Evaluation of the Anti-Inflammatory Activity of Raisins (Vitis vinifera L.) in Human Gastric Epithelial Cells: A Comparative Study
Abstract
:1. Introduction
2. Results
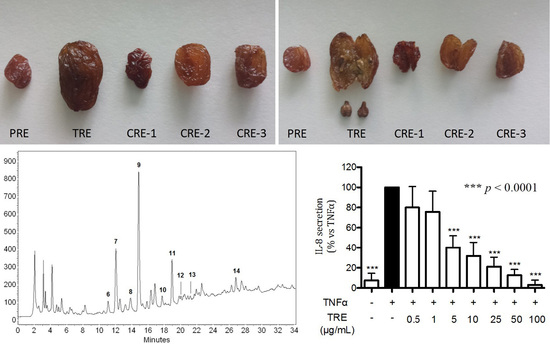
2.1. Total Phenol Content Assay
2.2. Validation of the HPLC-DAD Method
2.3. HPLC-DAD Characterization of Raisin Extracts
2.4. Effect of Hydro-Alcoholic Extracts from Different Raisins on IL-8 Release in Human Gastric Epithelial (AGS) Cells
2.5. TRE from Fruits Inhibits IL-8 Release and Promoter Activity by a NF-κB Independent Mechanism
2.6. TRE from Seeds Inhibits IL-8 Release and Promoter Activity by a NF-κB Dependent Mechanism
2.7. Contribution of Individual Compounds to the Anti-Inflammatory Activity of TRE from Seeds
3. Discussion
4. Materials and Methods
4.1. Reagents
4.2. Plant Material and Samples Preparation
4.3. Preparation of the Hydro-Alcoholic Extract
4.4. Cell Culture
4.5. Total Phenol Content Assay
4.6. HPLC-DAD Conditions and Method Validation
4.7. Measurement of IL-8 Secretion
4.8. NF-κB Driven Transcription and IL-8 Promoter Activity
4.9. NF-κB Nuclear Translocation
4.10. Cytotoxicity
4.11. Statistical Analysis
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
Abbreviations
| TNFα | Tumour necrosis factor alpha |
| NF-κB | Nuclear factor κB |
| IL-8 | Interleukin 8 |
| ELISA | Enzyme-linked immunosorbent assay |
| HPLC-DAD | High-performance liquid chromatography with Diode Array Detector |
| LDL | Low-density lipoprotein |
| IL-1β | Interleukin 1 β |
| ICAM-1 | Intercellular Adhesion Molecule 1 |
| GAE | Gallic acid equivalent |
| GA | Gallic acid |
| PRE | Portugal raisins extract sample |
| TRE | Turkey raisins extract sample |
| CRE1 | Commercial raisins extract 1 sample |
| CRE2 | Commercial raisins extract 2 sample |
| CRE3 | Commercial raisins extract 3 sample |
| FDA | Food and Drug Administration |
| CV | Coefficient of variation |
| LOD | Limit of Detection |
| LOQ | Limit of Quantitation |
| RSD | Relative standard deviation |
| s.d. | Standard deviation |
| EGCG | Epigallocatechin-3-gallate |
| DMEM F12 | Dulbecco’s Modified Eagle Medium F12 |
| FCS | Foetal calf serum |
| TMB | 3,3′,5,5′-tetramethylbenzidine |
| AP-1 | Activator protein 1 |
| MTT | 3,4,5-dimethylthiazol-2-yl-2-5-diphenyltetrazolium bromide |
References
- Williamson, G.; Carughi, A. Polyphenol content and health benefits of raisins. Nutr. Res. 2010, 30, 511–519. [Google Scholar] [CrossRef] [PubMed]
- Whiterspoon, B. Raisins to the rescue. Sch. Food Serv. Nutr. 2000, 54, 60–63. [Google Scholar]
- Carughi, A. Health Benefits of Sun-Dried Raisins; Health Research and Studies Center: Kingsburg, CA, USA, 2009. [Google Scholar]
- Karadeniz, F.; Durst, R.W.; Wrolstad, R.E. Polyphenolic composition of raisins. J. Agric. Food Chem. 2000, 48, 5343–5350. [Google Scholar] [CrossRef] [PubMed]
- Anderson, J.W.; Waters, A.R. Raisin consumption by humans: Effects on glycemia and insulinemia and cardiovascular risk factors. J. Food Sci. 2013, 78 (Suppl. S1), A11–A17. [Google Scholar] [CrossRef] [PubMed]
- Patel, B.P.; Bellissimo, N.; Luhovvyy, B.; Bennett, L.J.; Hurton, E.; Painter, J.E.; Anderson, G.H. A pre-meal raisin snack increases satiety and lowers cumulative food intake in normal weight children. Appl. Physiol. Nutr. Metab. 2011, 36, 475. [Google Scholar]
- Puglisi, M.J.; Vaishnav, U.; Shrestha, S.; Torres-Gonzalez, M.; Wood, R.J.; Volek, J.S.; Fernandez, M.L. Raisins and additional walking have distinct effects on plasma lipids and inflammatory cytokines. Lipids Health Dis. 2008, 7, 14. [Google Scholar] [CrossRef] [PubMed]
- Rankin, J.W.; Andreae, M.C.; Oliver Chen, C.Y.; O’Keefe, S.F. Effect of raisin consumption on oxidative stress and inflammation in obesity. Diabetes Obes. Metab. 2008, 10, 1086–1096. [Google Scholar] [CrossRef] [PubMed]
- Wong, A.; Young, D.A.; Emmanouil, D.E.; Wong, L.M.; Waters, A.R.; Booth, M.T. Raisins and oral health. J. Food Sci. 2013, 78 (Suppl. S1), A26–A29. [Google Scholar] [CrossRef] [PubMed]
- Bodger, K.; Crabtree, J.E. Helicobacter pylori and gastric inflammation. Br. Med. Bull. 1998, 54, 139–150. [Google Scholar] [CrossRef] [PubMed]
- Shimada, T.; Terano, A. Chemokine expression in Helicobacter pylori-infected gastric mucosa. J. Gastroenterol. 1998, 33, 613–617. [Google Scholar] [CrossRef] [PubMed]
- Van Den Brink, G.R.; ten Kate, F.J.; Ponsioen, C.Y.; Rive, M.M.; Tytgat, G.N.; van Deventer, S.J.; Peppelenbosch, M.P. Expression and activation of NF-kappa B in the antrum of the human stomach. J. Immunol. 2000, 164, 3353–3359. [Google Scholar] [CrossRef] [PubMed]
- Hsieh, Y.Y.; Shen, C.H.; Huang, W.S.; Chin, C.C.; Kuo, Y.H.; Hsieh, M.C.; Yu, H.R.; Chang, T.S.; Lin, T.H.; Chiu, Y.W.; et al. Resistin-induced stromal cell-derived factor-1 expression through toll-like receptor 4 and activation of p38 mapk/NF-κB signaling pathway in gastric cancer cells. J. Biomed. Sci. 2014, 21, 59. [Google Scholar] [CrossRef] [PubMed]
- Kaliora, A.C.; Kountouri, A.M.; Karathanos, V.T.; Koumbi, L.; Papadopoulos, N.G.; Andrikopoulos, N.K. Effect of greek raisins (Vitis vinifera L.) from different origins on gastric cancer cell growth. Nutr. Cancer 2008, 60, 792–799. [Google Scholar] [CrossRef] [PubMed]
- Breksa, A.P.; Takeoka, G.R.; Hildago, M.B.; Vilches, A.; Vasse, J.; Ramming, D.W. Antioxidant activity and phenolic content of 16 raisin grape (Vitis vinifera L.) cultivars and selections. Food Chem. 2010, 121, 740–745. [Google Scholar] [CrossRef]
- Yilmaz, Y.; Goksel, Z.; Erdogan, S.S.; Ozturk, A.; Atak, A.; Ozer, C. Antioxidant activity and phenolic content of seed, skin and pulp parts of 22 grape (Vitis vinifera L.) cultivars (4 common and 18 registered or candidate for registration). J. Food Process. Preserv. 2015, 39, 1682–1691. [Google Scholar] [CrossRef]
- Booth, B.; Arnold, M.E.; DeSilva, B.; Amaravadi, L.; Dudal, S.; Fluhler, E.; Gorovits, B.; Haidar, S.H.; Kadavil, J.; Lowes, S.; et al. Workshop report: Crystal city v—Quantitative bioanalytical method validation and implementation: The 2013 revised FDA guidance. AAPS J. 2015, 17, 277–288. [Google Scholar] [CrossRef] [PubMed]
- Sangiovanni, E.; Di Lorenzo, C.; Colombo, E.; Colombo, F.; Fumagalli, M.; Frigerio, G.; Restani, P.; Dell’Agli, M. The effect of in vitro gastrointestinal digestion on the anti-inflammatory activity of Vitis vinifera L. Leaves. Food Funct. 2015, 6, 2453–2463. [Google Scholar] [CrossRef] [PubMed]
- Grases, F.; Prieto, R.M.; Fernandez-Cabot, R.A.; Costa-Bauza, A.; Sanchez, A.M.; Prodanov, M. Effect of consuming a grape seed supplement with abundant phenolic compounds on the oxidative status of healthy human volunteers. Nutr. J. 2015, 14, 94. [Google Scholar] [CrossRef] [PubMed]
- Colombo, E.; Sangiovanni, E.; Dell’Agli, M. A review on the anti-inflammatory activity of pomegranate in the gastrointestinal tract. Evid. Based Complement. Altern. Med. 2013, 2013, 247145. [Google Scholar] [CrossRef] [PubMed]
- Safavi, M.; Shams-Ardakani, M.; Foroumadi, A. Medicinal plants in the treatment of Helicobacter pylori infections. Pharm. Biol. 2015, 53, 939–960. [Google Scholar] [CrossRef] [PubMed]
- Sério, S.; Rivero-Pèrez, M.D.; Correia, A.C.; Jordao, A.M.; Gonzàlez-San Josè, M.L. Analysis of commercial grape raisins: Phenolic content, antioxidant capacity and radical scavenger activity. Cienc. Tèc. Vitivinic. 2014, 29, 1–8. [Google Scholar] [CrossRef]
- Gessner, D.K.; Ringseis, R.; Siebers, M.; Keller, J.; Kloster, J.; Wen, G.; Eder, K. Inhibition of the pro-inflammatory NF-κB pathway by a grape seed and grape marc meal extract in intestinal epithelial cells. J. Anim. Physiol. Anim. Nutr. 2012, 96, 1074–1083. [Google Scholar] [CrossRef] [PubMed]
- Bak, M.J.; Truong, V.L.; Kang, H.S.; Jun, M.; Jeong, W.S. Anti-inflammatory effect of procyanidins from wild grape (Vitis amurensis) seeds in lps-induced raw 264.7 cells. Oxid. Med. Cell. Longev. 2013, 2013, 409321. [Google Scholar] [CrossRef] [PubMed]
- Adam, S.H.; Giribabu, N.; Kassim, N.; Kumar, K.E.; Brahmayya, M.; Arya, A.; Salleh, N. Protective effect of aqueous seed extract of Vitis vinifera against oxidative stress, inflammation and apoptosis in the pancreas of adult male rats with diabetes mellitus. Biomed. Pharmacother. 2016, 81, 439–452. [Google Scholar] [CrossRef] [PubMed]
- Mnaria, A.B.; Harzallaha, A.; Amria, Z.; Aguira, S.D.; Hammamia, M. Phyotchemical content, antioxidant properties, and phenolic profile of tunisian raisin varieties (Vitis vinifera L.). Int. J. Food Prop. 2016, 19, 578–590. [Google Scholar] [CrossRef]
- Mackenzie, G.G.; Carrasquedo, F.; Delfino, J.M.; Keen, C.L.; Fraga, C.G.; Oteiza, P.I. Epicatechin, catechin, and dimeric procyanidins inhibit PMA-induced NF-κB activation at multiple steps in jurkat t cells. FASEB J. 2004, 18, 167–169. [Google Scholar] [PubMed]
- Terra, X.; Pallares, V.; Ardevol, A.; Blade, C.; Fernandez-Larrea, J.; Pujadas, G.; Salvado, J.; Arola, L.; Blay, M. Modulatory effect of grape-seed procyanidins on local and systemic inflammation in diet-induced obesity rats. J. Nutr. Biochem. 2011, 22, 380–387. [Google Scholar] [CrossRef] [PubMed]
- Fraga, C.G.; Oteiza, P.I. Dietary flavonoids: Role of (−)-epicatechin and related procyanidins in cell signaling. Free Radic. Biol. Med. 2011, 51, 813–823. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Wang, J.; Chen, Y.; Agarwal, R. Anti-tumor-promoting activity of a polyphenolic fraction isolated from grape seeds in the mouse skin two-stage initiation-promotion protocol and identification of procyanidin B 5-3′-gallate as the most effective antioxidant constituent. Carcinogenesis 1999, 20, 1737–1745. [Google Scholar] [CrossRef] [PubMed]
- Singleton, V.L.; Rossi, J.A. Colorimetry of total phenolics with phosphomolybdic-phosphotungstic acid reagents. Am. J. Enol. Vitic. 1965, 16, 144–158. [Google Scholar]




| Measurement | PRE | TRE | TRE without Seeds | TRE Seeds | CRE1 | CRE2 | CRE3 |
|---|---|---|---|---|---|---|---|
| Total phenol content | 2.26 ± 0.12 | 15.04 ± 0.09 | 5.88 ± 0.78 | 147.78 ± 3.32 | 3.94 ± 0.12 | 3.77 ± 0.12 | 3.72 ± 0.03 |
| Compound | Precision | Linearity | Sensibility | |||
|---|---|---|---|---|---|---|
| Intraday (CV %) 1 | Interday (CV %) 1 | Linear Range (μg/mL) | Correlation Coefficient (R2) 1 | LOD 1 (ng/mL) | LOQ 1 (ng/mL) | |
| Catechin | 2.52 | 5.51 | 0.03–5 | 0.997 | 5.0 ± 0.6 | 16.6 ± 2.0 |
| Epicatechin | 3.44 | 3.90 | 0.03–5 | 0.999 | 4.2 ± 0.5 | 13.9 ± 1.8 |
| Epicatechin-3-gallate | 10.84 | 2.41 | 0.03–5 | 0.991 | 1.2 ± 0.1 | 3.9 ± 0.2 |
| Epigallocatechin-3-gallate | 3.87 | 6.63 | 0.1–5 | 0.999 | 2.1 ± 0.1 | 7.1 ± 0.3 |
| Procyanidin B1 | 2.29 | 4.10 | 0.1–5 | 0.998 | 6.9 ± 0.8 | 22.9 ± 2.8 |
| Procyanidin B2 | 2.16 | 4.19 | 0.1–5 | 0.995 | 5.2 ± 0.6 | 17.4 ± 2.1 |
| Procyanidin B3 | 3.72 | 6.14 | 0.1–5 | 0.996 | 8.9 ± 1.2 | 29.6 ± 4.1 |
| Procyanidin C1 | 2.66 | 7.19 | 0.075–5 | 0.993 | 5.6 ± 0.2 | 18.7 ± 0.5 |
| Chemical Class | Compounds | PRE | TRE | TRE Fruits | TRE Seeds | CRE1 | CRE2 | CRE3 |
|---|---|---|---|---|---|---|---|---|
| Organic acids | Caftaric acid | 59.40 ± 4.39 | 60.37 ± 1.05 | 51.48 ± 4.26 | N.Q. | 24.92 ± 1.50 | 74.93 ± 2.27 | 38.62 ± 0.73 |
| Flavonols | Rutin | 2.73 ± 0.34 | N.Q. | N.Q. | N.Q. | 4.32 ± 0.16 | 5.03 ± 0.23 | 5.54 ± 0.08 |
| Hyperoside | 0.59 ± 0.02 | N.Q. | N.Q. | 2.40 ± 0.13 | N.Q. | 0.50 ± 0.01 | 0.95 ± 0.02 | |
| Quercetin-3-O-glucoside | 10.99 ± 0.51 | 0.52 ± 0.07 | 2.18 ± 0.04 | 4.90 ± 0.26 | 5.00 ± 0.19 | 8.05 ± 0.21 | 23.34 ± 0.73 | |
| Quercetin-3-O-glucuronide | 21.68 ± 0.91 | N.D. | N.D. | N.D. | N.Q. | N.Q. | N.Q. | |
| Kaempferol-3-O-glucoside | 16.81 ± 0.49 | N.Q. | 0.70 ± 0.10 | 1.43 ± 0.01 | 1.03 ± 0.03 | 0.38 ± 0.03 | 4.48 ± 0.04 | |
| Flavan-3-ols monomers | Catechin | N.D. | 615.33 ± 42.84 | N.D. | 10231.06 ± 89.38 | N.D. | N.D. | N.D. |
| Epicatechin | N.D. | 148.14 ± 13.43 | N.D. | 3201.16 ± 156.90 | N.D. | N.D. | N.D. | |
| Epicatechin-3-gallate | N.D. | 10.86 ± 0.34 | N.D. | 300.50 ± 7.11 | N.D. | N.D. | N.D. | |
| Epigallocatechin-3-gallate | N.D. | 4.94 ± 0.22 | N.D. | 19.45 ± 0.62 | N.D. | N.D. | N.D. | |
| Flavan-3-ols dimers and trimers | Procyanidin B1 | N.D. | 345.46 ± 15.43 | N.D. | 1690.27 ± 76.26 | N.D. | N.D. | N.D. |
| Procyanidin B2 | N.D. | 41.82 ± 2.16 | N.D. | 579.60 ± 51.79 | N.D. | N.D. | N.D. | |
| Procyanidin B3 | N.D. | 138.93 ± 9.03 | N.D. | 1684.81 ± 34.85 | N.D. | N.D. | N.D. | |
| Procyanidin C1 | N.D. | 14.00 ± 1.24 | N.D. | 703.27 ± 4.85 | N.D. | N.D. | N.D. |
| Biological Assay | p65 Translocation | NF-κB Driven Transcription | IL-8 Promoter Activity | IL-8 Secretion |
|---|---|---|---|---|
| Turkey raisin fruits | >250 | >250 | 39.5 ± 3.1 | 37.8 ± 2.9 |
| Turkey raisin seeds | 1.81 ± 0.182 | 1.34 ± 0.14 | 0.86 ± 0.06 | 0.49 ± 0.05 |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Di Lorenzo, C.; Sangiovanni, E.; Fumagalli, M.; Colombo, E.; Frigerio, G.; Colombo, F.; Peres de Sousa, L.; Altindişli, A.; Restani, P.; Dell’Agli, M. Evaluation of the Anti-Inflammatory Activity of Raisins (Vitis vinifera L.) in Human Gastric Epithelial Cells: A Comparative Study. Int. J. Mol. Sci. 2016, 17, 1156. https://doi.org/10.3390/ijms17071156
Di Lorenzo C, Sangiovanni E, Fumagalli M, Colombo E, Frigerio G, Colombo F, Peres de Sousa L, Altindişli A, Restani P, Dell’Agli M. Evaluation of the Anti-Inflammatory Activity of Raisins (Vitis vinifera L.) in Human Gastric Epithelial Cells: A Comparative Study. International Journal of Molecular Sciences. 2016; 17(7):1156. https://doi.org/10.3390/ijms17071156
Chicago/Turabian StyleDi Lorenzo, Chiara, Enrico Sangiovanni, Marco Fumagalli, Elisa Colombo, Gianfranco Frigerio, Francesca Colombo, Luis Peres de Sousa, Ahmet Altindişli, Patrizia Restani, and Mario Dell’Agli. 2016. "Evaluation of the Anti-Inflammatory Activity of Raisins (Vitis vinifera L.) in Human Gastric Epithelial Cells: A Comparative Study" International Journal of Molecular Sciences 17, no. 7: 1156. https://doi.org/10.3390/ijms17071156
APA StyleDi Lorenzo, C., Sangiovanni, E., Fumagalli, M., Colombo, E., Frigerio, G., Colombo, F., Peres de Sousa, L., Altindişli, A., Restani, P., & Dell’Agli, M. (2016). Evaluation of the Anti-Inflammatory Activity of Raisins (Vitis vinifera L.) in Human Gastric Epithelial Cells: A Comparative Study. International Journal of Molecular Sciences, 17(7), 1156. https://doi.org/10.3390/ijms17071156