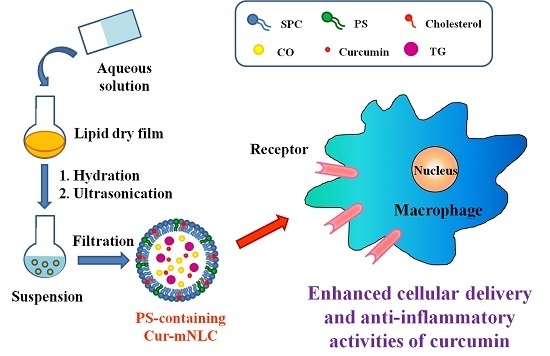
Enhancement of Anti-Inflammatory Activity of Curcumin Using Phosphatidylserine-Containing Nanoparticles in Cultured Macrophages
Abstract
:1. Introduction
2. Results
2.1. Particle Size, Zeta Potential, Entrapment Efficiency (EE), Drug Loading Capacity (DL), and Morphology
2.2. In Vitro Drug Release Studies
2.3. Hemocompatibility Assay
2.4. Cytotoxicity Studies
2.5. In Vitro Cellular Uptake
2.6. Enhanced Anti-Inflammatory Functions of Curcumin by PS in Cultured Macrophages
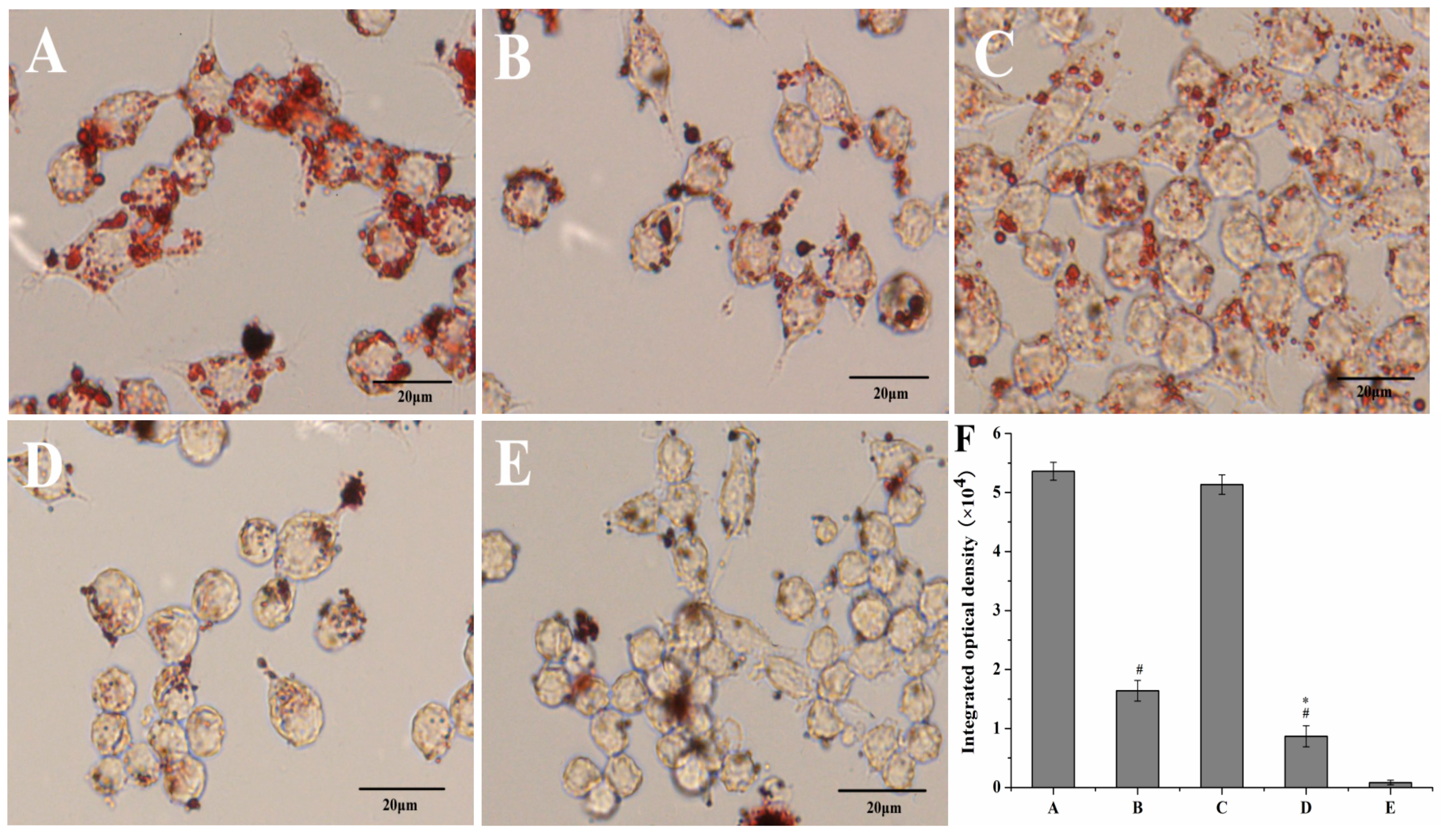
2.6.1. Lipid Uptake Behavior of Macrophages
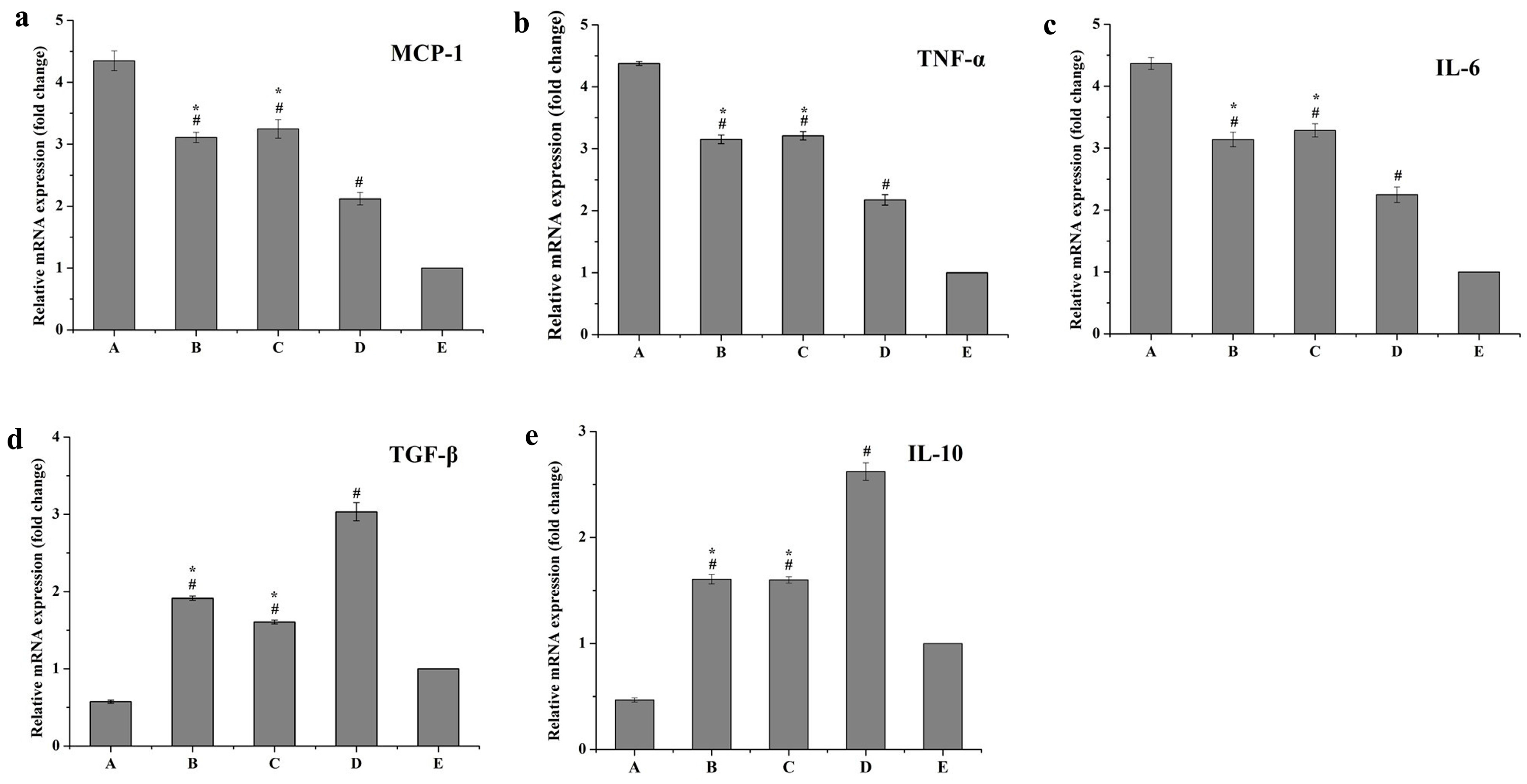
2.6.2. Inflammatory Responses of Macrophages
3. Discussion
4. Materials and Methods
4.1. Materials
4.2. Preparation of Different PS-Containing Cur-mNLCs
4.3. Particle Size and Zeta Potential
4.4. Entrapment Efficiency and Drug Loading Capacity
4.5. Transmission Electron Microscopy
4.6. In Vitro Drug Release Studies of Various PS-Containing Cur-mNLCs
4.7. Hemocompatibility Assay
4.8. Macrophage Culture
4.9. Cytotoxicity Assay
4.10. In Vitro Cellular Uptake
4.11. Enhanced Anti-Inflammatory Functions of Curcumin by PS in Cultured Macrophages
4.11.1. Lipid Uptake Behavior of Macrophages
Oil Red O Staining
Intracellular Cholesterol Measurement
4.11.2. Anti-Inflammatory Responses of Macrophages
Real-Time Quantitative PCR (RT-qPCR) Assay for mRNA Levels of Inflammatory Factors
Detection of Inflammatory Cytokines in Cell Culture Medium by ELISA
4.12. Statistical Analysis
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
Abbreviations
| AS | Atherosclerosis |
| C6 | Coumarin-6 |
| C6-mNLCs | PS-containing C6-loaded nanostructured lipid carriers |
| CLSM | Confocal laser scanning microscope |
| CO | Cholesteryl oleate |
| CT | Computed tomography |
| Cur-mNLCs | PS-containing curcumin-loaded nanostructured lipid carriers |
| DAPI | 4',6-diamidino-2-phenylindole |
| DL | Drug loading capacity |
| DLS | Dynamic light scattering |
| DMEM | Dulbecco’s modified Eagles’s medium |
| EE | Entrapment efficiency |
| FBS | Fetal bovine serum |
| FCM | Flow cytometry |
| GAPDH | Glyceraldehyde 3-phosphate dehydrogenase |
| HR | Hemolysis rate |
| IL-6 | Interleukin 6 |
| IL-10 | Interleukin 10 |
| MCP-1 | Monocyte chemoattractant protein 1 |
| MRI | Magnetic resonance imaging |
| MTT | 3-(4,5-dimethyl-thiazol-2-yl)-2,5-diphenyltetrazolium bromide |
| NLCs | Nanostructured lipid carriers |
| oxLDL | Oxidized low density lipoprotein |
| PBS | Phosphate buffer saline |
| PDI | Polydispersity index |
| PS | Phosphatidylserine |
| RT-qPCR | Real-time quantitative PCR |
| SDS | Sodium dodecyl sulfate |
| SPC | Soybean phosphatidylcholine |
| TEM | Transmission electron microscopy |
| TG | Trioleate glycerol |
| TGF-β | Transforming growth factor β |
| TNF | Tumor necrosis factor |
References
- Hyafil, F.; Cornily, J.C.; Feig, J.E.; Gordon, R.; Vucic, E.; Amirbekian, V.; Fisher, E.A.; Fuster, V.; Feldman, L.J.; Fayad, Z.A. Noninvasive detection of macrophages using a nanoparticulate contrast agent for computed tomography. Nat. Med. 2007, 13, 636–641. [Google Scholar] [CrossRef] [PubMed]
- Weissleder, R.; Nahrendorf, M.; Pittet, M.J. Imaging macrophages with nanoparticles. Nat. Mater. 2014, 13, 125–138. [Google Scholar] [CrossRef] [PubMed]
- Schiener, M.; Hossann, M.; Viola, J.R.; Ortega-Gomez, A.; Weber, C.; Lauber, K.; Lindner, L.H.; Soehnlein, O. Nanomedicine-based strategies for treatment of atherosclerosis. Trends Mol. Med. 2014, 20, 271–281. [Google Scholar] [CrossRef] [PubMed]
- Beloqui, A.; Coco, R.; Alhouayek, M.; Solinis, M.A.; Rodriguez-Gascon, A.; Muccioli, G.G.; Preat, V. Budesonide-loaded nanostructured lipid carriers reduce inflammation in murine DSS-induced colitis. Int. J. Pharm. 2013, 454, 775–783. [Google Scholar] [CrossRef] [PubMed]
- Beloqui, A.; Memvanga, P.B.; Coco, R.; Reimondez-Troitino, S.; Alhouayek, M.; Muccioli, G.G.; Alonso, M.J.; Csaba, N.; de la Fuente, M.; Preat, V. A comparative study of curcumin-loaded lipid-based nanocarriers in the treatment of inflammatory bowel disease. Colloid Surface B 2016, 143, 327–335. [Google Scholar] [CrossRef] [PubMed]
- Poon, I.K.; Lucas, C.D.; Rossi, A.G.; Ravichandran, K.S. Apoptotic cell clearance: Basic biology and therapeutic potential. Nat. Rev. Immunol. 2014, 14, 166–180. [Google Scholar] [CrossRef] [PubMed]
- Ravichandran, K.S. Beginnings of a good apoptotic meal: The find-me and eat-me signaling pathways. Immunity 2011, 35, 445–455. [Google Scholar] [CrossRef] [PubMed]
- Krysko, D.V.; Garg, A.D.; Kaczmarek, A.; Krysko, O.; Agostinis, P.; Vandenabeele, P. Immunogenic cell death and DAMPs in cancer therapy. Nat. Rev. Cancer 2012, 12, 860–875. [Google Scholar] [CrossRef] [PubMed]
- Fischer, U.; Schulze-Osthoff, K. Apoptosis-based therapies and drug targets. Cell Death Differ. 2005, 12, 942–961. [Google Scholar] [CrossRef] [PubMed]
- Bagalkot, V.; Deiuliis, J.A.; Rajagopalan, S.; Maiseyeu, A. “Eat me” imaging and therapy. Adv. Drug Deliv. Rev. 2016, 99, 2–11. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Fujii, S.; Wu, Z.; Hashioka, S.; Tanaka, Y.; Shiratsuchi, A.; Nakanishi, Y.; Nakanishi, H. Involvement of COX-1 and up-regulated prostaglandin E synthases in phosphatidylserine liposome-induced prostaglandin E2 production by microglia. J. Neuroimmunol. 2006, 172, 112–120. [Google Scholar] [CrossRef] [PubMed]
- Dvoriantchikova, G.; Agudelo, C.; Hernandez, E.; Shestopalov, V.I.; Ivanov, D. Phosphatidylserine-containing liposomes promote maximal survival of retinal neurons after ischemic injury. J. Cereb. Blood Flow Metab. 2009, 29, 1755–1759. [Google Scholar] [CrossRef] [PubMed]
- Otsuka, M.; Tsuchiya, S.; Aramaki, Y. Involvement of ERK, a MAP kinase, in the production of TGF-beta by macrophages treated with liposomes composed of phosphatidylserine. Biochem. Biophys. Res. Commun. 2004, 324, 1400–1405. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.A.; Jabeen, R.; Nasti, T.H.; Mohammad, O. Enhanced anticryptococcal activity of chloroquine in phosphatidylserine-containing liposomes in a murine model. J. Antimicrob. Chemother. 2005, 55, 223–228. [Google Scholar] [CrossRef] [PubMed]
- Shi, D.; Fu, M.; Fan, P.; Li, W.; Chen, X.; Li, C.; Qi, X.; Gao, T.; Liu, Y. Artificial phosphatidylserine liposome mimics apoptotic cells in inhibiting maturation and immunostimulatory function of murine myeloid dendritic cells in response to 1-chloro-2,4-dinitrobenze in vitro. Arch. Dermatol. Res. 2007, 299, 327–336. [Google Scholar] [CrossRef] [PubMed]
- Ichihashi, T.; Satoh, T.; Sugimoto, C.; Kajino, K. Emulsified phosphatidylserine, simple and effective peptide carrier for induction of potent epitope-specific T cell responses. PLoS ONE 2013, 8, e60068. [Google Scholar] [CrossRef] [PubMed]
- Ogawa, M.; Uchino, R.; Kawai, A.; Kosugi, M.; Magata, Y. PEG modification on (111)In-labeled phosphatidyl serine liposomes for imaging of atherosclerotic plaques. J. Nucl. Med. 2014, 55, 115–120. [Google Scholar] [CrossRef] [PubMed]
- Kansal, S.; Tandon, R.; Dwivedi, P.; Misra, P.; Verma, P.R.; Dube, A.; Mishra, P.R. Development of nanocapsules bearing doxorubicin for macrophage targeting through the phosphatidylserine ligand: A system for intervention in visceral leishmaniasis. J. Antimicrob. Chemother. 2012, 67, 2650–2660. [Google Scholar] [CrossRef] [PubMed]
- Adar, T.H.; Ben, M.T.; Amsalem, Y.; Feinberg, M.S.; Leor, J.; Cohen, S. Modulation of cardiac macrophages by phosphatidylserine-presenting liposomes improves infarct repair. Proc. Natl. Acad. Sci. USA 2011, 108, 1827–1832. [Google Scholar] [CrossRef] [PubMed]
- Maiseyeu, A.; Mihai, G.; Roy, S.; Kherada, N.; Simonetti, O.P.; Sen, C.K.; Sun, Q.; Parthasarathy, S.; Rajagopalan, S. Detection of macrophages via paramagnetic vesicles incorporating oxidatively tailored cholesterol ester: An approach for atherosclerosis imaging. Nanomedicine (London) 2010, 5, 1341–1356. [Google Scholar] [CrossRef] [PubMed]
- Maiseyeu, A.; Mihai, G.; Kampfrath, T.; Simonetti, O.P.; Sen, C.K.; Roy, S.; Rajagopalan, S.; Parthasarathy, S. Gadolinium-containing phosphatidylserine liposomes for molecular imaging of atherosclerosis. J. Lipid. Res. 2009, 50, 2157–2163. [Google Scholar] [CrossRef] [PubMed]
- Ogawa, M.; Umeda, I.O.; Kosugi, M.; Kawai, A.; Hamaya, Y.; Takashima, M.; Yin, H.; Kudoh, T.; Seno, M.; Magata, Y. Development of 111In-labeled liposomes for vulnerable atherosclerotic plaque imaging. Nucl. Med. Biol. 2015, 42, 299–304. [Google Scholar] [CrossRef] [PubMed]
- Geelen, T.; Yeo, S.Y.; Paulis, L.E.M.; Starmans, L.W.E.; Nicolay, K.; Strijkers, G.J. Internalization of paramagnetic phosphatidylserine-containing liposomes by macrophages. J. Nanobiotechnol. 2012, 10, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Liu, T.; Li, C.; Sun, H.; Luo, T.; Tan, Y.; Tian, D.; Guo, Z. Curcumin inhibits monocyte chemoattractant protein-1 expression and enhances cholesterol efflux by suppressing the c-Jun N-terminal kinase pathway in macrophage. Inflamm. Res. 2014, 63, 841–850. [Google Scholar] [CrossRef] [PubMed]
- Min, K.J.; Um, H.J.; Cho, K.H.; Kwon, T.K. Curcumin inhibits oxLDL-induced CD36 expression and foam cell formation through the inhibition of p38 MAPK phosphorylation. Food Chem. Toxicol. 2013, 58, 77–85. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.F.; Ching, L.C.; Huang, Y.C.; Chen, C.Y.; Chiang, A.N.; Kou, Y.R.; Shyue, S.K.; Lee, T.S. Molecular mechanism of curcumin on the suppression of cholesterol accumulation in macrophage foam cells and atherosclerosis. Mol. Nutr. Food Res. 2012, 56, 691–701. [Google Scholar] [CrossRef] [PubMed]
- Naksuriya, O.; Okonogi, S.; Schiffelers, R.M.; Hennink, W.E. Curcumin nanoformulations: A review of pharmaceutical properties and preclinical studies and clinical data related to cancer treatment. Biomaterials 2014, 35, 3365–3383. [Google Scholar] [CrossRef] [PubMed]
- Ji, G.; Yang, J.; Chen, J. Preparation of novel curcumin-loaded multifunctional nanodroplets for combining ultrasonic development and targeted chemotherapy. Int. J. Pharm. 2014, 466, 314–320. [Google Scholar] [CrossRef] [PubMed]
- Song, Z.; Zhu, W.; Liu, N.; Yang, F.; Feng, R. Linolenic acid-modified PEG-PCL micelles for curcumin delivery. Int. J. Pharm. 2014, 471, 312–321. [Google Scholar] [CrossRef] [PubMed]
- Esposito, E.; Ravani, L.; Mariani, P.; Huang, N.; Boldrini, P.; Drechsler, M.; Valacchi, G.; Cortesi, R.; Puglia, C. Effect of nanostructured lipid vehicles on percutaneous absorption of curcumin. Eur. J. Pharm. Biopharm. 2014, 86, 121–132. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.; Chen, H.; Zhao, J.; Pang, X.; Xi, Y.; Zhai, G. Development of a folate-modified curcumin loaded micelle delivery system for cancer targeting. Colloids Surf. B Biointerfaces 2014, 121, 206–213. [Google Scholar] [CrossRef] [PubMed]
- Kee, P.; Bagalkot, V.; Johnson, E.; Danila, D. Noninvasive detection of macrophages in atheroma using a radiocontrast-loaded phosphatidylserine-containing liposomal contrast agent for computed tomography. Mol. Imaging Biol. 2015, 17, 328–336. [Google Scholar] [CrossRef] [PubMed]
- Yu, T.; Xu, B.; He, L.; Xia, S.; Chen, Y.; Zeng, J.; Liu, Y.; Li, S.; Tan, X.; Ren, K.; et al. Pigment epithelial-derived factor gene loaded novel COOH-PEG-PLGA-COOH nanoparticles promoted tumor suppression by systemic administration. Int. J. Nanomed. 2016, 11, 743–759. [Google Scholar]
- Moore, K.J.; Sheedy, F.J.; Fisher, E.A. Macrophages in atherosclerosis: A dynamic balance. Nat. Rev. Immunol. 2013, 13, 709–721. [Google Scholar] [CrossRef] [PubMed]
- Chono, S.; Tauchi, Y.; Morimoto, K. Pharmacokinetic analysis of the uptake of liposomes by macrophages and foam cells in vitro and their distribution to atherosclerotic lesions in mice. Drug Metab. Pharmacokinet. 2006, 21, 37–44. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.H.; Termsarasab, U.; Cho, H.J.; Yoon, I.S.; Lee, J.Y.; Moon, H.T.; Kim, D.D. Preparation and characterization of self-assembled nanoparticles based on low-molecular-weight heparin and stearylamine conjugates for controlled delivery of docetaxel. Int. J. Nanomed. 2014, 9, 5711–5727. [Google Scholar] [CrossRef] [PubMed]
- Schrijvers, D.M.; De Meyer, G.R.; Herman, A.G.; Martinet, W. Phagocytosis in atherosclerosis: Molecular mechanisms and implications for plaque progression and stability. Cardiovasc. Res. 2007, 73, 470–480. [Google Scholar] [CrossRef] [PubMed]
- Gramatica, A.; Petazzi, R.A.; Lehmann, M.J.; Ziomkowska, J.; Herrmann, A.; Chiantia, S. alphaEnv-decorated phosphatidylserine liposomes trigger phagocytosis of HIV-virus-like particles in macrophages. Nanomedicine 2014, 10, 981–989. [Google Scholar] [PubMed]
- Greco, E.; Quintiliani, G.; Santucci, M.B.; Serafino, A.; Ciccaglione, A.R.; Marcantonio, C.; Papi, M.; Maulucci, G.; Delogu, G.; Martino, A.; et al. Janus-faced liposomes enhance antimicrobial innate immune response in Mycobacterium tuberculosis infection. Proc. Natl. Acad. Sci. USA 2012, 109, 1360–1368. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Birge, R.B.; Boeltz, S.; Kumar, S.; Carlson, J.; Wanderley, J.; Calianese, D.; Barcinski, M.; Brekken, R.A.; Huang, X.; Hutchins, J.T.; et al. Phosphatidylserine is a global immunosuppressive signal in efferocytosis, infectious disease, and cancer. Cell Death Differ. 2016, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Webb, M.S.; Johnstone, S.; Morris, T.J.; Kennedy, A.; Gallagher, R.; Harasym, N.; Harasym, T.; Shew, C.R.; Tardi, P.; Dragowska, W.H.; et al. In vitro and in vivo characterization of a combination chemotherapy formulation consisting of vinorelbine and phosphatidylserine. Eur. J. Pharm. Biopharm. 2007, 65, 289–299. [Google Scholar] [CrossRef] [PubMed]
- Mercanti, G.; Ragazzi, E.; Toffano, G.; Giusti, P.; Zusso, M. Phosphatidylserine and Curcumin Act Synergistically to Down-Regulate Release of Interleukin-1β from Lipopolysaccharide-Stimulated Cortical Primary Microglial Cells. CNS Neurol. Disord. Drug Targets 2014, 13, 792–800. [Google Scholar] [CrossRef] [PubMed]
- Peer, D. Immunotoxicity derived from manipulating leukocytes with lipid-based nanoparticles. Adv. Drug Deliv. Rev. 2012, 64, 1738–1748. [Google Scholar] [CrossRef] [PubMed]
- Allon, N.; Saxena, A.; Chambers, C.; Doctor, B.P. A new liposome-based gene delivery system targeting lung epithelial cells using endothelin antagonist. J. Control. Release 2012, 160, 217–224. [Google Scholar] [CrossRef] [PubMed]
- Bagalkot, V.; Badgeley, M.A.; Kampfrath, T.; Deiuliis, J.A.; Rajagopalan, S.; Maiseyeu, A. Hybrid nanoparticles improve targeting to inflammatory macrophages through phagocytic signals. J. Control. Release 2015, 217, 243–255. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; He, H.; Liu, J.; Wang, J.; Zhang, S.; Zhang, S.; Wu, Z. Pharmacokinetics and atherosclerotic lesions targeting effects of tanshinone IIA discoidal and spherical biomimetic high density lipoproteins. Biomaterials 2013, 34, 306–319. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Cui, J.; Wang, C.; Li, Y.; Zhang, H.; Wang, J.; Li, Y.; Zhang, L.; Zhang, L.; Guo, W.; et al. Encapsulation of mitoxantrone into pegylated SUVs enhances its antineoplastic efficacy. Eur. J. Pharm. Biopharm. 2008, 70, 657–665. [Google Scholar] [CrossRef] [PubMed]
- He, H.; Liu, L.; Bai, H.; Wang, J.; Zhang, Y.; Zhang, W.; Zhang, M.; Wu, Z.; Liu, J. Arachidonic acid-modified lovastatin discoidal reconstituted high density lipoprotein markedly decreases the drug leakage during the remodeling behaviors induced by lecithin cholesterol acyltransferase. Pharm. Res. 2014, 31, 1689–1709. [Google Scholar] [CrossRef] [PubMed]











| Samples | Particle Size (nm) | PDI | Zeta Potential (mV) | EE (%) | DL (%) |
|---|---|---|---|---|---|
| 0% PS | 212.1 ± 1.41 | 0.076 ± 0.002 | −1.33 ± 0.41 | 85.64 ± 0.67 | 4.04 ± 0.05 |
| 4% PS | 210.6 ± 1.27 | 0.060 ± 0.003 | −18.01 ± 0.54 | 86.13 ± 0.56 | 3.64 ± 0.03 |
| 8% PS | 207.6 ± 1.69 | 0.044 ± 0.005 | −45.31 ± 0.36 | 88.38 ± 0.34 | 3.96 ± 0.06 |
| 12% PS | 198.4 ± 1.74 | 0.047 ± 0.003 | −45.56 ± 0.41 | 88.78 ± 0.58 | 4.00 ± 0.04 |
| 20% PS | 156.8 ± 1.25 | 0.102 ± 0.006 | −45.85 ± 0.26 | 49.69 ± 0.33 * | 1.87 ± 0.08 * |
| Samples | 0% PS | 4% PS | 8% PS | 12% PS |
|---|---|---|---|---|
| Hemolysis rate (HR) (%) | 4.66 ± 0.96 | 3.36 ± 1.03 | 3.61 ± 0.87 | 4.23 ± 0.78 |
| Preparation | SPC (mol %) | PS (mol %) | CO (mol %) | TG (mol %) | Cholesterol (mol %) | Curcumin (mol %) |
|---|---|---|---|---|---|---|
| 0% PS | 45.39 | 0 | 12 | 24 | 10.46 | 8.15 |
| 4% PS | 41.39 | 4 | 12 | 24 | 10.46 | 8.15 |
| 8% PS | 37.39 | 8 | 12 | 24 | 10.46 | 8.15 |
| 12% PS | 33.39 | 12 | 12 | 24 | 10.46 | 8.15 |
| 20% PS | 25.39 | 20 | 12 | 24 | 10.46 | 8.15 |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, J.; Kang, Y.-X.; Pan, W.; Lei, W.; Feng, B.; Wang, X.-J. Enhancement of Anti-Inflammatory Activity of Curcumin Using Phosphatidylserine-Containing Nanoparticles in Cultured Macrophages. Int. J. Mol. Sci. 2016, 17, 969. https://doi.org/10.3390/ijms17060969
Wang J, Kang Y-X, Pan W, Lei W, Feng B, Wang X-J. Enhancement of Anti-Inflammatory Activity of Curcumin Using Phosphatidylserine-Containing Nanoparticles in Cultured Macrophages. International Journal of Molecular Sciences. 2016; 17(6):969. https://doi.org/10.3390/ijms17060969
Chicago/Turabian StyleWang, Ji, Yu-Xia Kang, Wen Pan, Wan Lei, Bin Feng, and Xiao-Juan Wang. 2016. "Enhancement of Anti-Inflammatory Activity of Curcumin Using Phosphatidylserine-Containing Nanoparticles in Cultured Macrophages" International Journal of Molecular Sciences 17, no. 6: 969. https://doi.org/10.3390/ijms17060969
APA StyleWang, J., Kang, Y.-X., Pan, W., Lei, W., Feng, B., & Wang, X.-J. (2016). Enhancement of Anti-Inflammatory Activity of Curcumin Using Phosphatidylserine-Containing Nanoparticles in Cultured Macrophages. International Journal of Molecular Sciences, 17(6), 969. https://doi.org/10.3390/ijms17060969