03 ± 1.02 | 7.48 ± 0.53 | 18.26 ± 0.81 | 2.4 |
| 8 | 8.11 ± 0.76 | 14.21 ± 1.22 | 30.84 ± 2.09 | 32.96 ± 2.19 | 24.37 ± 1.59 | 0.7 |
| 9a | 2.47 ± 0.16 | 1.83 ± 0.16 | 5.12 ± 0.42 | 1.33 ± 0.10 | 10.36 ± 0.61 | 7.7 |
| 9b | 2.31 ± 0.21 | 1.62 ± 0.08 | 4.83 ± 0.30 | 1.20 ± 0.08 | 16.73 ± 0.17 | 13.9 |
| 9c | 1.93 ± 0.10 | 1.34 ± 0.13 | 3.76 ± 0.37 | 0.83 ± 0.06 | 19.87 ± 0.18 | 23.9 |
| 9d | 2.15 ± 0.12 | 1.50 ± 0.10 | 4.98 ± 0.42 | 1.23 ± 0.08 | 13.58 ± 1.31 | 11.0 |
| 9e | 1.92 ± 0.09 | 1.29 ± 0.12 | 4.27 ± 0.35 | 0.92 ± 0.05 | 17.20 ± 0.77 | 18.6 |
| 9f | 1.68 ± 0.12 | 1.11 ± 0.05 | 3.60 ± 0.12 | 0.72 ± 0.04 | 18.80 ± 1.25 | 26.1 |
| 9g | 2.26 ± 0.08 | 1.43 ± 0.08 | 5.13 ± 0.22 | 1.27 ± 0.11 | 11.57 ± 0.39 | 9.1 |
| 9h | 2.11 ± 0.16 | 1.49 ± 0.11 | 4.68 ± 0.31 | 1.08 ± 0.05 | 12.09 ± 1.08 | 11.1 |
| 9i | 1.86 ± 0.18 | 1.25 ± 0.10 | 3.82 ± 0.19 | 0.78 ± 0.04 | 14.57 ± 0.86 | 18.6 |
| Taxol 1 | 0.41 ± 0.02 | 0.85 ± 0.06 | 0.43 ± 0.03 | 1.89 ± 0.09 | 3.73 ± 0.17 | 1.9 |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, D.; Hu, X.; Han, T.; Xu, S.; Zhou, T.; Wang, Z.; Cheng, K.; Li, Z.; Hua, H.; Xiao, W.; et al. Synthesis, Biological Activity, and Apoptotic Properties of NO-Donor/Enmein-Type ent-Kauranoid Hybrids. Int. J. Mol. Sci. 2016, 17, 747. https://doi.org/10.3390/ijms17060747
Li D, Hu X, Han T, Xu S, Zhou T, Wang Z, Cheng K, Li Z, Hua H, Xiao W, et al. Synthesis, Biological Activity, and Apoptotic Properties of NO-Donor/Enmein-Type ent-Kauranoid Hybrids. International Journal of Molecular Sciences. 2016; 17(6):747. https://doi.org/10.3390/ijms17060747
Chicago/Turabian StyleLi, Dahong, Xu Hu, Tong Han, Shengtao Xu, Tingting Zhou, Zhenzhong Wang, Keguang Cheng, Zhanlin Li, Huiming Hua, Wei Xiao, and et al. 2016. "Synthesis, Biological Activity, and Apoptotic Properties of NO-Donor/Enmein-Type ent-Kauranoid Hybrids" International Journal of Molecular Sciences 17, no. 6: 747. https://doi.org/10.3390/ijms17060747
APA StyleLi, D., Hu, X., Han, T., Xu, S., Zhou, T., Wang, Z., Cheng, K., Li, Z., Hua, H., Xiao, W., & Xu, J. (2016). Synthesis, Biological Activity, and Apoptotic Properties of NO-Donor/Enmein-Type ent-Kauranoid Hybrids. International Journal of Molecular Sciences, 17(6), 747. https://doi.org/10.3390/ijms17060747